Abstract
BACKGROUND:
Lithium is a benchmark treatment for bipolar disorder in adults. Definitive studies of lithium in pediatric bipolar I disorder (BP-I) are lacking.
METHODS:
This multicenter, randomized, double-blind, placebo-controlled study of pediatric participants (ages 7–17 years) with BP-I/manic or mixed episodes compared lithium (n = 53) versus placebo (n = 28) for up to 8 weeks. The a priori primary efficacy measure was change from baseline to the end of study (week 8/ET) in the Young Mania Rating Scale (YMRS) score, based on last-observation-carried-forward analysis.
RESULTS:
The change in YMRS score was significantly larger in lithium-treated participants (5.51 [95% confidence interval: 0.51 to 10.50]) after adjustment for baseline YMRS score, age group, weight group, gender, and study site (P = .03). Overall Clinical Global Impression–Improvement scores favored lithium (n = 25; 47% very much/much improved) compared with placebo (n = 6; 21% very much/much improved) at week 8/ET (P = .03). A statistically significant increase in thyrotropin concentration was seen with lithium (3.0 ± 3.1 mIU/L) compared with placebo (–0.1 ± 0.9 mIU/L; P < .001). There was no statistically significant between-group difference with respect to weight gain.
CONCLUSIONS:
Lithium was superior to placebo in reducing manic symptoms in pediatric patients treated for BP-I in this clinical trial. Lithium was generally well tolerated in this patient population and was not associated with weight gain, distinguishing it from other agents commonly used to treat youth with bipolar disorder.
What’s Known on This Subject:
Strictly-defined pediatric bipolar I disorder (BP-I) is a serious condition. Although lithium is a benchmark treatment and has shown effectiveness in adults for decades, no definitive efficacy or long-term safety studies had been performed in pediatric patients with BP-I.
What This Study Adds:
This study provides evidence to support the efficacy of lithium in the acute treatment of youths with BP-I who are currently in a manic or mixed state. Lithium had an adverse effect profile that was acceptable for most patients.
Bipolar I disorder (BP-I) is a highly impairing mood disorder that often has its onset before adulthood.1 It is a psychiatric condition that occurs in pediatric patients worldwide.2 This chronic disorder is characterized by periods of spontaneous, abnormally elevated mood and abnormally irritable mood.3 BP-I is associated with substantial disability,4 suicide attempts,5,6 reduced quality of life, and significant functional impairment.7–9
Lithium has long been a benchmark treatment of adults with BP-I.10–14 Despite lithium’s use for BP-I in adults, definitive placebo-controlled, methodologically stringent studies of efficacy have not been available for children.15
The Best Pharmaceuticals for Children Act was signed into law in 2002 and re-authorized in 2007 under the Food and Drug Administration Amendments Act and in 2012 under the Food and Drug Administration Safety and Innovation Act.16 The law incorporates as its key legislative goals: (1) prioritizing the study of off-patent drugs used in children; and (2) sponsoring clinical trials when a pharmaceutical company declines to perform them. In 2005, a written request from the US Food and Drug Administration was sent to the National Institutes of Health, who, in turn, developed a contract with a consortium of experts in the field of child and adolescent psychiatry to conduct a rigorous and comprehensive set of clinical studies of lithium in children with BP-I. Hence, the Collaborative Lithium Trials were conducted.
One of the foremost purposes of the Collaborative Lithium Trials was to examine the acute efficacy and long-term safety of lithium in participants aged 7 to 17 years with BP-I. After establishing the pharmacokinetics, the empirically determined dosing strategy, and tolerability through an initial set of studies,17,18 the first randomized, double-blind, placebo-controlled lithium acute efficacy trial was conducted in a different patient sample. The present article describes the key findings of that trial and the impact the results may have on pediatric mental health.
Methods
We conducted a multicenter, randomized, placebo-controlled outpatient trial to examine lithium in the acute treatment of pediatric patients with BP-I. Details of the study design and methods are presented elsewhere19 and are briefly summarized here. Outpatient participants were enrolled at 1 of 10 academic medical centers in the United States that are experienced in pediatric psychiatric care. The study duration for each participant in this efficacy clinical trial was up to 8 weeks, with visits completed at weeks 1, 2, 3, 4, 6, and 8 and telephone assessments at day 3 of week 1, week 5, and week 7. The first participant was enrolled on June 2, 2010, and date of study completion for the last participant was February 7, 2013.
Study Participants
Children aged 7 to 17 years meeting unmodified Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for BP-I currently in a manic or mixed episode, scoring ≥20 on the Young Mania Rating Scale (YMRS),20 having a negative drug screen at baseline and remaining drug-free through the study period, and willing and clinically able to undergo a washout period for all psychotropic medications were eligible. Children were ineligible if they: were clinically stable on a medication regimen for BP-I; diagnosed with schizophrenia or schizoaffective disorder, a pervasive developmental disorder, anorexia nervosa, bulimia nervosa, obsessive-compulsive disorder, substance dependence, symptoms of mania that were attributable to a general medical condition or secondary to use of medications or general medical condition including neurologic disease, diabetes mellitus, thyroid dysfunction, or renal dysfunction that might be adversely affected by lithium; had clinically significant abnormal laboratory assessments that could influence the efficacy or safety of lithium or would complicate interpretation of study results; had evidence of serious homicidal/suicidal ideation or active hallucinations and delusions such that in the treating physician's opinion it would not be appropriately safe for the subject to participate in this study; or had concomitant prescription of over-the-counter medication or nutritional supplements that would interact with lithium or affect the participant’s physical or mental status.
Initially, the prescription of concomitant psychostimulants was precluded. However, starting in June 2011, to enhance recruitment and retention, participants with comorbid attention-deficit/hyperactivity disorder were able to receive psychostimulants after 4 weeks of double-blind therapy at the treating physician’s discretion. Melatonin (up to 3 mg) at bedtime was permitted to treat insomnia.
Institutional Review Board Review and Informed Consent
Local institutional review board approval of the protocol, informed consent, advertising, and all amendments were obtained at each of the 10 study sites before implementation. Before the initiation of any study-related procedures, the informed consent statement was signed by the participant’s parent or legal guardian and by the person who was authorized to administer the informed consent. Children who could read and understand the assent form were asked to give written assent.
Diagnostic Procedures
Eligible participants underwent a psychiatric interview with a board-certified or board-eligible child and adolescent psychiatrist. This interview was followed by an assessment with an interviewer/rater trained on study-specific procedures using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL)21 to confirm the clinician’s diagnosis.
Initial training presentations were provided for the K-SADS-PL, the YMRS, the Children's Depression Rating Scale–Revised (CDRS-R),22,23 and the Columbia Suicide Severity Rating Scale24 for raters across all 10 sites. Raters were considered trained if they scored within 20% of the gold standard score for the YMRS and CDRS-R videos (established by the lead clinical site Principal Investigator) in addition to completing a written examination regarding administration of the K-SADS-PL. Inter-rater reliability was completed on the CDRS-R and the YMRS every 6 months to ensure consistency between raters at all sites.
Randomization, Masking, and Drug Administration
Participants were enrolled into the study and were randomized to receive lithium or matching placebo in a 2 (lithium):1 (placebo) allocation ratio. Stratification factors included study site, age at randomization (7–11 years and 12–17 years), and gender (male and female). The randomization list was created by an unblinded BPCA data coordinating center (DCC) statistician. Unblinded site staff members were provided randomization assignments via an electronic data capture system.
The dosing of lithium used in the present study was based on previous research conducted by this investigative group.17,18 The starting dose of lithium (supplied as 300-mg, regular-release capsules) was either 600 or 900 mg/d. Participants weighing <30 kg started with 600 mg/d; all other participants began lithium therapy with 900 mg/d. Dose increases of 300 mg/d could occur at study visits and via telephone call during the middle of the first week of randomized treatment unless the participant had the following: had met dosing response criteria (defined as a Clinical Global Impression–Improvement scale [CGI-I]25 score ≤2 and a 50% decrease in the YMRS score from baseline assessment); experienced ≥1 adverse effect that significantly affected functioning that was at least of moderate severity; had a serum lithium level >1.4 mEq/L; or if the dose exceeded 40 mg/kg/d (with the exception of participants weighing <23 kg, who could receive up to 900 mg/d). Participants randomized to receive placebo were preassigned to a maximum dose at randomization to maintain the integrity of the blind. Adherence to study medication was monitored by using a dosing diary and pill counts.
Study Assessments
The a priori primary outcome measure was the change from baseline to the end of study (week 8/early termination [ET]) on the YMRS score, based on last-observation-carried-forward (LOCF) values. Beginning at baseline, psychometric assessments performed at study visits included the YMRS, the CDRS-R, and the Clinical Global Impression–Severity scale (CGI-S).24 Starting at week 1, the CGI-I was obtained at study visits. The Columbia Suicide Severity Rating Scale was completed at each study visit to assess for suicidal behavior and ideation by a trained rater. Baseline scores were compared with subsequent scores during the 8-week trial. Treatment safety at each visit was evaluated based on the incidence of adverse events (AEs), treatment-emergent AEs, serious AEs, treatment-emergent AEs leading to study drug discontinuation, clinically significant laboratory findings, vital signs, electrocardiogram investigations, physical examination abnormalities, and trough lithium serum levels. Lithium levels were obtained at all study visits.
AE Monitoring
Participants were monitored for the presence of treatment-emergent AEs by open-ended inquiry and use of the Side Effects Form for Children and Adolescents,26 the Neurological Examination for Lithium,18 and the Neurological Rating Scale27 at each study visit. A 13-item expanded version of the Neurological Rating Scale was used to assess for potential additional extrapyramidal adverse effects. These additional items include: (1) cogwheeling; (2) acute dystonic reaction; and (3) subjective sense of stiffness.
Items from the Side Effects Form for Children and Adolescents, the Neurological Examination for Lithium, the Neurological Rating Scale, or open-ended inquiry that were reported as being present since the last visit were documented at each study visit. The study physician who conducted the visit determined whether the effects that were reported constituted an AE and whether the AE was related to study medication.
The intensity or severity of AEs was graded as follows: mild (awareness of sign or symptom but easily tolerated; not expected to have a clinically significant effect on the participant’s overall health and well-being; not likely to require medical attention); moderate (discomfort enough to cause interference with usual activity or affects clinical status; may require medical intervention); or severe (incapacitating or significantly affecting clinical status; likely requires medical intervention and/or close follow-up).
Statistical Analyses and Sample Size Determinations
Sample size determination was based on interim conditional power analyses. These analyses suggested that with a total sample size of 100, there would be 94% power to detect a statistically significant difference in the mean change from baseline to week 8/ET in YMRS scores in the 2 treatment arms, based on LOCF values.
Efficacy variables that were based on assessment instruments administered over the course of weekly assessments in the study, including the YMRS, the CDRS-R, and the Children's Global Assessment Scale (CGAS),28 were analyzed primarily on the basis of mean change from baseline to end-of-study scores according to LOCF methods. These changes were assessed by using an analysis of covariance model, with change from baseline score as the dependent variable, baseline score as a covariate, and age stratum, gender, weight (<30 kg and ≥30 kg), study site (pooled), and treatment group as factors.
The CGI-S and CGI-I (overall illness scores) are measured on a Likert scale and were analyzed by using a logistic regression model. A reduction of at least 2 points from baseline to week 8/ET on the CGI-S was considered an improvement for this measure. A score of 1 or 2 on the CGI-I measured at week 8/ET was classified as an improvement on this scale. Independent factors in the logistic regression model were age stratum, gender, weight (<30 kg or ≥30 kg), and treatment group.
The categorical end points of response and remission were compared according to treatment group. Response was defined as a reduction in baseline YMRS score ≥50% and a CGI-I score of 1 or 2. Remission was defined as a YMRS score ≤12 and a CGI-S score ≤2. Cochran-Mantel-Haenszel tests were performed to determine if there was a significant difference in proportions between the treatment groups for each of these end points.
All analyses were conducted by using SAS version 9.2 or higher (SAS Institute, Inc, Cary, NC).
Results
Study Participants and Characteristics
A total of 153 participants were screened for possible inclusion into this clinical trial. Of these 153, 81 (53%) were randomized to receive lithium (n = 53) or placebo (n = 28) based on a 2:1 allocation ratio. Fig 1 summarizes participants screened and enrolled into the study. Enrollment across the sites ranged from 1 patient to 24 patients randomized to study.
FIGURE 1.
Participant flow. aParticipant also experienced previous serious AE of aggression.
Table 1 displays the baseline characteristics of the participants. There were no statistically significant differences between the lithium and placebo groups regarding the baseline variables of age group, gender, race, and ethnicity. Furthermore, there were no statistically significant differences between treatment groups at baseline with regard to the most recent episode (manic or mixed) and YMRS, CDRS-R, CGAS, or CGI-S overall illness scores.
TABLE 1.
Baseline Demographic Characteristics, Symptoms, and Comorbid Diagnoses
| Characteristic | 7–11 Years of Age | 12–17 Years of Age | Total | |||
|---|---|---|---|---|---|---|
| Lithium (n = 32) | Placebo (n = 17) | Lithium (n = 21) | Placebo (n = 11) | Lithium (n = 53) | Placebo (n = 28) | |
| Age at baseline, y | ||||||
| Mean ± SD | 9.5 ± 1.6 | 9.1 ± 1.3 | 14.6 ± 1.5 | 14.5 ± 1.6 | 11.5 ± 2.9 | 11.2 ± 3.0 |
| Median (Min–Max) | 9.8 (7.3–11.9) | 9.0 (7.0–11.6) | 14.1 (12.3–17.3) | 14.3 (12.5–16.7) | 11.3 (7.3–17.3) | 10.6 (7.0–16.7) |
| Gender, n (%) | ||||||
| Male | 14 (43.8) | 10 (58.8) | 8 (38.1) | 5 (45.5) | 22 (41.5) | 15 (53.6) |
| Female | 18 (56.3) | 7 (41.2) | 13 (61.9) | 6 (54.5) | 31 (58.5) | 13 (46.4) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 6 (18.8) | 1 (5.9) | 3 (14.3) | 2 (18.2) | 9 (17.0) | 3 (10.7) |
| Not Hispanic or Latino | 26 (81.3) | 16 (94.1) | 17 (81.0) | 8 (72.7) | 43 (81.1) | 24 (85.7) |
| Not reported | 0 | 0 | 1 (4.8) | 1 (9.1) | 1 (1.9) | 1 (3.6) |
| Race, n (%) | ||||||
| White | 18 (56.3) | 10 (58.8) | 12 (57.1) | 4 (36.4) | 30 (56.6) | 14 (50.0) |
| African American | 10 (31.3) | 6 (35.3) | 5 (23.8) | 5 (45.5) | 15 (28.3) | 11 (39.3) |
| Asian | 0 | 0 | 2 (9.5%) | 0 | 2 (3.8) | 0 |
| >1 race | 3 (9.4) | 1 (5.9) | 1 (4.8) | 1 (9.1) | 4 (7.5) | 2 (7.1) |
| Not reported | 1 (3.1) | 0 | 1 (4.8) | 1 (9.1) | 2 (3.8) | 1 (3.6) |
| Most recent episode, n (%) | ||||||
| Manic | 15 (46.9) | 10 (58.8) | 8 (38.1) | 8 (72.7) | 23 (43.4) | 18 (64.3) |
| Mixed | 17 (53.1) | 7 (41.2) | 13 (61.9) | 3 (27.3) | 30 (56.6) | 10 (35.7) |
| YMRS total score | ||||||
| Mean ± SD | 28.9 ± 4.6 | 31.4 ± 6.3 | 30.5 ± 6.9 | 27.9 ± 4.9 | 29.5 ± 5.6 | 30.0 ± 6.0 |
| CDRS-R total score | ||||||
| Mean ± SD | 34.2 ± 8.8 | 40.5 ± 11.3 | 38.2 ± 11.7 | 34.6 ± 7.3 | 35.8 ± 10.1 | 38.2 ± 10.2 |
| CGI-S score (overall illness), n (%) | ||||||
| 4, moderately ill | 17 (53.1) | 4 (23.5) | 10 (47.6) | 8 (72.7) | 27 (50.9) | 12 (42.9) |
| 5, markedly ill | 13 (40.6) | 12 (70.6) | 9 (42.9) | 3 (27.3) | 22 (41.5) | 15 (53.6) |
| 6, severely ill | 2 (6.3) | 1 (5.9) | 2 (9.5) | 0 | 4 (7.5) | 1 (3.6) |
| CGAS score | ||||||
| Mean ± SD | 51.2 ± 6.2 | 50.4 ± 7.2 | 47.7 ± 6.9 | 49.6 ± 4.3 | 49.8 ± 6.7 | 50.1 ± 6.1 |
| Columbia Suicide Severity Rating Scale–Lifetime Ratings, n (%) | ||||||
| Suicidal ideation | ||||||
| Thoughts of their death | 16 (50.0) | 8 (47.1) | 12 (57.1) | 6 (54.6) | 28 (52.8) | 14 (50.0) |
| Nonspecific suicidal thoughts | 4 (12.5) | 3 (17.7) | 6 (28.6) | 1 (9.1) | 10 (18.9) | 4 (14.3) |
| Active thoughts of methods but no clear plan | 1 (3.1) | 1 (5.9) | 2 (9.5) | 0 | 3 (5.7) | 1 (3.6) |
| Active ideation with plan | 1 (3.1) | 3 (17.7) | 3 (14.3) | 2 (18.2) | 4 (7.6) | 5 (17.9) |
| History of suicide attempts | 0 | 1 (5.9) | 1 (4.8) | 1 (9.1) | 1 (1.9) | 2 (7.1) |
| History of interrupted attempts | 0 | 1 (5.9) | 2 (9.5) | 0 | 2 (3.8) | 1 (3.6) |
| History of aborted attempts | 0 | 1 (5.9) | 0 | 0 | 0 | 1 (3.6) |
| Comorbid diagnoses, n (%) | ||||||
| ADHD | 22 (68.8) | 11 (64.7) | 12 (57.1) | 7 (63.6) | 34 (64.2) | 18 (64.3) |
| Disruptive behavior | 7 (21.9) | 3 (17.7) | 4 (19.1) | 3 (27.3) | 11 (20.8) | 6 (21.4) |
| Enuresis | 1 (3.1) | 2 (11.8) | 0 | 0 | 1 (1.9) | 2 (7.1) |
| Anxiety disorder | 8 (25.0) | 5 (29.4) | 6 (28.6) | 0 | 14 (26.4) | 5 (17.9) |
| Othera | 0 | 2 (11.8) | 1 (4.8) | 0 | 1 (1.9) | 2 (7.1) |
ADHD, attention-deficit/hyperactivity disorder; Min–Max, minimum–maximum.
Other includes 1 each of marijuana abuse, motor tic disorder, and trichotillomania.
There was no statistically significant difference between the treatment groups with respect to length of study participation. The mean ± SD length of study participation for lithium-treated participants and placebo participants was 47.5 ± 16.6 days and 48.6 ± 15.3 days, respectively. The mean lithium serum level at study’s end was 0.98 ± 0.47 mEq/L.
Details regarding end-of-study lithium dosing are summarized in Table 2.The mean daily dose for participants aged 7 to 11 years (n = 49) was 1292 ± 420 mg and 1716 ± 606 mg for participants aged 12 to 17 years (n = 32). With regard to weight, the mean daily dose was 956 ± 225 mg for participants weighing <30 kg (n = 16) and 1583 ± 524 mg for participants weighing ≥30 kg (n = 65).
TABLE 2.
Dosing and Weight at Last Study Visit According to Randomized Study Group
| Variable | Lithium (n = 53) | Placebo (n = 28) | Total (N = 81) |
|---|---|---|---|
| Dose, mg/d | |||
| Mean ± SD | 1483 ± 584 | 1414 ± 454 | 1459 ± 540 |
| Median (Min–Max) | 1500 (300–3600) | 1350 (600–2700) | 1500 (300–3600) |
| Dose, mg/kg/d | |||
| Mean ± SD | 30.5 ± 8.7 | 29.2 ± 10.1 | 30.0 ± 9.2 |
| Median (Min–Max) | 30.0 (5.9–50.0) | 27.6 (10.0– 47.1) | 29.0 (5.9–50.0) |
| Weight, kg | |||
| Mean ± SD | 51.8 ± 22.5 | 52.7 ± 19.8 | 52.1 ± 21.5 |
| Median (Min–Max) | 47.2 (19.5–115.7) | 50.5 (25.8–105.7) | 47.8 (19.5–115.7) |
End dose is the last nonzero dose reported. Min–Max, minimum–maximum.
The overall mean adherence rate for medication dosing was 91.9 ± 11.8%. The adherence rates for the lithium and placebo groups were 92.0 ± 11.8% and 91.6 ± 12.1%, respectively.
Mania Response
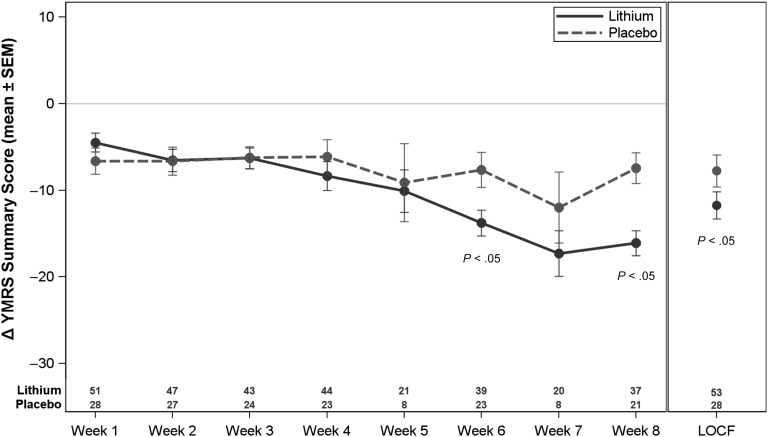
The mean YMRS LOCF score at week 8 for lithium was 17.8 ± 11.0, and for placebo it was 22.3 ± 9.7. The mean change from baseline to week 8 LOCF is illustrated in Figure 3. The change in YMRS score at week 8 (ie, the primary efficacy measure) was significant in favor of lithium (P = .03). After adjusting for baseline YMRS score, age group, weight group, gender, and study site, the treatment effect size was 5.51 (95% confidence interval: 0.51 to 10.50).
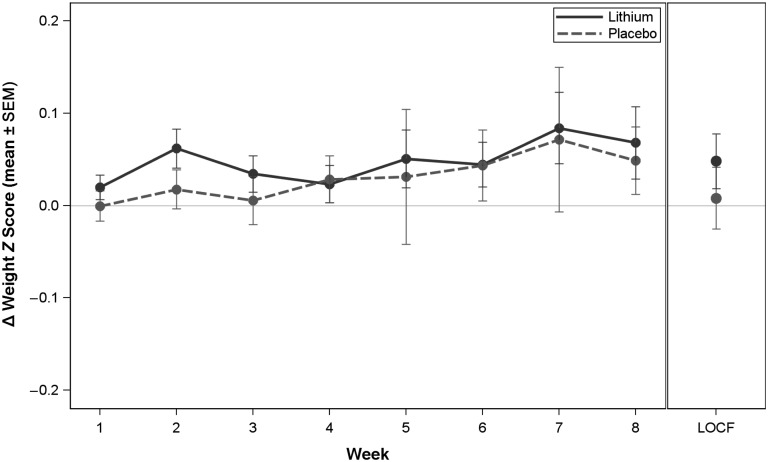
FIGURE 3.
Change in weight z score during the efficacy phase. Change score is the week value minus the baseline value; baseline is defined as the first visit of the efficacy phase.
FIGURE 2.
Change in YMRS summary score according to visit in the intention-to-treat population. Baseline was defined as the first visit of the efficacy phase. Visits at weeks 5 and 7 were changed from clinic-based visits to telephone calls with a protocol amendment on July 15, 2011 (with no YMRS data collected).
The adjusted standardized effect size (Cohen’s d) and corresponding 95% confidence interval, adjusting for baseline factors in the primary efficacy analysis, was 0.53 (0.06 to 0.99).29 The unadjusted standardized effect size was 0.37 (–0.10 to 0.83).
Secondary Measures
For the CDRS-R, the mean decrease in scores (reflecting reduced depressive symptoms) was 5.5 ± 12.2 on lithium and 6.8 ± 8.5 on placebo (P = .49). CGAS scores increased (indicative of improved global functioning) for patients receiving lithium (9.5 ± 13.8) and for those receiving placebo (8.5 ± 12.1) (P = .63). Scores in 22 (42%) of the lithium group participants decreased at least 2 points (from baseline to week 8/ET) on the CGI-S for overall illness severity, whereas 6 (21%) participants receiving placebo decreased by at least 2 points. There was no statistically significant between-group difference (P = .11). However, overall CGI-I scores favored lithium (n = 25; 47% very much/much improved) compared with placebo (n = 6; 21% very much/much improved) at week 8/ET (P = .03).
Response and Remission
The number of participants meeting a priori response criteria (reduction in baseline YMRS score ≥50% and CGI-I score of 1 or 2) was 17 (32%) for lithium and 6 (21%) for placebo. The number of participants meeting criteria for remission (YMRS score ≤12 and CGI-S score ≤2) at the end of study was 14 (26%) for lithium and 4 (14%) for placebo. There was no statistically significant difference between treatment groups for either response or remission.
Adverse Events
No participants discontinued the study due to lack of tolerability. The mean number of AEs per participant was 6.4 (7.7 in the lithium group and 4.0 in the placebo group).
Seven AEs met the definition of a serious AE. These 7 events occurred among 6 participants (5 events in 4 lithium participants and 2 events in 2 placebo participants). None of the serious AEs were believed to be related to study medication.
Two lithium-treated participants discontinued the study due to persistent psychosis. Two lithium participants and 1 placebo participant discontinued because of suicidality; all 3 of these discontinuations were considered unrelated to the study medication. Three additional AEs led to study discontinuation: 1 lithium participant with agitation, 1 lithium participant with mood instability, and 1 placebo participant with aggressive behavior. All of these discontinuations were unrelated to study medication. Table 3 lists those AEs occurring at a rate of ≥5% and twice as frequently on lithium as placebo. Most AEs were mild to moderate in severity.
TABLE 3.
AEs Seen at a Rate of ≥5% (n ≥ 5) in the Total Population and Twice as Frequently With Lithium as Placebo According to Age Strata and Treatment Group
| System Organ Class/Preferred Term | 7–11 Years of Age | 12–17 Years of Age | Total | |||
|---|---|---|---|---|---|---|
| Lithium (n = 32) | Placebo (n = 17) | Lithium (n = 21) | Placebo (n = 11) | Lithium (n = 53) | Placebo (n = 28) | |
| Eye disorders | ||||||
| Blurred vision | 4 (12.5%) | 0 | 1 (4.8%) | 0 | 5 (9.4%) | 0 |
| Gastrointestinal disorders | ||||||
| Abdominal pain | 4 (12.5%) | 0 | 2 (9.5%) | 1 (9.1%) | 6 (11.3%) | 1 (3.6%) |
| Diarrhea | 10 (31.3%) | 3 (17.6%) | 5 (23.8%) | 1 (9.1%) | 15 (28.3%) | 4 (14.3%) |
| Nausea | 12 (37.5%) | 3 (17.6%) | 11 (52.4%) | 2 (18.2%) | 23 (43.4%) | 5 (17.9%) |
| Vomiting | 15 (46.9%) | 3 (17.6%) | 9 (42.9%) | 0 | 24 (45.3%) | 3 (10.7%) |
| General disorders and administration site conditions | ||||||
| Fatigue | 0 | 0 | 5 (23.8%) | 1 (9.1%) | 5 (9.4%) | 1 (3.6%) |
| Thirst | 12 (37.5%) | 0 | 3 (14.3%) | 3 (27.3%) | 15 (28.3%) | 3 (10.7%) |
| Laboratory tests | ||||||
| Blood thyroid-stimulating hormone increased | 4 (12.5%) | 0 | 5 (23.8%) | 0 | 9 (17.0%) | 0 |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 5 (15.6%) | 1 (5.9%) | 0 | 0 | 5 (9.4%) | 1 (3.6%) |
| Nervous system disorders | ||||||
| Dizziness | 7 (21.9%) | 0 | 5 (23.8%) | 2 (18.2%) | 12 (22.6%) | 2 (7.1%) |
| Sedation | 6 (18.8%) | 0 | 0 | 0 | 6 (11.3%) | 0 |
| Tremor | 9 (28.1%) | 2 (11.8%) | 8 (38.1%) | 0 | 17 (32.1%) | 2 (7.1%) |
| Renal and urinary disorders | ||||||
| Pollakiuria (abnormally frequent urination) | 10 (31.3%) | 0 | 4 (19.0%) | 2 (18.2%) | 14 (26.4%) | 2 (7.1%) |
| Skin and subcutaneous tissue disorders | 0 | |||||
| Rash | 3 (9.4%) | 0 | 3 (14.3%) | 0 | 6 (11.3%) | 0 |
The most common AEs with lithium were vomiting (n = 24 [45%]), nausea (n = 23 [43%]), and headache (n = 19 [36%]). With placebo, headache (n = 9 [32%]), upper abdominal pain (n = 9 [32%]), and nausea and increased appetite (both at n = 5 [18%]) were most common. Fourteen of the 24 lithium-treated participants who experienced vomiting had their first episode during week 1. The dose was reduced in 12 of the 24 participants after a vomiting episode. The mean ± SD length of time from onset of vomiting to resolution (or end of phase) for lithium-treated participants was 7.3 ± 11.4 days; the mean length of time for resolution of nausea was 14.7 ± 17.3 days.
A weight gain of 0.9 ± 1.6 kg was reported in the lithium-treated participants and a weight gain of 1.2 ± 1.7 kg was observed in participants receiving placebo (Figure 3). There was no statistically significant between-group difference with respect to weight gain. A statistically significant increase in thyrotropin concentration of 3.0 ± 3.1 mIU/L was observed in those participants who received lithium, compared with –0.1 ± 0.9 mIU/L in participants receiving placebo (P < .001).
No participants discontinued treatment as a result of any clinically significant findings related to vital signs, physical examination, or electrocardiography.
Discussion
The present study comprises the largest prospective, randomized, double-blind, placebo-controlled study to-date of lithium in youth aged 7 to 17 years with BP-I mixed or manic episodes. The data provide evidence that lithium was effective in reducing manic symptoms in approximately one-half of these participants.
Lithium was superior to placebo in reducing manic symptoms in these patients, albeit with a delay of between-group separation. Whether the primary and secondary efficacy analyses would differ if this study was a longer clinical trial remains an empirical question that warrants further study. Ethical considerations during the design of the study precluded a longer clinical trial with one of the arms being placebo. The dropout rates during this clinical trial accentuate the challenges of performing a double-blind, placebo-controlled study in this population.
In addition to participant age, the study results are similar to those of a meta-analysis of lithium-controlled trials in adults.12 The calculated mean standardized effect size was reported to be 0.40 with a range of 0.11 to 0.55. The clinical trial reported herein suggests that the efficacy of lithium in children is similar to that reported in adults and highlights that rigorous study of an older drug can improve the armamentarium of drugs used in the pediatric population. These study results add to the findings of the other large pediatric bipolar study, the National Institute of Mental Health–funded TEAM (Treatment of Early Age Mania) study, which compared treatment with lithium, divalproex, and risperidone in outpatients.30 However, in that study, lithium and divalproex were each found to be less effective than risperidone.
Lithium was generally well tolerated in the present study. The adverse effect profile was consistent with what has been previously reported in adults. Of note, lithium was not associated with weight gain relative to placebo. This observation distinguishes lithium from the antipsychotic agents,31–34 some of which have been shown to be effective in the acute treatment of manic and/or mixed states in this population but with a risk of substantial weight gain and metabolic derangements.
A potential limitation of the present study is that LOCF was used in the analyses. LOCF may bias an estimate of the treatment and underestimate variability of the estimated result. Sensitivity analyses have been performed by using mixed model repeated measures, which do not include imputed data for missing values and use all available YMRS scores at each visit. Results of both methods of analyses indicate the statistically significant superiority of lithium versus placebo. The mixed model repeated measures analyses showed significance only at the week 6 and week 8 time points.
The study does have some limitations. It was relatively brief, and BP-I is a chronic, recurrent condition; therefore, definitive conclusions about long-term efficacy cannot be made from these data. Another shortcoming of this research is that current scientific methods preclude the absolute certainty of the diagnosis of BP-I in this cohort. In addition, the study enrolled a relatively small sample. Therefore, uncommon adverse effects during acute treatment were likely not observed. The relatively modest sample size may also explain why between-group differences were not found on the secondary efficacy measures. In addition, sample size considerations limit the ability to perform analyses on subpopulations and extrapolation to a more general pediatric population.
Conclusions
Lithium exhibited efficacy in the acute treatment of pediatric BP-I. With the dosing regimen used, lithium was found to have a generally acceptable adverse effect profile. Although use of the sustained-release formulation of lithium may obviate vomiting, this question is empirical and has not yet been tested.
Glossary
- AE
adverse event
- BP-I
bipolar disorder I
- CDRS-R
Children's Depression Rating Scale–Revised
- CGAS
Children's Global Assessment Scale
- CGI-I
Clinical Global Impression–Improvement
- CGI-S
Clinical Global Impression–Severity
- K-SADS-PL
Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version
- LOCF
last-observation-carried-forward
- YMRS
Young Mania Rating Scale
Footnotes
Dr Findling was responsible for study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, and/or material support; and study supervision. Dr Findling had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Dr Robb was responsible for study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, and/or material support; and study supervision. Dr McNamara was responsible for acquisition of data and administrative, technical, and/or material support. Drs Pavuluri, Kafantaris, and Frazier were responsible for study concept and design; acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, and/or material support; and study supervision. Drs Scheffer, Rynn, DelBello, and Kowatch were responsible for study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, and/or material support; and study supervision. Ms Rowles was responsible for drafting of the manuscript and administrative, technical, and/or material support. Ms Lingler was responsible for acquisition of data; drafting of the manuscript; and administrative, technical, and/or material support. Ms Martz and Dr Anand were responsible for analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and administrative, technical, and/or material support. Dr Clemons was responsible for analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; and administrative, technical, and/or material support. Dr Taylor-Zapata was responsible for drafting of the manuscript; critical revision of the manuscript for important intellectual content; administrative, technical, and/or material support; study supervision; and contributions regarding the study design, interpretation of data, and the decision to submit the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01166425).
FINANCIAL DISCLOSURE: Dr Findling receives or has received research support, acted as a consultant, and/or served on a speakers bureau for Alcobra, the American Academy of Child and Adolescent Psychiatry, the American Physician Institute, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, CogCubed, Cognition Group, Coronado Biosciences, the Dana Foundation, Elsevier, Forest, GlaxoSmithKline, Guilford Press, Johnson and Johnson, Jubilant Clinsys, KemPharm, Lilly, Lundbeck, Merck, Neurim, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Dr Robb receives or has received research support, acted as a consultant, received royalties from, payment for manuscript preparation or development of educational presentations from, and/or served on a speakers bureau for Bristol-Myers Squibb, Epocrates, Forest Pharmaceuticals, GlaxoSmithKline, Johnson & Johnson, Lilly, Lundbeck, Merck/Schering Plough, Otsuka, Pfizer, Sepracor, Supernus, and Takeda. Dr Kafantaris has received research support from AstraZeneca, the Brain and Behavior Foundation, Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Pfizer, and Sunovion. Dr Frazier has received research support from GlaxoSmithKline, Pfizer, Neuren, Roche, and Seaside Therapeutics and has served on a Data Safety Monitoring Board for Forest Pharmaceuticals. Dr Rynn receives or has received research support, acted as a consultant, received royalties from, and/or served on a speakers bureau for APPI, Oxford Press, Merck, Shire, Pfizer, and Lilly. Dr DelBello receives or has received research support, acted as a consultant, received travel support from, and/or served on a speakers bureau for Amylin, Astra Zeneca, Bristol-Myers Squibb, Dey, Lilly, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Shire, Somerset, and Sunovion. Dr Kowatch is a consultant for AstraZeneca, Forest Pharmaceuticals, Sunovion, and the REACH Foundation. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, “Best Pharmaceuticals for Children Act Pediatric Off-Patent Drug Study (PODS): Lithium in the Treatment of Pediatric Mania” (contract HHSN275200503406C). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Findling has consulted for Bristol-Myers Squibb, Lilly, Merck, Otsuka, Pfizer, Sunovion, and Validus; he receives or has received research support from AstraZeneca, Johnson & Johnson, Bristol-Myers Squibb, Lilly, Merck, Otsuka, Pfizer, Sunovion, and Validus. Dr Robb has served on speakers bureaus and/or advisory boards for Actavis and Pfizer and receives or has received research support from Pfizer, Sunovion, and SyneuRx; Dr Kantafaris has consulted for Bracket and receives or has received research support from Merck, Pfizer, and Sunovion; Dr Frazier has received research support from Pfizer; Dr DelBello receives or has received research support, acted as a consultant, received travel support from, and/or served on a speakers bureau for Amylin, AstraZeneca, Bristol-Myers Squibb, Dey, Lilly, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Shire, Somerset, and Sunovion; and Dr Kowatch has consulted for AstraZeneca and Sunovion. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Perlis RH, Miyahara S, Marangell LB, et al. STEP-BD Investigators . Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2004;55(9):875–881 [DOI] [PubMed] [Google Scholar]
- 2.Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250–1256 [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 4.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JPA, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser M, Galling B, Correll CU. Suicidal ideation and suicide attempts in children and adolescents with bipolar disorder: a systematic review of prevalence and incidence rates, correlates, and targeted interventions. Bipolar Disord. 2013;15(5):507–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein TR, Ha W, Axelson DA, et al. Predictors of prospectively examined suicide attempts among youth with bipolar disorder. Arch Gen Psychiatry. 2012;69(11):1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein TR, Birmaher B, Axelson D, et al. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114(1–3):174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148 [DOI] [PubMed] [Google Scholar]
- 9.Baldessarini RJ, Tondo L, Vazquez GH, et al. Age at onset versus family history and clinical outcomes in 1,665 international bipolar-I disorder patients. World Psychiatry. 2012;11(1):40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):349–352 [DOI] [PubMed] [Google Scholar]
- 11.Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;161(2):217–222 [DOI] [PubMed] [Google Scholar]
- 12.Muzina DJ, Calabrese JR. Maintenance therapies in bipolar disorder: focus on randomized controlled trials. Aust N Z J Psychiatry. 2005;39(8):652–661 [DOI] [PubMed] [Google Scholar]
- 13.Storosum JG, Wohlfarth T, Schene A, Elferink A, van Zwieten BJ, van den Brink W. Magnitude of effect of lithium in short-term efficacy studies of moderate to severe manic episode. Bipolar Disord. 2007;9(8):793–798 [DOI] [PubMed] [Google Scholar]
- 14.Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findling RL, Pavuluri MN. Lithium. In: Geller B, DelBello MP, eds. Treatment of Bipolar Disorder in Children and Adolescents. New York, NY: Guilford Press; 2008:43–68 [Google Scholar]
- 16.Eunice Kennedy Shriver National Institute of Child Health and Human Development. Best Pharmaceuticals for Children Act. Available at: http://bpca.nichd.nih.gov. Accessed November 12, 2013
- 17.Findling RL, Landersdorfer CB, Kafantaris V, et al. First-dose pharmacokinetics of lithium carbonate in children and adolescents. J Clin Psychopharmacol. 2010;30(4):404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findling RL, Kafantaris V, Pavuluri M, et al. Dosing strategies for lithium monotherapy in children and adolescents with bipolar I disorder. J Child Adolesc Psychopharmacol. 2011;21(3):195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findling RL, Frazier JA, Kafantaris V, et al. The Collaborative Lithium Trials (CoLT): specific aims, methods, and implementation. Child Adolesc Psychiatry Ment Health. 2008;2(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435 [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988 [DOI] [PubMed] [Google Scholar]
- 22.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23(2):191–197 [DOI] [PubMed] [Google Scholar]
- 23.Overholser JC, Brinkman DC, Lehnert KL, Ricciardi AM. Children’s Depression Rating Scale-Revised: development of a short form. J Clin Child Psychol. 1995;24(4):443–452 [Google Scholar]
- 24.Posner K, Melvin GA, Stanley B, Oquendo MA, Gould M. Factors in the assessment of suicidality in youth. CNS Spectr. 2007;12(2):156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute of Mental Health Clinical global impression scale. Psychopharmacol Bull. 1985;21:839–843 [Google Scholar]
- 26.Klein RG, Abikoff H, Barkley RA, et al. Clinical trials in children and adolescents. In: Prien RF, Robinson DS, eds. Clinical Evaluation of Psychotropic Drugs: Principles and Guidelines. New York, NY: Raven Press, Ltd; 1994:501–546 [Google Scholar]
- 27.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19 [DOI] [PubMed] [Google Scholar]
- 28.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231 [DOI] [PubMed] [Google Scholar]
- 29.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001 [Google Scholar]
- 30.Geller B, Luby JL, Joshi P, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry. 2012;69(5):515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547–1556 [DOI] [PubMed] [Google Scholar]
- 32.Findling RL, Nyilas M, Forbes RA, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2009;70(10):1441–1451 [DOI] [PubMed] [Google Scholar]
- 33.Haas M, DelBello MP, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2009;11(7):687–700 [DOI] [PubMed] [Google Scholar]
- 34.Pathak S, Findling RL, Earley WR, Acevedo LD, Stankowski J, DelBello MP. Efficacy and safety of quetiapine in children and adolescents with mania associated with bipolar I disorder: a 3-week, double-blind, placebo-controlled trial. J Clin Psychiatry. 2013;74(1):e100–e109 [DOI] [PubMed] [Google Scholar]