Abstract
Congenital airway obstruction poses a life-threatening challenge to the newborn. We present the first case of three-dimensional (3D) modeling and 3D printing of complex fetal maxillofacial anatomy after prenatal ultrasound indicated potential upper airway obstruction from a midline mass of the maxilla. Using fetal MRI and patient-specific computer-aided modeling, the craniofacial anatomy of the fetus was manufactured using a 3D printer. This model demonstrated the mass to be isolated to the upper lip and maxilla, suggesting the oral airway to be patent. The decision was made to deliver the infant without a planned ex utero intrapartum treatment procedure. The neonate was born with a protuberant cleft lip and palate deformity, without airway obstruction, as predicted by the patient-specific model. The delivery was uneventful, and the child was discharged without need for airway intervention. This case demonstrates that 3D modeling may improve prenatal evaluation of complex patient-specific fetal anatomy and facilitate the multidisciplinary approach to perinatal management of complex airway anomalies.
Congenital anomalies of the fetal airway represent a unique management challenge at birth and carry a significant risk of morbidity and mortality to the newborn. Congenital high airway obstruction syndrome (often referred to as CHAOS) may be identified on prenatal ultrasound by the presence of polyhydramnios, large echogenic lungs, and flattened diaphragms. These findings suggest complete obstruction of the fetal airway.1 However, in circumstances in which airway obstruction is not complete, such as with head and neck masses, predicting the severity of airway obstruction at birth is challenging. When significant upper airway obstruction is identified before delivery, maintaining placental support via ex utero intrapartum treatment (EXIT) to perform bronchoscopy, intubation, or tracheostomy can be lifesaving for the fetus.1–3 Despite potential fetal advantages for perinatal management with EXIT, the procedure is associated with increased risks and morbidity for the mother and significantly alters her delivery experience. Therefore, the procedure should only be offered if there is an experienced multidisciplinary team and prenatal imaging demonstrates a high likelihood of difficulty in securing an airway.
Current prenatal imaging modalities include ultrasound and fetal MRI to delineate the anatomy of suspected airway obstruction and predict severity.4,5 The ability to successfully diagnose and manage fetal airway obstruction depends primarily on the resolution of these imaging studies and experience of the delivery team. Rarely, the two-dimensional representation of these imaging modalities provides incomplete or unclear visualization of suspected anatomic defects. With recent advancements in 3D modeling and printing technologies, preliminary efforts at detailing complex fetal anatomy has shown promising results.6 Werner et al used 3D computer-aided-design to perform a virtual bronchoscopy on fetuses with large cervical tumors, confirming airway patency.7 We describe the first use of prenatal 3D modeling and 3D printing to characterize the detailed maxillofacial fetal airway in a case in which prenatal ultrasound and fetal MRI was concerning for upper airway obstruction from a congenital mass but was insufficient to establish a definitive perinatal airway management strategy.
Case Report
The patient was a 22-year-old G1P0 who was referred for a new diagnosis of an abnormal masslike lesion involving the fetal premaxilla at 30 weeks’ gestation. Previous ultrasounds performed at 20 and 28 weeks’ gestation were reported as normal, and images were not available for review. On referral, ultrasound revealed a facial mass concerning for a complex cleft lip and palate deformity, infantile hemangioma or lymphangioma of the philtrum, or an epignathus, a rare teratoma arising from the midline oral cavity which can cause life-threatening airway obstruction in the neonate (Fig 1). However, visualization of the face during the ultrasound remained suboptimal due to fetal positioning and advanced gestational age. Normal amniotic fluid volume was noted. Fetal MRI was performed at 32 weeks, which confirmed a heterogeneous mass of the anterior maxilla. The mass and craniofacial skeleton were again poorly visualized, and it remained unclear if a secure airway could be established after birth.
FIGURE 1.
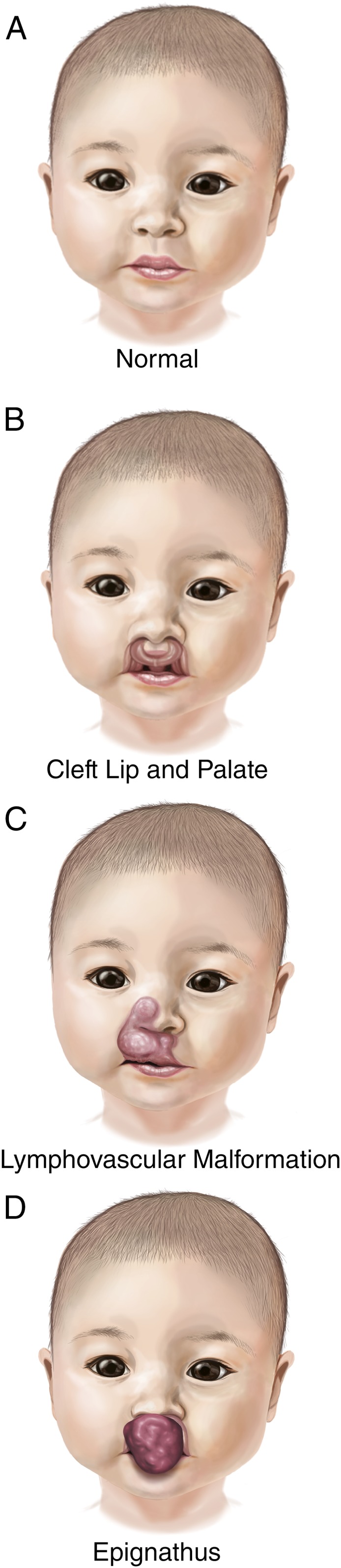
Differential diagnoses: Illustrations of (A) normal neonatal maxillofacial anatomy and (B–D) differential diagnoses entertained. Epignathus (D) shows obliteration of the oral cavity and nasal airway with potential for upper airway obstruction.
We hypothesized that patient-specific computer-aided modeling in conjunction with 3D printing of the fetal maxillofacial anatomy would better characterize this mass lesion to determine if an EXIT procedure would be required. Fetal MRI was repeated using single shot T2 turbo spin echo–weighted images reformatted in axial, coronal, and sagittal planes to the fetal head on a 1.5-T scanner using a slice thickness of 3.0 mm (Fig 2A). Digital Imaging and Communications in Medicine images from the reformatted MRI were imported into the Mimics Innovation Suite (Materialise, Belgium) computer-aided design software. The axially formatted 3.0-mm images were interpolated into 1.0-mm slices using the Online Reslice function to improve triangle resolution. A 3D model of the fetal craniofacial anatomy was then generated via segmentation using the thresholding function, where pixels of variable signal intensity are used to segment voxels into a set or “mask,” and then used to generate a 3D model. The subsequent model was refined using the multiple slice edit function in which voxels were excluded or included in the mask by user selection. This process was performed for both soft tissue anatomy and the maxillomandibular skeleton. The 3D models were then printed using a combination of fused deposition modeling and stereolithography 3D printers.
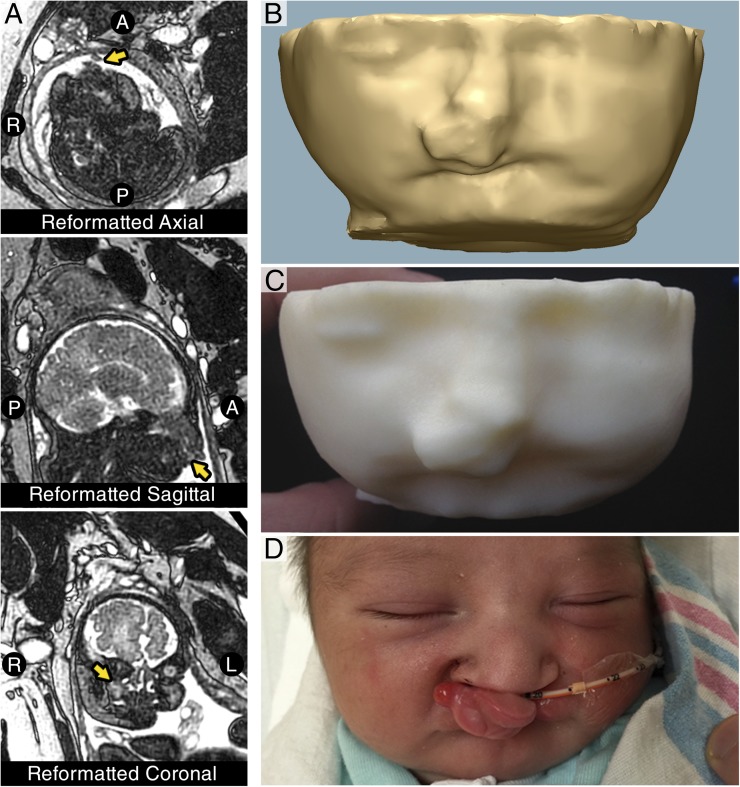
FIGURE 2.
Segmentation, modeling, and 3D printing of fetal MRI. (A) Representative single-shot T2 turbo spin echo-weighted MRI of the fetus reformatted in axial, sagittal, and coronal planes, which fail to clearly demonstrate the anatomy, making clinical decision-making difficult. (B) 3D model of the fetal facial soft tissues. (C) Final 3D-printed model of the fetal soft tissues demonstrating protuberant, isolated mass of the upper lip that does not involve the oral aperture. (D) Patient after successful delivery by cesarean delivery demonstrating bilateral cleft lip and palate with anteriorly displaced premaxilla.
The 3D-printed models revealed the relationship of the craniofacial skeleton to the soft tissue mass. The mass was noted to involve the anterior face of the premaxilla but spare the lower lip and not extend into the oral cavity (Fig 2B and 2C). There was no evidence of limitations to the oral aperture. These findings confirmed that an EXIT procedure was not indicated.
With the results of the 3D-printed models, the decision was made to proceed with a scheduled cesarean delivery. The neonate was successfully delivered without complication and was able to breathe spontaneously without intervention. Anatomic examination revealed a protuberant bilateral cleft lip and palate with a prominent anteriorly displaced premaxilla and philtrum (Fig 2D). No other anomalies were noted, and the postnatal course has been uncomplicated. The infant was placed in the ICU for 1 day and ultimately discharged from the hospital without further interventions once an oral feeding regimen was established on day of life 5. Plans have been made for repair of the cleft lip at 3 to 4 months of age, and subsequent repair of the cleft palate between 9 and 12 months.
Discussion
Perinatal management of complicated and potentially life-threatening congenital anomalies remains a challenging task for the obstetrician, neonatologist, and surgeon. We present the first reported medical case of antenatal 3D printing for use in multidisciplinary perinatal management of the airway. Recent advances in 3D printing in medicine have hinged on the improvements in resolution of traditional imaging modalities such as MRI. Traditionally, cross-sectional imaging tools such as computed tomography and MRI are visualized in a series of 2D planar images. However, for the clinician to visualize the 3D anatomic relationships, they must mentally reconstruct the 2D planar images into a 3D picture. For our team of clinicians, the 2D images were insufficient in allowing the team to fully appreciate the anatomy and could not depict whether airway obstruction would be present. However, the 3D models provided a greatly improved appreciation of the maxillofacial anatomy and showed the lesion was isolated to the upper lip and premaxilla, indicating the airway was likely to be patent. Although 3D printing does not provide new data, it filters and processes the raw data matrix from the MRI in a way that enables improved visualization and clinical decision-making for the clinician. Additionally, the hands-on experience provided by the 3D printed model allowed the team to plan potential interventions (ie, intubation with a specific laryngoscope) in a fashion that would not have been possible with purely digital models. This experience allowed the team to proceed without a planned EXIT procedure having more confidence in the anatomy and potential interventions necessary.
This case highlights the potential utility of 3D printing technologies in the field of fetal diagnosis and treatment. The use of 3D printing to rapidly manufacture anatomic models used for presurgical planning of complex anatomy in adult and pediatric patients has been well described, particularly for orthopedic and congenital cardiac applications.8–13 Prenatal printing of fetal surface anatomy as a social product is being explored commercially in Japan, and other groups have preliminarily examined 3D modeling of fetal imaging for characterizing the central nervous system.14–16 Initial studies by Werner and colleagues have demonstrated that airway patency can be evaluated by fetal MRI and 3D modeling in cases of large cervical tumors that could cause airway compression.7 However, this technique has not been previously used in detailing the maxillofacial anatomy of a fetus because of the relatively low resolution of the fetal MRI and fetal motion artifact. We demonstrate that using specific MRI sequencing, slice interpolation, and appropriate segmentation can result in an anatomic model of sufficient accuracy to contribute to successful perinatal multidisciplinary planning and management.
Conclusions
We present a proof-of-concept case of successful 3D modeling and 3D printing of the maxillofacial skeleton and facial anatomy of a 35 5/7-week fetus based on fetal MRI. The 3D models of the facial soft tissue and maxillomandibular skeleton accurately predicted the appropriate airway management plan before delivery. This technique has broad potential applications in fetal modeling of complex airway, cardiac, and other anatomic anomalies to assist in perinatal management strategies and newborn safety at birth.
Acknowledgments
We thank Mr Khaled N. Kashlan for his assistance with modeling.
Glossary
- 3D
three-dimensional
- EXIT
ex utero intrapartum treatment
Footnotes
Dr VanKoevering drafted the initial manuscript, and all revisions were completed by Dr Morrison. Dr VanKoevering, Dr Morrison and Dr Hollister performed the 3D modeling. Dr Prabhu provided the protocol and detailed information regarding the fetal MRI, which was performed and read by Dr Ladino Torres. Drs Myhaliska, Treadwell, and Green were the primary members of the multidisciplinary delivery team and provided the care for this patient. All authors critically reviewed and approved the manuscript.
FINANCIAL DISCLOSURES: Drs Green and Hollister have patents pending for three-dimensional printed medical devices (surgically implanted devices and external scaffolds). The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Morrison is supported by National Institutes of Health grant T32 DC005356. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hedrick MH, Ferro MM, Filly RA, Flake AW, Harrison MR, Adzick NS. Congenital high airway obstruction syndrome (CHAOS): a potential for perinatal intervention. J Pediatr Surg. 1994;29(2):271–274 [DOI] [PubMed] [Google Scholar]
- 2.DeCou JM, Jones DC, Jacobs HD, Touloukian RJ. Successful ex utero intrapartum treatment (EXIT) procedure for congenital high airway obstruction syndrome (CHAOS) owing to laryngeal atresia. J Pediatr Surg. 1998;33(10):1563–1565 [DOI] [PubMed] [Google Scholar]
- 3.Mychaliska GB, Bealer JF, Graf JL, Rosen MA, Adzick NS, Harrison MR. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg. 1997;32(2):227–230, discussion 230–231 [DOI] [PubMed] [Google Scholar]
- 4.Coakley FV, Hricak H, Filly RA, Barkovich AJ, Harrison MR. Complex fetal disorders: effect of MR imaging on management—preliminary clinical experience. Radiology. 1999;213(3):691–696 [DOI] [PubMed] [Google Scholar]
- 5.Mong A, Johnson AM, Kramer SS, et al. Congenital high airway obstruction syndrome: MR/US findings, effect on management, and outcome. Pediatr Radiol. 2008;38(11):1171–1179 [DOI] [PubMed] [Google Scholar]
- 6.Werner H, dos Santos JR, Fontes R, et al. Additive manufacturing models of fetuses built from three-dimensional ultrasound, magnetic resonance imaging and computed tomography scan data. Ultrasound Obstet Gynecol. 2010;36(3):355–361 [DOI] [PubMed] [Google Scholar]
- 7.Werner H, Lopes dos Santos JR, Fontes R, et al. Virtual bronchoscopy for evaluating cervical tumors of the fetus. Ultrasound Obstet Gynecol. 2013;41(1):90–94 [DOI] [PubMed] [Google Scholar]
- 8.Sodian R, Schmauss D, Markert M, et al. Three-dimensional printing creates models for surgical planning of aortic valve replacement after previous coronary bypass grafting. Ann Thorac Surg. 2008;85(6):2105–2108 [DOI] [PubMed] [Google Scholar]
- 9.Sodian R, Weber S, Markert M, et al. Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg. 2008;136(4):1098–1099 [DOI] [PubMed] [Google Scholar]
- 10.Sodian R, Weber S, Markert M, et al. Stereolithographic models for surgical planning in congenital heart surgery. Ann Thorac Surg. 2007;83(5):1854–1857 [DOI] [PubMed] [Google Scholar]
- 11.Giovinco NA, Dunn SP, Dowling L, et al. A novel combination of printed 3-dimensional anatomic templates and computer-assisted surgical simulation for virtual preoperative planning in Charcot foot reconstruction. J Foot Ankle Surg. 2012;51(3):387–393 [DOI] [PubMed] [Google Scholar]
- 12.Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivieri L, Krieger A, Chen MY, Kim P, Kanter JP. 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int J Cardiol. 2014;172(2):e297–e298 [DOI] [PubMed] [Google Scholar]
- 14.Werner H, Rolo LC, Araujo Júnior E, Dos Santos JR. Manufacturing models of fetal malformations built from 3-dimensional ultrasound, magnetic resonance imaging, and computed tomography scan data. Ultrasound Q. 2014;30(1):69–75 [DOI] [PubMed] [Google Scholar]
- 15.Werner H, Lopes J, Tonni G, Araujo Júnior E. Physical model from 3D ultrasound and magnetic resonance imaging scan data reconstruction of lumbosacral myelomeningocele in a fetus with Chiari II malformation. Childs Nerv Syst. 2015;31(4):511–513 [DOI] [PubMed] [Google Scholar]
- 16.Velasco-Annis C, Gholipour A, Afacan O, Prabhu SP, Estroff JA, Warfield SK. Normative biometrics for fetal ocular growth using volumetric MRI reconstruction. Prenat Diagn. 2015;35(4):400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]