Abstract
The purpose of this study was to investigate the effects of diabetes on mesenchymal stem cells (MSCs) in terms of their angiogenic and therapeutic potential for repairing tissue ischemia. We culture-isolated MSCs from streptozotocin-induced diabetic rats (D-MSCs) and compared their proliferation, differentiation, and angiogenic effects with those from normal rats (N-MSCs). The angiogenic effects of MSCs were evaluated by real-time PCR, in vitro tube formation assay, and transplantation of the MSCs into a hindlimb ischemia model followed by laser Doppler perfusion imaging. The number of MSCs derived from diabetic rats was smaller, and their proliferation rate was slower than N-MSCs. Upon induction of differentiation, the osteogenic and angiogenic differentiation of D-MSCs were aberrant compared to N-MSCs. The expression of angiogenic factors was lower in D-MSCs than N-MSCs. D-MSCs cocultured with endothelial cells resulted in decreased tube formation compared to N-MSCs. D-MSCs were ineffective to improve hindlimb ischemia and showed lower capillary density and angiogenic gene expression in ischemic limbs than N-MSCs. D-MSCs have defective proliferation and angiogenic activities and are ineffective for repairing hindlimb ischemia. Newer measures are needed before MSCs can be employed as a source for autologous cell therapy.
Keywords: Diabetes mellitus, Mesenchymal stem cells (MSCs), Ischemic, Angiogenesis
INTRODUCTION
Diabetes mellitus is a major risk factor for cardiovascular disease (17). Cardiovascular disease is the principal cause of death in diabetic patients, accounting for about 50% of all diabetes fatalities (49). As the number of people with diabetes worldwide is expected to increase from 171 million in 2000 to 366 million by 2030 (57), cardiovascular disease in diabetic patients is rapidly emerging as a global health care problem (17,57). In addition to cardiovascular diseases, diabetic patients have a higher risk of developing bone fracture, wound healing problems, nephropathy, and retinopathy (22,56).
Mesenchymal stem cells (MSCs), also known as multi-potent stromal cells, hold great promise for treating intractable diseases because they are easily expandable and can differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes (44,55). MSCs also secrete various angiogenic and cell survival factors and were shown to be effective for treating ischemic cardiovascular diseases and diabetic complications in animal models and human patients (15,28,30–32,34,36,58). However, the proliferation and differentiation capacity of MSCs derived from diabetic patients have yet to be determined.
Although MSCs are known to be less immunogenic than other cells when transplanted into nonautologous recipients, allogeneic approaches for cell therapy have clear limitations due to the immunological reactions and the possibility of disease transmission. Thus, an autologous approach using MSCs for cell therapy would be more optimal. However, MSCs derived from subjects having cardiovascular risk factors have been poorly characterized. All the studies using MSCs for treatment of cardiovascular diseases were conducted with MSCs obtained from healthy subjects, and little is known about the effects of diabetes on MSCs. Given that diabetic patients are more prone to advanced cardiovascular diseases, cell therapy is likely to be more beneficial. It is crucial to elucidate the effects of diabetes on MSCs, particularly from the standpoint of their angiogenic and ischemic tissue-repairing potential.
In fact, recent studies have shown that other bone marrow (BM)-derived cells such as endothelial progenitor cells (EPCs) (7,37,53,54) and BM-mononuclear cells (MNCs) (52) isolated from diabetic subjects are defective in their angiogenic activities and are ineffective in the treatment of ischemic cardiovascular diseases. However, it was unknown whether diabetic MSCs have impaired angiogenic activities and reduced therapeutic effects on ischemic diseases.
Here we investigated the characteristics of BM-derived MSCs from diabetic rats (D-MSCs) focusing on their in vitro proliferation and multilineage differentiation capability, in vitro and in vivo angiogenic effects, and their revascularizing effects. This study demonstrated that D-MSCs had aberrant cell biologic characteristics, reduced angiogenic effects, and impaired therapeutic effects in recovering limb ischemia.
MATERIALS AND METHODS
Induction of Diabetes Mellitus
All protocols for animal experiments were approved by the Institutional Animal Care and Use Committees of St. Elizabeth Medical Center and Emory University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We induced diabetes in 8-week-old male Fischer 344 rats (Harlan, Indianapolis, IN, USA) by intravenous injection of streptozotocin (65 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). Blood glucose levels were measured using an Accu-Check glucometer (Roche Diagnostics, Indianapolis, IN, USA) after 1 week and 2 months. If blood glucose was higher than 200 mg/dl, rats were considered diabetic. Rats with glucose levels lower than 200 mg/dl were excluded from the study. Age- and sex-matched normal rats were used as controls.
MSC Culture
Two months after induction of diabetes using streptozotocin (Sigma-Aldrich), BMMNCs were isolated as previously described (26) using HISTOPAQUE®-1083 (Sigma-Aldrich) according to the manufacturer’s instructions. Whole BMMNCs were plated in Minimum Essential Media-α media (Life Technologies, Grand Island, NY, USA) supplemented with 20% FBS and 1% antibiotics (all from Life Technologies; final concentration: 100 U/ml penicillin and 100 µg/ml streptomycin). After 3 days of incubation in a humidified incubator at 37°C with 5% CO2, nonadherent hematopoietic cells were removed by changing the media. The adherent cells were further cultured as previously described, (35) and their MSC identity was confirmed by flow cytometry analysis using anti-CD29-APC (1:100; BD Biosciences, San Jose, CA, USA), anti-CD44-PE (1:100; BD Biosciences), anti-CD90-PE (1:100; eBioscience, San Diego, CA, USA), and anti-CD45-PE (1:100; BD Biosciences) antibody (4,33). MSCs in passages two to four were used for study.
MSC Proliferation and Colony Forming Unit Assay
For the cell proliferation assay, MSCs were plated at 200 cells/cm2, and the number of resultant cells was determined. To evaluate proliferative cells, immunocytochemistry with anti-Ki-67 antibody (EMD Millipore, Billerica, MA, USA) was performed. For the colony-forming unit assay, 100 MSCs were plated onto 100-mm dishes (Falcon®; Corning, Corning, NY, USA) and cultured for 14 days. The cells were stained with crystal violet (Sigma-Aldrich), and the number of colonies were counted as previously described (12).
Differentiation of MSCs
Osteogenic differentiation of MSCs was induced using osteogenic medium as previously described (21,43,44). Briefly, MSCs were plated at a density of 3,000 cells/cm2 in six-well plates and cultured in osteogenic induction medium [low-glucose DMEM (Life Technologies) supplemented with 100 nM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), 0.05 mM l-ascorbic acid-2-phosphate (Sigma-Aldrich), 10% FBS (Life Technologies), and 1% antibiotics (Life Technologies)] for 2 weeks. von Kossa (Abcam, Cambridge, UK) staining (21) and quantification of calcium was performed as previously described (19,27). Adipogenic differentiation of MSCs was induced using adipogenic induction and maintenance media as previously described (44,47). Briefly, MSCs were plated at a density of 10,000 cells/cm2 in six-well plates (Costar®; Corning). After confluence, the cells were cultured for an additional 5 days and cultured in adipogenic induction medium [high-glucose DMEM supplemented with 1 mM dexamethasone, 0.5 mM methyl-isobutylxanthine (Sigma-Aldrich), 10 µg/ml insulin (Sigma-Aldrich), 100 mM indomethacin (Sigma-Aldrich), 10% FBS, and 1% antibiotics] for 2 days and in adipogenic maintenance medium (high-glucose DMEM supplemented with 10 µg/ml insulin, 10% FBS, and 1% antibiotics) for 2 days. After two more cycles of culture in adipogenic induction and maintenance media, cells were cultured in adipogenic maintenance media until analyzed. The level of adipogenic differentiation was determined by Oil red O (Sigma-Aldrich) staining as previously described (44,46,47). Briefly, the cells were fixed using 10% formalin (Sigma-Aldrich) and stained using 3 mg/ml Oil red O solution in 60% isopropanol. After rinsing with 60% isopropanol, the cells were counterstained using hematoxylin (Sigma-Aldrich).
Quantitative Real-Time RT-PCR
Total RNA was extracted from tissue or cells with TRIzol® (Life Technologies) according to the manufacturer’s instructions. The tissues were collected from the hindlimbs at 2 weeks after cell transplantation. First-strand cDNA was generated using the TaqMan® Multiscribe Reverse Transcription Kit (Life Technologies) primed with a mix of oligo dT and random hexamers. Gene expression was determined by TaqMan® real-time quantitative PCR on the 7300 Sequence Detection System (Life Technologies) using TaqMan® PCR Master Mix (Life Technologies). Relative mRNA expression of target gene normalized to GAPDH was calculated using the formula Relative Expression Level = 2−ΔCT, where ΔCT = CT gene of interest −CT GAPDH as previously described (25). The primers and probes were designed using Primer Express 3.0 (Life Technologies) and are described in Table 1.
Table 1.
Sequences of Primers and Probes Used for Real-Time RT-PCR
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Angptl | CAGATACAACAGAATGCGGTTCA | TGAGACAAGAGGCTGGTTCCTAT | AACCACACGGCCACCATGCTGG |
| Angpt2 | CTACAGGATTCACCTTACAGGACTCA | CTTCCTGGTTGGCTGATGCT | TGATTTTGCCCGCCGTGCCT |
| Fgf2 | AAGGATCCCAAGCGGCTCTA | CGGCCGTCTGGATGGA | ACGGCGGCTTCTTCCTGCGC |
| Gapdh | CCGAGGGCCCACTAAAGG | TGCTGTTGAAGTCACAGGAGACA | CATCCTGGGCTACACTGAGGACCAGG |
| Hgf | CTCAGTGTTCAGAAGTTGAATGCAT | TGCCTGATTCTGTGTGATCCA | CTGCAACGGTGAAAGCTACAGAGGTCCC |
| Pdgfb | TCCAGATCTCGCGGAACCT | GCAGGGCGGCCACA | ATCGATCGCACCAATGCCAACTTCC |
| Pgf | ATGCTGGCCATGAAGCTGTT | GCCCCCTGGGAGTGTACAG | ACCCAGCTAGGACCTGCAAGAAGCAAG |
| Vegfa | GCGGGCTGCTGCAATG | CATAGTGACGTTGCTCTCCGAC | AGCCCTGGAGTGCGTGCCCA |
| Vegfc | ACGTTCTCTGCCAGCAACATT | CACATGTAGTTATTCCACACGTAGTTTG | CAGTGTCAGGCAGCTAACAAGACTTGTCCA |
| Igf | CCTACAAAGTCAGCTCGTTCCA | GGGCTGGGACTTCTGAGTCTT | CGGGCCCAGCGCCACACT |
| Tgfb | AGAAGTCACCCGCGTGCTA | TGTGTGATGTCTTTGGTTTTGTCA | TGGTGGACCGCAACAACGCAATC |
Tube Formation Assay
Glass chamber slides (Nunc™ Lab-Tek™ II; Thermo Scientific, Waltham, MA, USA) were coated with Matrigel™ (BD Biosciences) and overlaid with 7 × 104 human umbilical vein endothelial cells together with 2.5 × 105 MSCs in endothelial basal medium (Lonza, Basel, Switzerland) in the absence of additional growth factors or cytokines and incubated at 37°C with 5% CO2 for 16 h. Tube formation was evaluated using a phase-contrast microscope (Eclipse Ti; Nikon, Tokyo, Japan).
Transplantation of MSCs Into Rats With Ischemic Hindlimbs
MSCs were trypsinized (Life Technologies) and labeled with DiI (Molecular Probes®; Life Technologies) as described by our group (23,29). Unilateral hindlimb ischemia was created in normal F344 rats as previously described by our group by ligating the femoral artery and removing all arterial branches (10,23,25,29,51). Three million DiI-labeled MSCs in 500 µl PBS (Cellgro®; Corning) were intramuscularly injected into the ischemic hindlimbs.
Blood Flow Measurement in Hindlimbs
Blood flow in the hindlimb was measured using a Laser Doppler perfusion imager (LDPI; Moor Instrument, Delaware, DE, USA) as previously described (10,23,25, 29,51). Mean values of perfusion were calculated from the stored digital color-coded images. The level of blood flow of the ischemic (left) limb was normalized to that of the nonischemic (right) limb to avoid data variations caused by ambient light and temperature as we previously described (10,23,25,29,51).
Measurement of Capillary Density
Four weeks after the injection of N- or D-MSCs into ischemic hindlimbs, the hindlimb muscles were harvested, fixed with 4% paraformaldehyde (Sigma-Aldrich) at 4°C overnight, and frozen in OCT compound (Tissue-Tek®; Sakura Finetek USA, Torrance, CA, USA) and cryosectioned at 10-µm intervals using cryostat (Cryotome FE/FSE; Thermo Scientific). To visualize capillaries, the sections were stained using isolectin B4 (Vector Laboratories, Burlingame, CA, USA) (9,23,25,29), and the capillary density was determined under conventional epifluorescence microscopy (Eclipse Ti; Nikon) as previously described (9,23,25,29).
Western Blotting of AKT1
Western blots were performed as described previously (61) using monoclonal antibody against AKT1 (1:500; Cell Signaling Technology, Danvers, MA, USA) and phosphorylated AKT1 (1:500; Cell Signaling Technology). Densitometric analyses for the blots were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
All results were expressed as a mean value ± SEM. Statistical analysis was performed by Student’s t-test for comparisons between two groups and repeated-measures ANOVA for more than two groups followed by Tukey’s multiple comparison test using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). A value of p < 0.05 was considered to be statistically significant.
RESULTS
Fewer MSCs Are Culture-Isolated From Diabetic Than Normal Rats
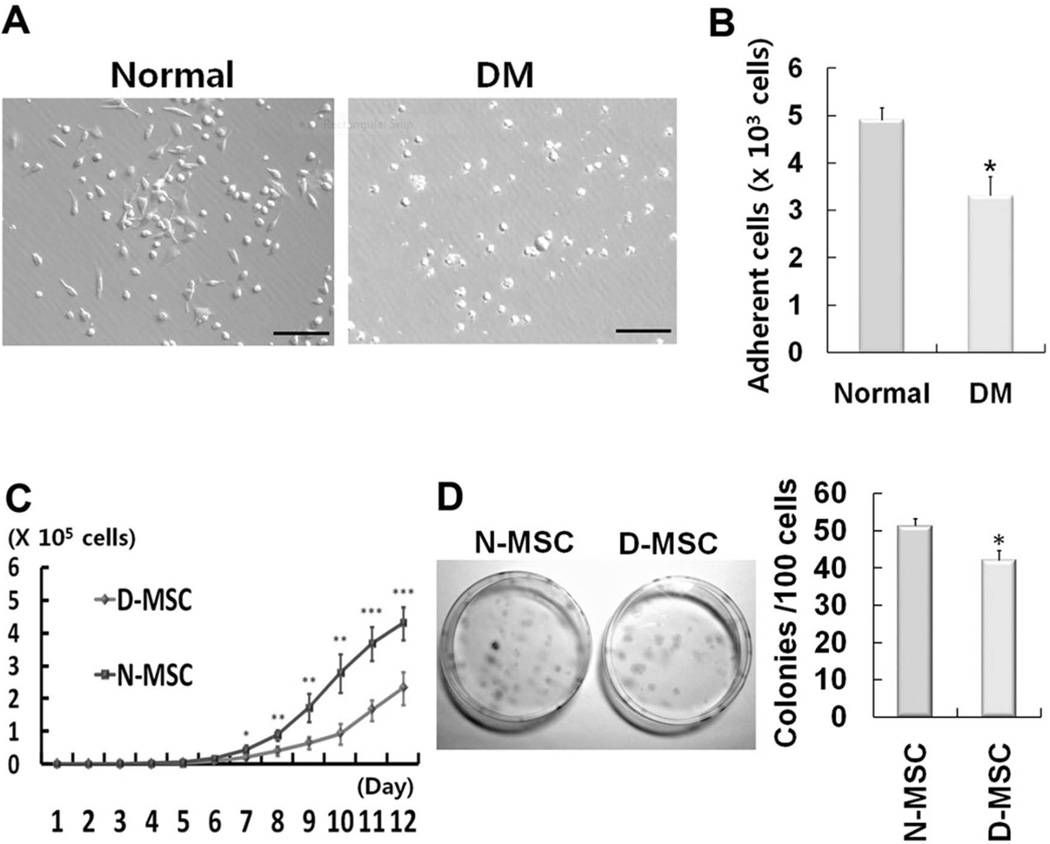
Hyperglycemia was induced by the injection of streptozotocin in the rats (Table 2). BMMNCs were isolated from normal and diabetic rats and subjected to MSC culture. Most of the adherent cells from normal BM were spindle or satellite shaped, whereas many of the adherent cells from diabetic BM were round (Fig. 1A). The number of adherent cells was 33% less in diabetic rats compared to normal rats (p < 0.05) at day 4 after culture of BM (Fig. 1B).
Table 2.
Blood Glucose Levels and Body Weights of Normal and Diabetic Rats Used in This Study (n = 16)
| Normal Rats | Diabetic Rats | p Value | |
|---|---|---|---|
| Blood glucose (mg/dl) | 251 ± 49 | 459 ± 76 | <0.001 |
| Body weight (g) | 333 ± 59 | 169 ± 52 | <0.001 |
Figure 1.
Impaired proliferation of mesenchymal stem cells (MSCs) derived from diabetic rats. (A) Representative photomicrographs of MSC culture at 4 days. Most adherent cells from normal rats were spindle shaped, whereas many of those from diabetic rats (DM) were rounded up, suggestive of aberrancy. Scale bars: 100 µm. (B) The numbers of adherent cells, most of which are MSCs, were significantly higher in the normal rat group than the DM group (*p < 0.05, n = 3) at 10 days of culture. (C) Growth curve of MSCs. The numbers of MSCs were counted daily. D-MSCs showed impaired proliferation compared to N-MSCs. (*p < 0.05, **p < 0.01, ***p < 0.001, n = 4). (D) One hundred MSCs were plated per 100-mm dish, and the number of colonies was counted at 14 days using crystal violet staining. The number of colony-forming cells was significantly lower in D-MSCs than N-MSCs (*p < 0.05, n = 4).
The Proliferation of D-MSCs Is Impaired
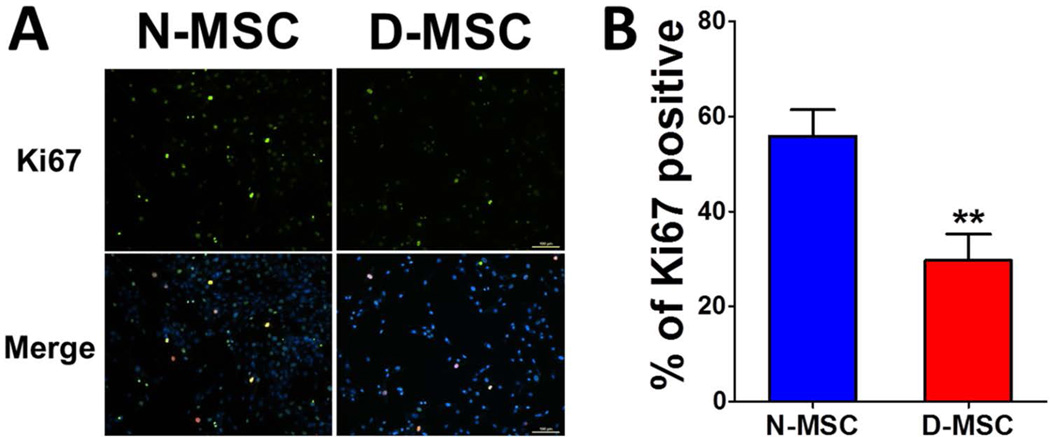
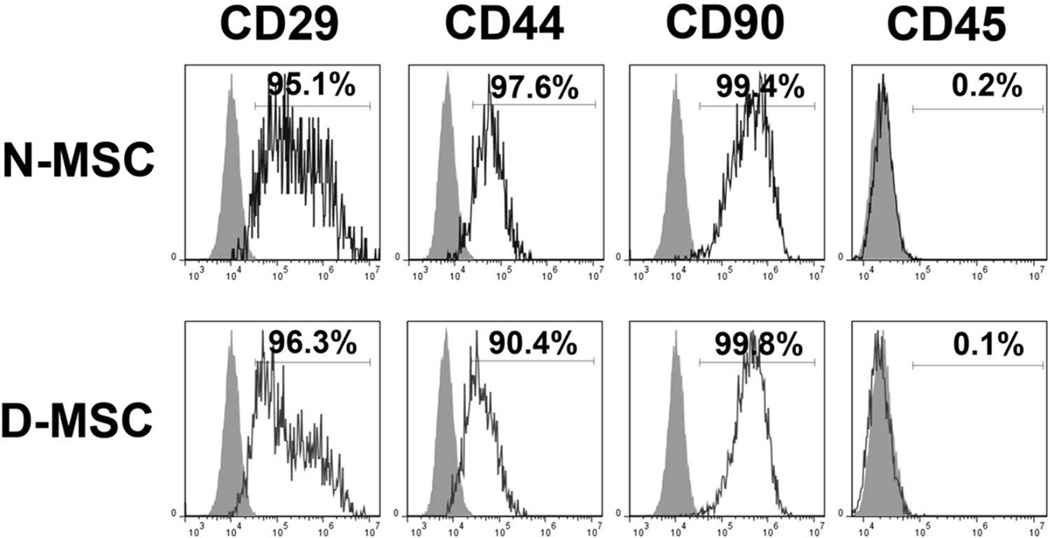
We next determined the proliferation potential of diabetic MSCs. Normal or diabetic MSCs were plated at 200 cells/cm2, and the cell number was determined by cell counting every day. The cell number became significantly smaller in D-MSCs than in N-MSCs from day 7, and the difference became greater over the next 5 days (Fig. 1C). Furthermore, we performed Ki-67 staining to verify these results. The number of Ki-67-positive cells was 53% lower in D-MSCs compared to N-MSCs (Fig. 2). To determine whether this was due to slow proliferation of D-MSCs compared to N-MSCs, we performed a colony-forming unit assay, which determines actively proliferating cell portions. The number of colony-forming units was 18% smaller in D-MSCs compared to N-MSCs (p < 0.05) (Fig. 1D), suggesting that the proportion of actively proliferating cells is smaller in D-MSCs than N-MSCs. This impaired proliferation of D-MSCs indicates that the self-renewal potential of D-MSCs is lower compared to N-MSCs. We next examined expression of the surface epitopes in these cells. Flow cytometry analysis demonstrated that the adherent cells derived from both normal or diabetic rats expressed typical MSC markers, such as CD29, CD44, and CD90, but not a pan-hematopoietic cell marker, CD45 (Fig. 3), confirming that these cells are MSCs regardless of diabetic status.
Figure 2.
Decreased Ki-67-positive cells in D-MSCs. Culture-expanded N-MSCs and D-MSCs were immunostained with anti-Ki-67 antibody to examine their proliferation capacity. (A) Representative immunocytochemical findings for Ki-67-positive cells. The nuclei of the proliferating cells were demonstrated by double positivity for green (Ki-67) and blue fluorescence (DAPI). Scale bar: 100 µm. (B) Percentages of Ki-67-positive cells in N-MSC and D-MSC (**p < 0.01).
Figure 3.
Flow cytometric characterization of N- and D-MSCs. MSCs were culture isolated from BM of normal and diabetic rats. N-MSCs and D-MSCs were equally positive for MSC markers such as CD29, CD44, and CD90 and were negative for a pan-hematopoietic cell marker, CD45. Gray curves indicate the isotype controls.
D-MSCs Have Aberrant Differentiation Propensity
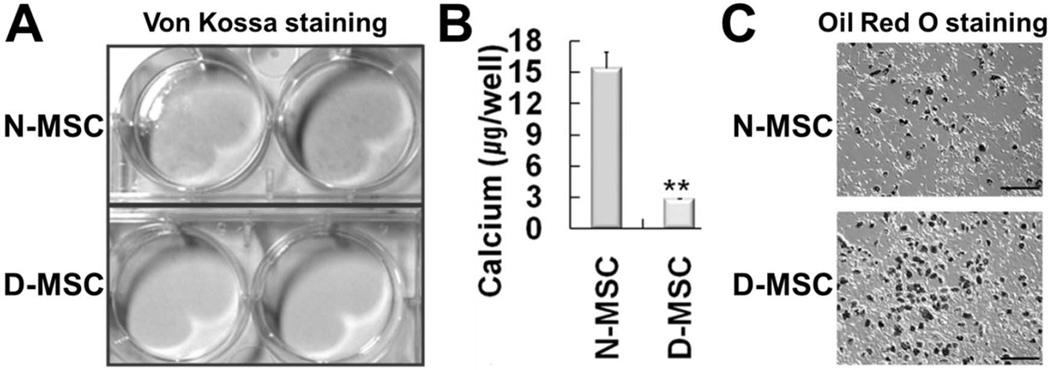
To determine whether diabetic conditions affect the differentiation propensity of MSCs, we exposed the N- or D-MSCs to known osteogenic and adipogenic differentiation conditions. After N- or D-MSCs were induced to differentiate into the osteogenic lineage, we performed von Kossa staining, which stains mineralized bone tissue with a black color. Whereas a significant proportion of N-MSCs were positive for von Kossa staining, no D-MSCs were (Fig. 4A). When we quantified the level of osteogenic differentiation by determining the calcium deposition, the amount of deposited calcium was more than fivefold lower in D-MSCs than in N-MSCs (p < 0.01) (Fig. 4B), indicating that the osteogenic differentiation potential of D-MSCs is impaired.
Figure 4.
D-MSCs have aberrant differentiation propensity. von Kossa staining (A) and quantification of the deposited calcium (B) after osteogenic differentiation of N- or D-MSCs. D-MSCs showed weaker von Kossa staining and lower levels of deposited calcium than N-MSCs (**p < 0.01, n = 3), suggesting impaired osteogenic differentiation. (C) Oil red O staining after adipogenic differentiation for 3 weeks. D-MSCs showed stronger Oil red O staining than N-MSCs, suggesting enhanced adipogenic differentiation. Scale bars: 400 µm.
To evaluate their adipogenic differentiation potential, we exposed N- or D-MSCs to well-known adipogenic differentiation conditions for 3 weeks and stained cells with Oil red O staining, which stains intracellular lipid with a dark color. D-MSCs showed higher numbers of Oil red O-stained cells than N-MSCs (Fig. 4C), suggesting that D-MSCs have an enhanced adipogenic differentiation capability compared to N-MSCs. Taken together, the differentiation propensity of D-MSCs into osteogenic and adipogenic lineages are aberrant and the reverse of that of N-MSCs.
The Expression of Angiogenic Factors Is Lower in D-MSCs Than N-MSCs
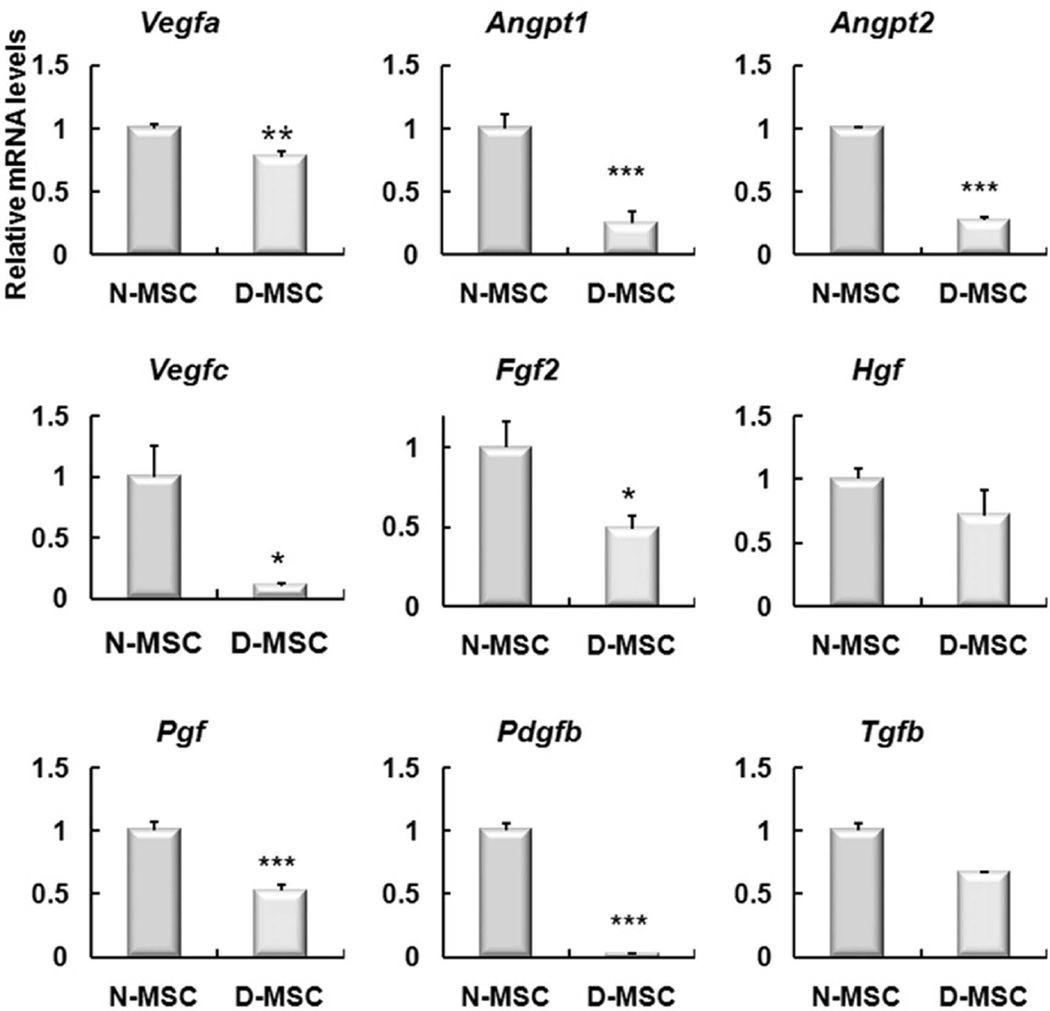
To examine the expression levels of angiogenic genes, we isolated RNAs from N- or D-MSCs and performed real-time RT-PCR. The expression of major angiogenic genes, such as vascular endothelial growth factor A (Vegfa), angiopoietin 1 (Angpt1), angiopoietin 2 (Angpt2), vascular endothelial growth factor C (Vegfc), fibroblast growth factor 2 (Fgf2), placental growth factor (Pgf), and platelet-derived growth factor β polypeptide (Pdgfb) were significantly lower in D-MSCs than in N-MSCs, while the levels of hepatocyte growth factor (Hgf) and transforming growth factor, β1 (Tgfb) were not significantly different (Fig. 5).
Figure 5.
The expression levels of angiogenic genes were lower in D-MSCs than N-MSCs. Real-time RT-PCR showed that the expression levels of Vegfa, Angpt1, Angpt2, Vegfc, Fgf2, Pgf, and Pdgfb were significantly lower in D-MSCs than N-MSCs (*p < 0.05, **p < 0.01, ***p < 0.001), whereas the levels of Hgf and Tgfb were not significantly different between the two groups. n = 4–6.
D-MSCs Show Reduced Angiogenic Potential In Vitro
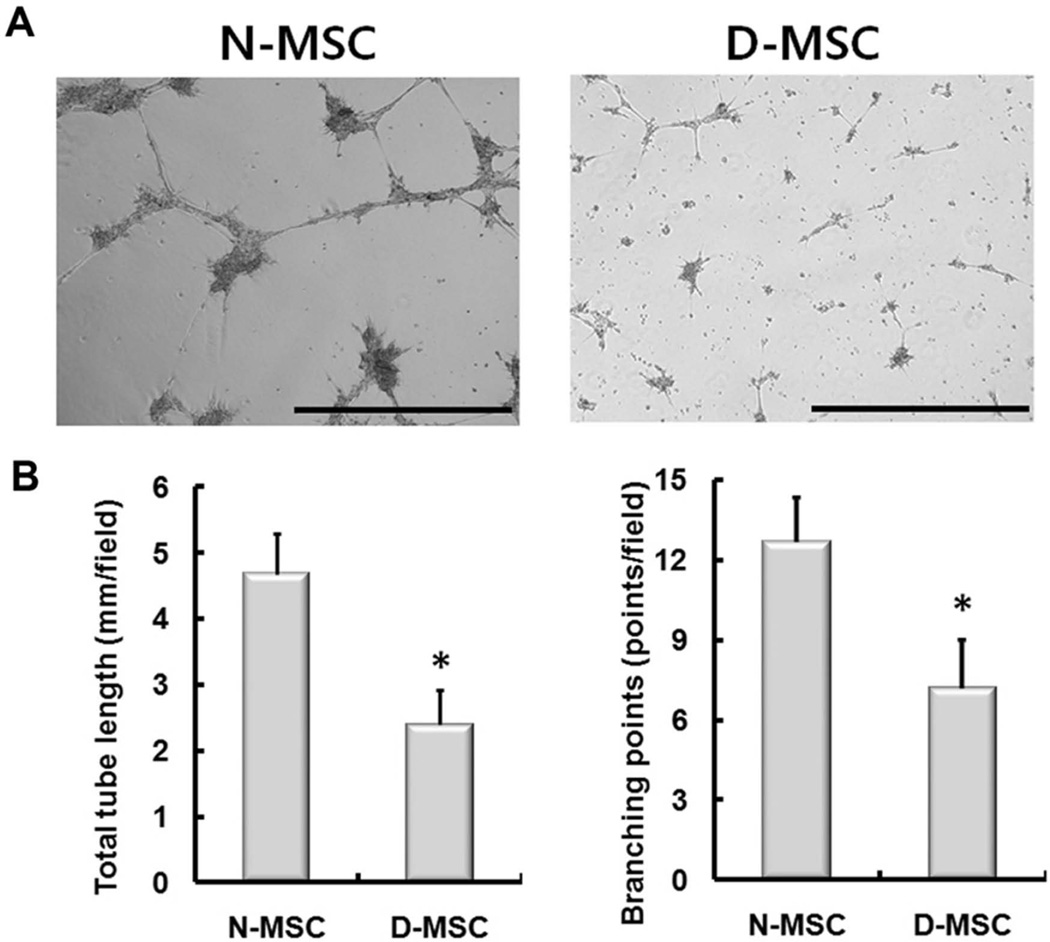
To determine the angiogenic capacity of D-MSCs on endothelial cells through their paracrine-angiogenic activities (13), we compared the tube formation capability of diabetic and nondiabetic MSCs in coculture with endothelial cells. We plated human umbilical vein endothelial cells onto Matrigel™ together with N- or D-MSCs in the absence of additional angiogenic factors. After 16 h, tube formation was evaluated under the microscope. The tubular structures were not clearly formed in the D-MSC group (Fig. 6A). Quantitatively, total tube length and branching points were 48% and 43% lower in the D-MSC group than in the N-MSC group, respectively (both p < 0.01) (Fig. 6B), suggesting that tube-forming ability is impaired in D-MSCs.
Figure 6.
In vitro tube formation by coculture of MSCs and endothelial cells. Human umbilical vein endothelial cells, 7 × 104, were cocultured with 2.5 × 105 N- or D-MSCs and plated onto Matrigel™. Tube formation was observed after 16 h. (A) Representative photomicrographs showing the in vitro tube formation. (B) The total tube length and number of branching points of the tubular network per field were higher in the N-MSC group than the D-MSC group (*p < 0.05, n = 6). Scale bars: 1 mm.
Implantation of D-MSCs Did Not Improve Hindlimb Ischemia In Vivo
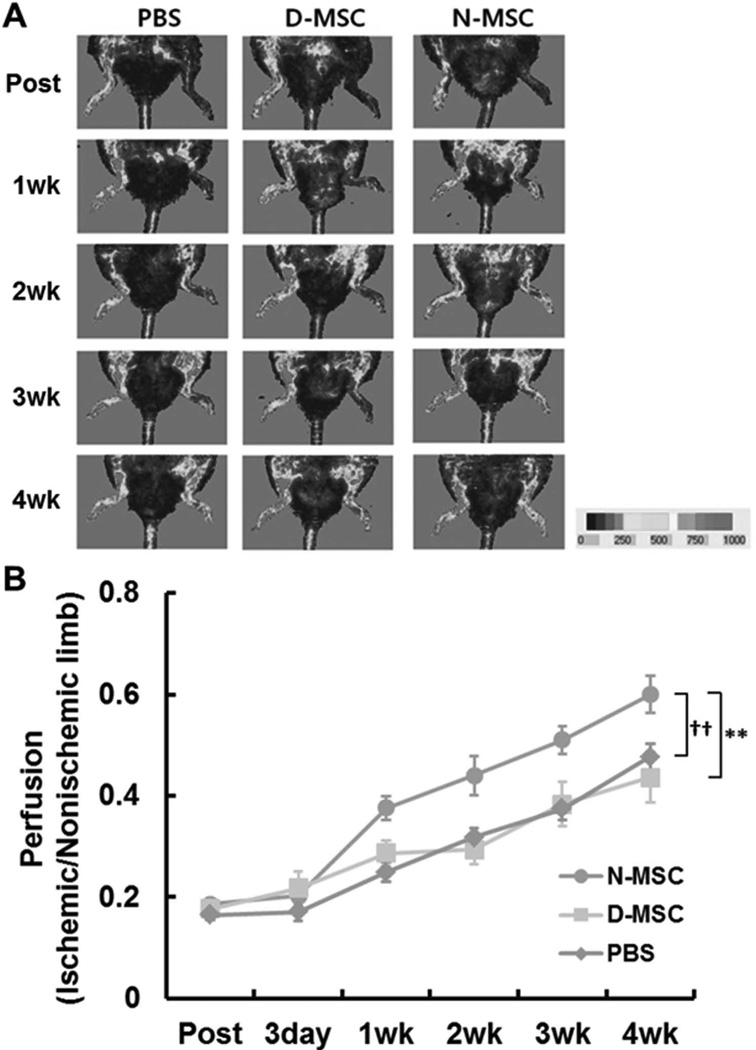
To test the therapeutic and regenerative effects of MSCs, we injected three million N- or D-MSCs into ischemic hindlimbs of rats and monitored blood flow using LDPI (23,25,29,32,41,51). PBS injection served as a control. Compared to the PBS-injected group, only the N-MSC-implanted group showed significantly improved blood flow (Fig. 7). Together, these results imply that the capacity for improving limb ischemia is impaired in D-MSCs.
Figure 7.
Impaired improvement of hindlimb ischemia by D-MSCs. Three million N- or D-MSCs were intramuscularly injected into the ischemic hindlimbs. Blood perfusion of the limb was determined using LDPI. Representative LDPI images (A) and quantification of the perfusion (B) showed that hindlimb blood flow was not significantly enhanced in the D-MSC group compared to the PBS group. The N-MSC group showed higher blood flow compared to the D-MSC group (**p < 0.01) and the PBS group (††p < 0.01). n = 5–14.
Capillary Densities Were Not Increased by Implantation of Diabetic MSCs
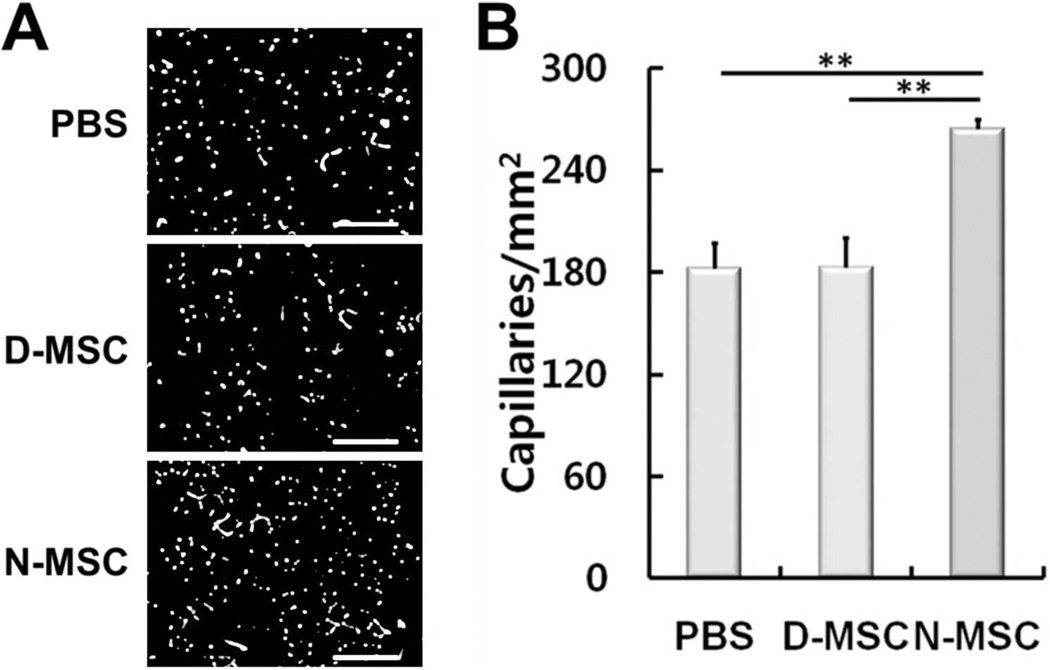
To further determine the neovascularization effects of D-MSCs, we quantified capillary density in the ischemic muscle at 4 weeks after cell implantation. MSCs were reported to increase capillary density through paracrine-angiogenic activities (31,32). Capillary density, determined by isolectin B4 (ILB4) staining, was not significantly different between the D-MSC and PBS groups, but was 45% higher in the N-MSC-injected group compared to the PBS group (p < 0.01) (Fig. 8). These data indicate that in vivo angiogenic effects of D-MSCs are impaired compared to N-MSCs.
Figure 8.
Impaired neovascularization in hindlimb ischemia by D-MSCs. Four weeks after implantation of N- or D-MSCs into ischemic limbs, the limb tissues were harvested and stained with ILB4. Representative fluorescent micrographs (A) and quantification of the capillary density (B) showed that capillary density was not increased in the D-MSCs compared to the PBS control, but did increase in the N-MSC group compared to the D-MSC (**p < 0.01) and PBS groups (**p < 0.01). n = 4. Scale bars: 200 µm.
Reduced Levels of Paracrine Factors in Ischemic Limbs Injected With D-MSCs
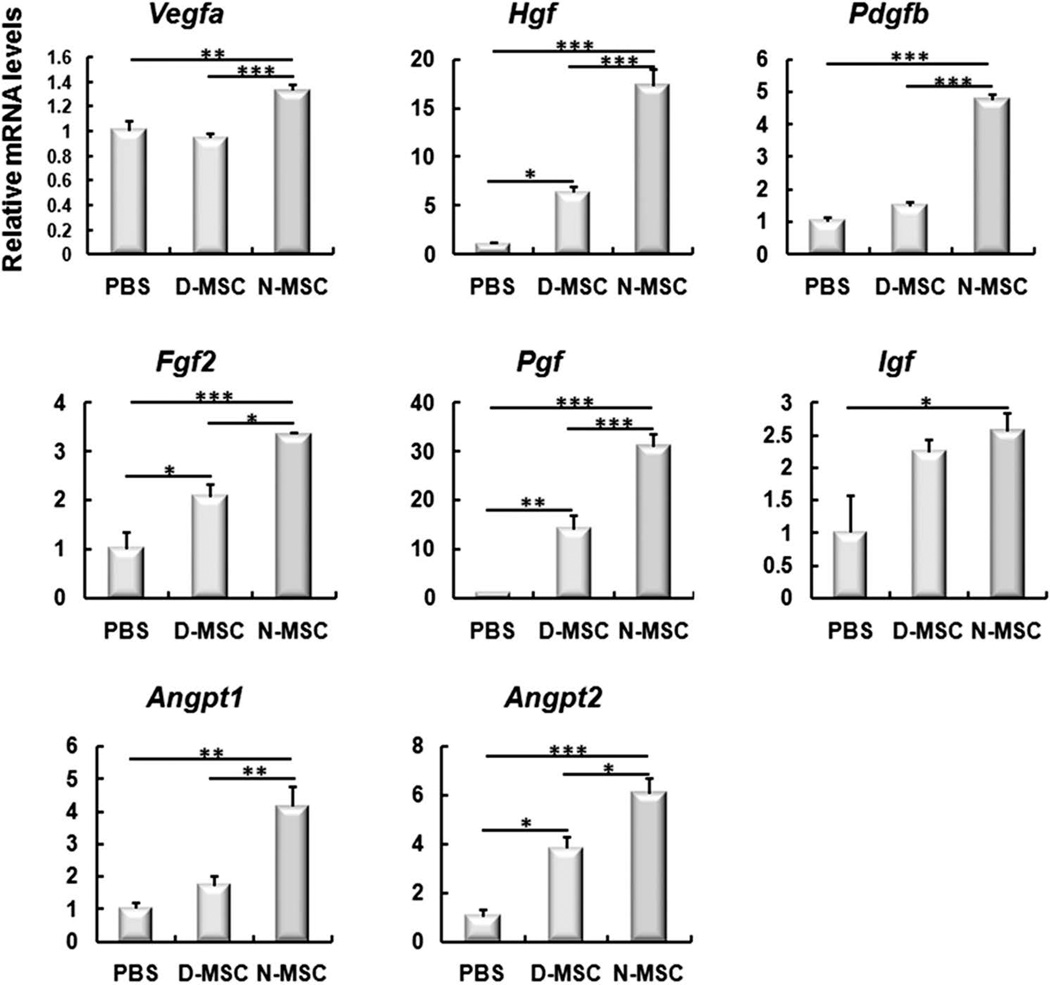
To investigate the changes of paracrine factors involved in neovascularization and apoptosis in vivo, we measured various gene expression levels by real-time RT-PCR using muscles harvested at 2 weeks after cell implantation. Among eight major angiogenic factors we selected and measured, the levels of all but insulin-like growth factor 1 (Igf) were significantly lower in the D-MSC group than in the N-MSC group (Fig. 9). However, Hgf, Fgf2, Pgf, and Angpt2 levels were higher in the D-MSC group compared to the PBS group. Together, these results suggest that although D-MSCs still express higher levels of certain angiogenic and antiapoptotic factors than the control, D-MSCs have significant impairment in exerting paracrine or humoral factor expression.
Figure 9.
Upregulation of angiogenic, antiapoptotic, and vessel-stabilizing factors in ischemic hindlimbs injected with D-MSCs and controls. The hindlimb tissues were harvested 2 weeks after cell implantation. Total RNA was isolated and subjected to quantitative RT-PCR. Gene levels were normalized to GAPDH. Data are presented as fold differences compared to the value of the PBS group. *p < 0.05, **p < 0.01, ***p < 0.001, n = 3–8.
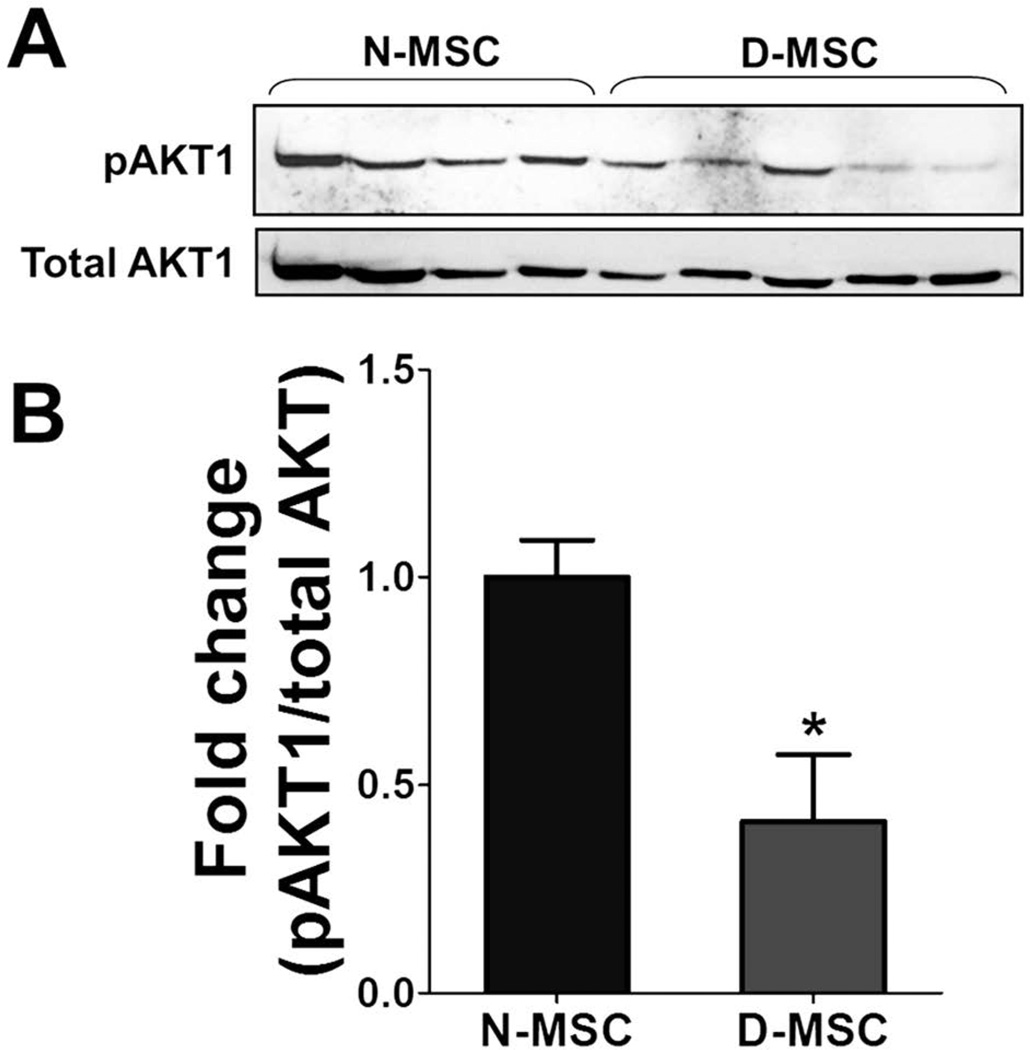
AKT1 Activity Is Decreased in D-MSCs Compared to N-MSCs
To further investigate the mechanisms underlying the reduced angiogenic effects of D-MSCs, we determined the protein levels of AKT1 and phosphorylated AKT1 (pATK1) in D- and N-MSCs (Fig. 10A). It was shown that AKT1 function is important for paracrine effects and angiogenesis mediated by BM-derived stem or progenitor cells (1,38,42). However, no studies investigated the effects of diabetes on AKT1 in MSCs. Western blotting analysis showed that the levels of pAKT1 normalized to total AKT1 were significantly lower in D-MSCs than N-MSCs, indicating that AKT1 activity is substantially reduced in D-MSCs (Fig. 10B).
Figure 10.
ATK1 levels in N- and D-MSCs. (A) Total proteins were isolated from N-MSCs derived from four normal rats and D-MSCs from five diabetic rats and subjected to Western blotting analysis. (B) The levels of phosphorylated AKT1 normalized to total AKT1 were lower in D-MSCs than in N-MSCs (*p < 0.05; n = 4 for N-MSCs, n = 5 for D-MSCs).
DISCUSSION
We demonstrated that MSCs derived from diabetic rats have significant defects in cell biologic, gene expression, and in vivo therapeutic effects for recovering from hindlimb ischemia. The salient findings of this study are as follows: First, we showed that the number and the proliferation rate of MSCs derived from diabetic rats were reduced compared to normal control rats despite a similar surface phenotype of both MSCs. Second, D-MSCs had an aberrant differentiation propensity, with reduced osteogenic and enhanced adipogenic differentiation potential compared to N-MSCs. Third, the expression of angiogenic factors and activity of AKT1 were reduced in D-MSCs. Fourth, implantation of D-MSCs, but not N-MSCs, was ineffective in recovering hindlimb ischemia.
We, for the first time, report that the neovascularizing and ischemia-recovering capabilities of MSCs derived from diabetic subjects are impaired. This has important clinical implications as the demand for cell therapy using MSCs for ischemic cardiovascular diseases is growing in diabetic patients. Previously, it has been reported that diabetic patients have decreased numbers and functionality of EPCs (7,37,53,54). Moreover, BMMNCs from diabetic mice were reported to have reduced ischemia-improving capacity (52). Both BMMNCs and EPCs were primarily isolated or short-term cultured (within 7 days) so that the finding that the effects of diabetes remained on these cells was not so surprising. However, in contrast to EPCs and BMMNCs, MSCs used in this study were extensively expanded by ex vivo culture for at least 3 weeks at normal glucose levels. D-MSCs used for our experiments were generated through multiple cell divisions in vitro under normal glucose conditions, but the defects were not repaired after cell divisions. This suggests that the exposure of the parent MSCs to a diabetic environment is passed onto the daughter cells even in a euglycemic environment over cell divisions. This is in line with the recent reports that high glucose can cause epigenetic changes that persist even if the cells are then exposed to normal glucose levels (5,14) and provides a novel insight into the effect of diabetic conditions on stem and progenitor cells.
It is apparent that D-MSCs have impaired paracrine activities. Given the complexities of the pathogenesis of diabetic cell dysfunction, it is hard to fully address the mechanisms underlying these paracrine defects. However, we found that AKT1 can be an important factor for determining such activities in diabetes. Prior studies suggested the role of AKT1 in paracrine activities of MSCs (15,38,42) or angiogenic activities in stromal or tumor cells (24), but no studies have explored these roles in relation to diabetes. Our data suggest that AKT1 activity is decreased in D-MSCs, which may underlie the impaired paracrine and vessel-forming activities of the D-MSCs. Although this is one potential mechanism, further investigations are required to fully address the molecular mechanisms responsible for the effects of diabetes on MSCs.
One study suggested a role for oxidative stress as a mechanism for impaired diabetic MSCs (60). This study demonstrated that type 2 diabetes induces oxidative stress and restricts the multipotency of MSCs, leading to impaired differentiation of MSCs into endothelial cells and reduced blood flow recovery after limb ischemia. Although this study and ours showed impaired capacity of diabetic MSCs, the study design addressed mechanisms that are quite dissimilar. First, that study used db/db mice, a model for type 2 diabetes, while our study used streptozotocin-induced diabetic rats, which is a model for type 1 diabetes. More importantly, the approach and the proposed mechanisms are different. In the other study, diabetic MSCs were transplanted into bone marrow 24 h after inducing hindlimb ischemia, and it was demonstrated that the mobilized MSCs from BM differentiated more into adipocytes than endothelial cells in the ischemic site, resulting in adipocyte infiltration in hindlimbs and reducing the neovascularization. Strictly speaking, this study addressed the pathophysiologic role of MSCs, rather than direct therapeutic effects of MSCs. Moreover, the effects of transdifferentiation of MSCs into endothelial cells are reported to be low (less than 1%) and may not explain the impaired neovascularization with MSCs (2,59). On the other hand, our study injected diabetic MSCs into ischemic hindlimb muscle to test their direct therapeutic effects and demonstrated impaired paracrine angiogenic effects of diabetic MSCs. Accumulating evidence suggests that the MSCs promote neovascularization mainly through paracrine molecules secreted by MSCs (6). In this regard, our finding reporting defects in paracrine factors of D-MSCs can better explain the impairment of neovascularization in ischemic tissue via diabetic MSCs.
Our data also showed enhanced adipogenic differentiation and reduced osteogenic differentiation of D-MSCs compared to N-MSCs. MSCs have been used for bone and adipose tissue engineering or regeneration. Diabetic patients have a higher risk for bone defects or fracture (11) and, thus, would have more demand for bone tissue engineering. Our data predict the efficacy of MSCs derived from diabetic subjects for bone and adipose tissue generation. In addition, the aberrant differentiation propensity of D-MSCs may also provide a novel mechanistic insight into the bone loss associated with diabetes. Previously, it has been shown that diabetic patients (11,20) and animals (50) have reduced bone mass. However, exact mechanisms underlying diabetic bone loss remain to be elucidated, although reduced osteoblastic activity without alteration in osteoclast activity and enhanced marrow adiposity have been reported to be involved (39). The enhanced adipogenic and reduced osteogenic differentiation of D-MSCs observed in our study is well in line with the above findings in diabetic subjects. Together, the evidence suggests that the diabetic environment favors adipogenic differentiation of MSCs residing in bone marrow while inhibiting their osteoblastic differentiation, leading to reduced bone formation.
Growing evidence has demonstrated favorable effects of MSCs on patients with acute and chronic cardiac dysfunction and peripheral vascular diseases (3,8,16,18,31, 32,40,45,48). These studies used MSCs isolated from healthy or normal donors. Although many such clinical studies adopted an allogeneic approach due to feasibility and cost effectiveness, an autologous approach is more ideal. As diabetic populations are ever increasing, particularly among patients with advanced cardiovascular diseases who are the most likely candidates for cell therapy, it is imperative to test the therapeutic potential of MSCs derived from such patients or animals. This study, for the first time, suggests that MSCs derived from diabetic subjects have limitations in their therapeutic potential, mainly attributable to their impaired vessel formation capabilities. This study further emphasizes the need for new approaches that can restore the function of defective MSCs isolated from diabetic subjects. However, since we used MSCs isolated from rats, not human patients, in this study, we cannot readily rule out the possibility that MSCs from diabetic patients might have different therapeutic potential, which awaits further investigation.
In conclusion, we showed that MSCs derived from diabetic subjects have impaired cell biologic function and reduced neovascularization capabilities. Implantation of N-MSCs, but not D-MSCs, recovered hindlimb ischemia and increased vascularization in vivo. Thus, further investigation is required before using MSCs derived from diabetic patients for the treatment of ischemic cardiovascular diseases.
ACKNOWLEDGMENTS
We are grateful to Yong-Wook Jung for his support for statistical analysis. This work was supported in part by NIH grants (DP3DK094346 and HHSN268201000043C) and a NSF-EBICS (Emergent Behaviors of Integrated Cellular Systems) grant.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10(8):621–629. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- 3.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003;89(6):1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 5.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58(5):1229–1236. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014;143(2):186–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Capla JM, Grogan RH, Callaghan MJ, Galiano RD, Tepper OM, Ceradini DJ, Gurtner GC. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast. Reconstr. Surg. 2007;119(1):59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 8.Chang W, Song BW, Lim S, Song H, Shim CY, Cha MJ, Ahn DH, Jung YG, Lee DH, Chung JH, Choi KD, Lee SK, Chung N, Jang Y, Hwang KC. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27(9):2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 9.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007;204(13):3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am. J. Pathol. 1998;152(6):1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 11.de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab. Res. Rev. 2010;26(8):622–630. doi: 10.1002/dmrr.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999;107(2):275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 13.Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014;3(1):32–41. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008;205(10):2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 16.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27(4):971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--A growing challenge. N. Engl. J. Med. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 18.Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, Iwasaki K, Fujiwara M, Kitamura S, Nagaya N, Ikeda T. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26(10):2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 19.Ishizeki K, Saito H, Shinagawa T, Fujiwara N, Nawa T. Histochemical and immunohistochemical analysis of the mechanism of calcification of Meckel’s cartilage during mandible development in rodents. J. Anat. 1999;194(Pt 2):265–277. doi: 10.1046/j.1469-7580.1999.19420265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isidro ML, Ruano B. Bone disease in diabetes. Curr. Diabetes Rev. 2010;6(3):144–155. doi: 10.2174/157339910791162970. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 22.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: Challenges and solutions. Circ. Res. 2010;106(5):854–869. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA. 2000;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: Novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ. Res. 2010;107(5):602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, Han JW, Lee J, Kim S, Houge MA, Ii M, Yoon YS. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27(7):1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Suh H, Jo SA, Kim HW, Lee JM, Kim EH, Reinwald Y, Park SH, Min BH, Jo I. In vivo bone formation by human marrow stromal cells in biodegradable scaffolds that release dexamethasone and ascorbate-2-phosphate. Biochem. Biophys. Res. Commun. 2005;332(4):1053–1060. doi: 10.1016/j.bbrc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 28.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24(6):1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J. Am. Coll. Cardiol. 2010;56(7):593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: Mechanisms, animal data, and initial clinical experiences. Circ. Res. 2004;95(4):354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 31.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 32.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 33.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp. Hematol. 2006;34(11):1606–1607. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: Changes in effects after ex vivo culturing. Cell Transplant. 2008;17(9):1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- 37.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53(1):195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 38.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 39.McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J. Cell. Biochem. 2007;102(6):1343–1357. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- 40.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 41.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Invest. 1998;101(11):2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. In vitro bone formation by rat marrow cell culture. J. Biomed. Mater. Res. 1996;32(3):333–340. doi: 10.1002/(SICI)1097-4636(199611)32:3<333::AID-JBM5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 44.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 45.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman A, W, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 47.Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs) J. Bone Miner. Res. 2004;19(2):256–264. doi: 10.1359/JBMR.0301220. [DOI] [PubMed] [Google Scholar]
- 48.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 49.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 50.Stolzing A, Sellers D, Llewelyn O, Scutt A. Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs. 2010;191(6):453–465. doi: 10.1159/000281826. [DOI] [PubMed] [Google Scholar]
- 51.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 1994;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Levy BI. Impairment in ischemia-induced neovascularization in diabetes: Bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am. J. Pathol. 2004;164(2):457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 54.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 55.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 56.Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29(1):5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 58.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ. Res. 2011;108(7):792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 60.Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J. Am. Heart Assoc. 2012;1(6):e002238. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: Restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111(16):2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]