Abstract
BACKGROUND
The association between body mass index as a measure of obesity and rectal cancer outcomes has been inconsistent. Radiologic measures of visceral adiposity using CT scans have not been well characterized among rectal cancer patients. The objective of this study was to examine quantitative radiologic measures of visceral obesity compared with body mass index in predicting patient outcomes among patients undergoing neoadjuvant chemoradiation and resection for locally advanced rectal cancers.
STUDY DESIGN
We identified 99 rectal adenocarcinoma patients treated with neoadjuvant chemoradiation and surgical resection. Visceral and subcutaneous fat areas, as well as perinephric fat thickness (PNF), were recorded and categorized as obese (body mass index ≥30, visceral fat area to subcutaneous fat area ratio [V/S] ≥0.4, or median PNF). The Kaplan-Meier method, log-rank test, and Cox proportional hazards models evaluated overall and disease-free survival differences by adiposity.
RESULTS
Viscerally obese rectal cancer patients (V/S >0.4 or PNF) were more likely to be older, male, and have pre-existing obesity-related conditions (eg, diabetes, hypertension, and/or hyper-cholesterolemia). Elevated V/S or PNF was associated with shorter disease-free survival (p = 0.02) or overall survival time (p = 0.047), respectively. Among patients with well to moderately differentiated tumors, visceral obesity was associated with poorer disease-free survival (V/S >0.4: adjusted hazard ratio = 5.0; 95% CI, 1.2–22.0).
CONCLUSIONS
Visceral fat area to subcutaneous fat area ratio and PNF were strongly associated with key preoperative metabolic comorbidities, and body mass index was not. Findings suggests that elevated visceral adiposity was associated with an increased risk of recurrence, which was most evident among patients with well to moderately differentiated tumors and those with incomplete response to neoadjuvant chemoradiation treatment. Quantitative measures of visceral adiposity warrant large-scale prospective evaluation.
Obesity is a major public health problem of epidemic proportions and is linked to the development of a number of malignancies, including colorectal cancer (CRC).1–3 Nearly 66% of the US population is overweight or obese, as defined by body mass index (BMI) ≥25.1 More than 90,000 cancer deaths per year are attributable to obesity or being overweight in the United States, and obesity plays a role in >20% of the approximately 150,000 CRC cases diagnosed each year.4
Obesity has been associated with increased risk for CRC recurrence and death.5–10 However, there have been a number of studies that have reported no association between BMI and CRC outcomes,11–14 and of those with significant findings, there are inconsistencies about level of obesity, outcomes (eg, overall survival [OS] or disease-free survival [DFS]), and the role of sex.6–8 Factors clustering with insulin-resistance syndrome (or metabolic syndrome) have also been associated with increased CRC mortality and recurrence.15–17
Additionally, when focusing on the select population of patients with rectal adenocarcinoma (rather than all CRC patients) the data become even more unclear. The most recent studies involving rectal cancer patients reported no difference in survival in patients with higher BMI after total mesorectal excision and neoadjuvant chemoradiation, and one study even reported a survival advantage in obese patients.3,18 Others have reported obese men have a significantly higher risk of locoregional recurrence; however, no associations were observed for women or OS, regardless of sex. One explanation for these inconsistencies could be that a majority of studies use BMI as a measure of obesity, which does not provide a consistent or accurate measure of abdominal (eg, visceral) obesity. It is possible that visceral obesity can have an unrecognized detrimental impact on optimal dosing and/or delivery of chemotherapy and radiation.6,19,20 (Although increased BMI has not been associated with increased rates of positive surgical radial margins, it is possible that visceral obesity might better reflect greater technical challenges with total mesorectal excision.)18 In addition, from a biological standpoint, excess abdominal adipose tissue promotes a greater degree of obesity-related metabolic derangements, including insulin resistance, perturbations in adipokines, and chronic inflammation compared with subcutaneous adipose tissue.21–24 Visceral adipose mass might be a more accurate measure of dysfunctional adipose tissue that facilitates cancer development and progression than BMI.
Quantitative radiologic measures of visceral adiposity using standard CT scans have been reported as the gold-standard method for assessing visceral adiposity.25,26 This precise and reliable measure of abdominal fat compartments allows for the possibility of redefining obesity in terms of visceral fat rather than BMI. In a heterogeneous group of both colon and rectal cancer patients, Moon and colleagues demonstrated that individuals with high visceral adiposity had a considerably shorter DFS compared with those with low visceral adiposity, and BMI had no influence.27 To date, there have been no investigations focused on the association between visceral adiposity and oncologic outcomes in patients specifically undergoing neoadjuvant therapy and resection for locally advanced rectal cancers. In undertaking this study, we hypothesized that quantitative CT measures of visceral adiposity would be associated with key pre- and postoperative clinicopathologic variables and significantly associated with patient outcomes, DFS and OS. We also hypothesized that visceral adiposity variables might have stronger associations with patient outcomes than BMI.
METHODS
Patient selection and chart review
A retrospective database of surgical cases performed at the Moffitt Cancer Center between 1998 and 2010 for rectal cancer was developed with IRB approval. Patients with stage II or III rectal adenocarcinoma who were treated with neoadjuvant chemoradiation followed by radical resection (low anterior or abdominoperineal resection) were identified. Data were collected on patient demographics, preoperative comorbidities, TNM stage, histopathologic features, perioperative complications, disease recurrence, and survival. Chart reviews were performed solely by experienced clinicians and data were abstracted on standardized abstraction forms. Clinical response to neoadjuvant treatment was defined as “no response” if there was no clinical change in the tumor; “partial response” if a residual palpable lesion was present but with a clinical reduction in size; and “complete response” if no tumor or very minor scar tissue was present on completion of treatment. Pathologic response was defined as a “complete response” if tumor regressed to T0N0 after neoadjuvant therapy; “partial response” if there was a reduction in tumor size and/or nodal status with residual tumor cells; and “no response” if there was no change in the tumor or progression of stage after neoad-juvant treatment. Data were entered into a secure Microsoft Access database.
Adiposity measures
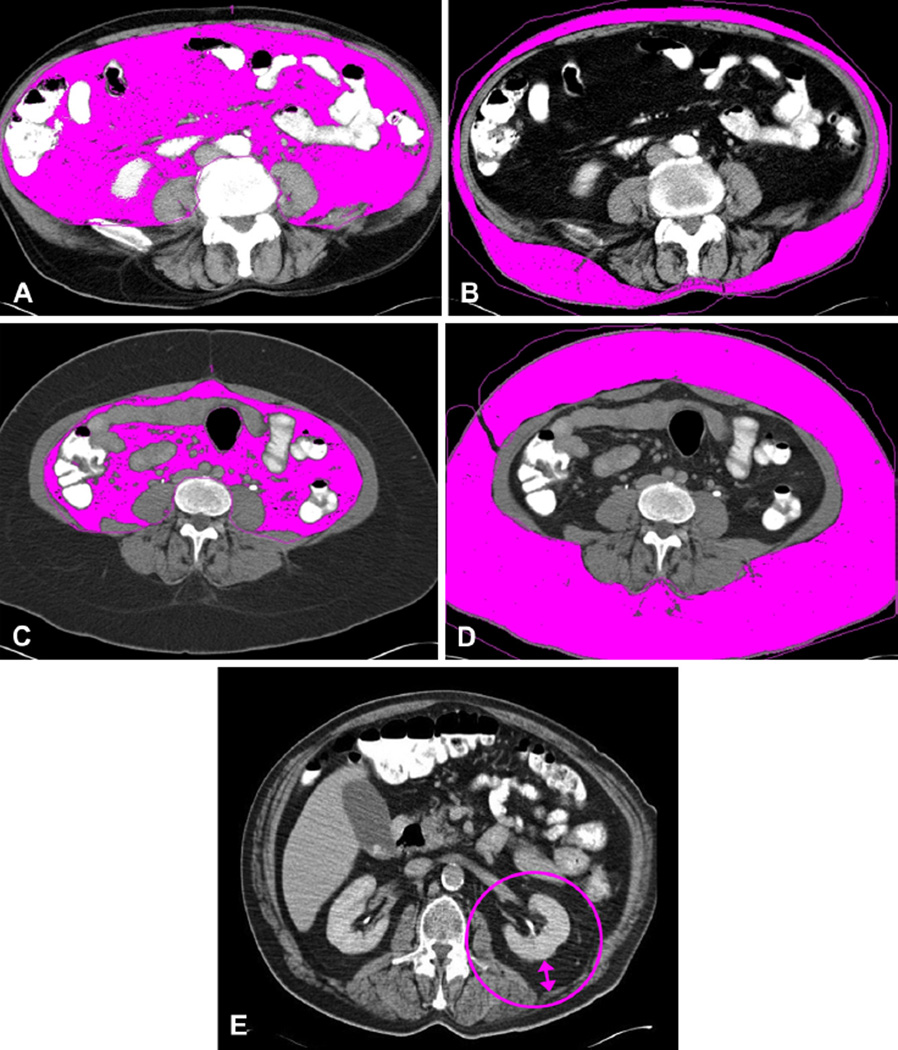
Patients with a pretreatment CT scan archived at Moffitt were included in this study (n = 99). Radiologic measures of adiposity were obtained from routine diagnostic CT scans using a Siemens CT Leonardo digital workstation (Siemens Medical Solutions). Visceral and subcutaneous fat areas (VFA and SFA, respectively) were measured from a single axial slice at the L4–L5 interver-tebral space.27,28 The CT attenuation level was set between −190 to −30 Hounsfield units, regions of adipose tissue were delineated, and areas of total, VFA, and SFA were calculated (Fig. 1A–D). Linear perinephric fat thickness (PNF) is defined as the shortest distance (in mm) between the kidney and abdominal wall at the level of the renal vein (Fig. 1E).29 The VFA to SFA ratio (V/S) was calculated to provide a single measure of abdominal fat, as published previously.27,30 Elevated V/S indicated higher visceral fat compared with subcutaneous fat, and a threshold was set at V/S = 0.4 to define visceral obesity.31 Quantitative CT adiposity measurements have been validated in colon cancer, with a measurement error rate of 0.5%.28,32 Height and weight recorded before surgery were used to calculate BMI (weight in kg divided by height in m2).
Figure 1.
Radiological measurement of visceral (VFA) and subcutaneous fat area (SFA) and linear perinephric fat thickness (PNF). Measurement of VFA (A, C) and SFA (B, D) by CT analysis software for 2 representative patients (A, B, and C, D). Visceral fat to subcutaneous fat ratio (V/S) differences observed for patient with (A) high VFA vs (D) high SFA. Patient in (A) and (B) is viscerally obese, with a V/S of 1.55 but a body mass index (BMI) of 27. Patient in (C) and (D) is obese when defined by BMI (BMI = 37), but is not viscerally obese (V/S = 0.23). (E) PNF measurement taken at level of the left renal vein.
Statistical analysis
Adiposity-related variables were evaluated as continuous measures and also categorized according to a priori cut points (BMI ≥30 and V/S ≥0.4)27,31 or at the median (PNF). Linear or nonlinear correlations among measures of obesity (BMI, VFA, SFA, V/S, and PNF) were examined as reported previously,33 and the interaction between obesity, age, and sex was examined using an analysis of covariance (not shown). Differences in adiposity by clinicopathologic factors and postoperative complications were evaluated using the chi-square or Fisher’s exact tests (categorical) or 2 sample t-tests with equal or unequal variance (continuous), as appropriate. Survival end points were OS, defined as time from surgery to death from any cause, and DFS, defined as time from surgery to either rectal cancer recurrence or death. Survival time was censored if patients were lost-to-follow-up or after 7 years. The Kaplan-Meier product limit method and log-rank test were used to compare the survival difference by categorized adiposity variables.
Multivariable Cox proportional hazard models were developed using the backward elimination to determine clinical adjustment factors (significance level = 0.1) to include in each model, with each of the adiposity measures forced into the models. Variables included for backward elimination were sex, age, presurgical N-stage, presurgical T-stage, down-staging of N- or T-stage, node positivity (positive vs negative), clinical response to treatment (complete response vs partial response/no response), grade (well/moderate vs poorly/undifferentiated), CEA level (≤5.0 vs >5), and any complication after surgery (yes vs no). Factors retained in the models were post-treatment grade and clinical response to treatment; however, to increase clinical accuracy of the model, pathologic response was included instead of clinical response in final models. Adjusted hazard ratio (AHR) and 95% CI were estimated for each adiposity variable (ie, BMI, VFA, SFA, V/S, and PNF) and outcomes (ie, OS and DFS). We conducted stratified analyses to investigate the impact of clinical parameters (eg, differentiation and response to neoadjuvant treatment) on the association between adiposity and outcomes. All statistical tests were 2-sided and considered as statistically significant at the level of 0.05 unless otherwise specified. Analyses were performed using SAS (SAS 9.2., SAS Institute) and Matlab R2011b (Mathwork Inc).
RESULTS
Demographics and clinical features
The study population consisted of 99 patients with stage II/III rectal cancer, with a mean age of 61 ± 13 years (Table 1). Median follow-up time was 39.4 months for all patients and 41.1 months for patients who were alive (range 2.3 to 109.5 months). There were slightly more men (n = 57) than women (n = 42), and almost all patients were white (94%). The frequency of diabetes, hypertension, and hypercholesterolemia (ie, obesity-associated comorbid conditions) among this population was 16%, 41%, and 19%, respectively. Before neoadjuvant chemoradiation, most patients (92%) had disease extending beyond the muscularis propria (T3 or T4) and had nodal disease (n = 52 [57%]). All patients included in this study underwent neoadjuvant chemoradiation treatment followed by either low anterior resection (n = 77) or abdominoperineal resection (n = 22). Twenty-five percent of patients had a complete pathological response, 49% had partial response, and 26% had no response to neoadjuvant chemoradiation. Clinical and pathologic response to neoadjuvant treatment were highly correlated (chi-square = 43.56; p < 0.0001). Mean number of lymph nodes in the surgical specimen was similar, irrespective of BMI or V/S. Patients with BMI ≥30 had 13.3 ± 8 nodes harvested vs 12.8 ± 9 in patients with BMI <30 (p = 0.41). Similarly, patients with V/S ≥0.4 had 13.3 ± 7 nodes harvested vs 12.8 ± 7 nodes in patients with V/S <0.4 (p = 0.84) (data not shown). Only one patient in our series had a positive radial margin. Postoperative complications occurred in 43% of patients, which, for the most part, were infection-related, including minor surgical site infections (n = 19), but also anastomotic leaks (n = 4) (Table 1). There were no patients reported to have MI, pulmonary embolism, or deep vein thrombosis.
Table 1.
Characteristics of Patients with Locally Advanced Rectal Cancer Treated at the Moffitt Cancer Center (n = 99)
| Characteristics | n* | % |
|---|---|---|
| Age, y, mean ± SD | 60.86 ± 12.98 | |
| Sex | ||
| Male | 57 | 57.58 |
| Female | 42 | 42.42 |
| Race | ||
| White | 93 | 93.94 |
| Non-white | 5 | 5.05 |
| Unknown | 1 | 1.01 |
| Comorbid conditions (yes) | ||
| Diabetes | 16 | 16.33 |
| Hypertension | 40 | 40.82 |
| Hypercholesterol | 19 | 19.39 |
| Pretreatment stage (overall) | ||
| Stage II | 38 | 43.18 |
| Stage III | 50 | 56.82 |
| Pretreatment T-stage | ||
| T1 | 1 | 1.03 |
| T2 | 7 | 7.22 |
| T3 | 79 | 81.44 |
| T4 | 10 | 10.31 |
| Pretreatment N-stage | ||
| N0 | 39 | 42.86 |
| N1 | 50 | 54.95 |
| N2 | 2 | 2.20 |
| Pathologic T-stage post-neoadjuvant treatment | ||
| Tis-T0 | 27 | 27.27 |
| T1 | 9 | 9.09 |
| T2 | 30 | 30.30 |
| T3 | 27 | 27.27 |
| T4 | 6 | 6.06 |
| Pathologic N-stage post-neoadjuvant treatment | ||
| N0 | 74 | 74.75 |
| N1 | 16 | 16.16 |
| N2 | 6 | 6.06 |
| NX | 3 | 3.03 |
| Down-staging in T-stage | ||
| Yes | 61 | 62.89 |
| No | 36 | 37.11 |
| Down-staging in N-stage | ||
| Yes | 39 | 43.82 |
| No | 50 | 56.18 |
| Clinical response to neoadjuvant treatment | ||
| None | 44 | 44.44 |
| Partial | 13 | 13.13 |
| Complete | 42 | 42.42 |
| Pathologic response to neoadjuvant treatment | ||
| None | 26 | 26.26 |
| Partial | 48 | 48.48 |
| Complete | 25 | 25.25 |
| Histological grade | ||
| Well to moderate | 88 | 88.89 |
| Poorly to undifferentiated | 6 | 6.06 |
| Cannot be accessed | 3 | 3.03 |
| No tumor present | 2 | 2.02 |
| Adjuvant treatment | ||
| Yes | 75 | 78.95 |
| No | 20 | 21.05 |
| Any postoperative complications | ||
| Yes | 42 | 42.86 |
| No | 56 | 57.14 |
| Surgical site infection | ||
| Yes | 19 | 19.39 |
| No | 79 | 80.61 |
Total number might be <99 due to missing data points.
Adiposity measures by sex
A complete characterization of BMI and abdominal adiposity variables is presented in Table 2 by sex. Overall mean BMI of these rectal cancer patients was 28.8 ± 6.6 and was not different by sex (p = 0.14). Alternatively, mean VFA, V/S, and PNF were significantly higher among men than women (p < 0.001). Sex differences in V/S and PNF increased with age (p < 0.001); the largest differences between men and women were observed in those older than age 60 years (data not shown). Forty-one percent of patients had a BMI ≥30, the conventional classification of obesity; and 62% of patients had a V/S ≥0.4, the cut point for classification as viscerally obese. A significantly higher proportion of patients were defined as obese when using V/S vs BMI (p = 0.004).
Table 2.
Descriptive Statistics of Adiposity Variables among Rectal Cancer Patients
| Mean | SD | Media | Minimum | Maximum | |
|---|---|---|---|---|---|
| Overall (n = 99) | |||||
| Weight, kg | 83.5 | 22.9 | 80.7 | 32.5 | 141.0 |
| Height, cm | 170.1 | 10.5 | 170.0 | 147.3 | 190.5 |
| BMI* | 28.8 | 6.6 | 27.7 | 15.0 | 48.1 |
| VFA | 156.7 | 91.5 | 136.2 | 12.4 | 570.0 |
| SFA | 314.5 | 151.4 | 291.2 | 43.4 | 718.0 |
| V/S | 0.6 | 0.3 | 0.5 | 0.1 | 1.8 |
| PNF | 13.4 | 9.6 | 12.2 | 1.2 | 52.0 |
| Males (n = 57) | |||||
| Weight, kg | 93.2 | 19.3 | 89.9 | 48.6 | 134.8† |
| Height, cm | 177.2 | 7.1 | 177.8 | 164.3 | 190.5† |
| BMI | 29.7 | 5.6 | 29.2 | 15.0 | 40.5 |
| VFA | 188.0 | 89.5 | 169.0 | 12.4 | 570.0† |
| SFA | 307.3 | 143.7 | 287.2 | 80.1 | 653.3 |
| V/S | 0.7 | 0.3 | 0.6 | 0.1 | 1.8† |
| PNF | 18.0 | 9.0 | 18.2 | 1.8 | 52.0† |
| Females (n = 42) | |||||
| Weight, kg | 71.2 | 21.3 | 65.9 | 32.5 | 141.0 |
| Height, cm | 160.6 | 5.8 | 160.0 | 147.3 | 177.8 |
| BMI | 27.6 | 7.5 | 25.4 | 15.0 | 48.1 |
| VFA | 114.2 | 76.7 | 105.6 | 18.4 | 472.2 |
| SFA | 324.3 | 162.5 | 299.1 | 43.4 | 718.0 |
| V/S | 0.4 | 0.2 | 0.3 | 0.1 | 0.9 |
| PNF | 7.1 | 6.3 | 4.6 | 1.2 | 33.7 |
Preoperative BMI was not available in 3 patients (n = 96).
One-way ANOVA testing for mean differences in adiposity variables by sex; p < 0.0001.
BMI, body mass index; PNF, perinephric fat thickness; SFA, subcutaneous fat area; VFA, visceral fat area; V/S, visceral fat to subcutaneous fat ratio.
Adiposity measures by clinicopathological characteristics
A summary of selected pre- and postoperative clinicopathologic variables as a function of BMI, V/S, and PNF is presented in Table 3. Obese patients defined by BMI (≥30) were significantly younger than nonobese patients (BMI <30; p = 0.03). Patients with pre-existing diabetes had significantly higher mean BMI compared with patients without diabetes (32 vs 28; p = 0.03); however, there were no significant differences in BMI among those that were hypertensive or hypercholesterolemic. In contrast to BMI, rectal cancer patients with a V/S ≥0.4 were significantly older (p = 0.006), more likely to be male (p ≤ 0.001), and have pre-existing hypertension (50% vs 26.3%; p = 0.02) and hypercholesterolemic (26.7% vs 7.9%; p = 0.03) than patients with V/S <0.4. In addition, patients with higher visceral fat had less down-staging of T-stage after neoadjuvant therapy (p = 0.04) (data not shown). Similar results were observed when the V/S and BMI were evaluated as continuous variables. Mean PNF was higher among males (18 mm vs 7 mm; p < 0.001) and those with hypertension (16.2 mm vs 11.2 mm; p = 0.01) or diabetes (19.1 mm vs 12.1 mm; p = 0.04).
Table 3.
Characterization of the Relationship between Clinicopathologic Factors and Adiposity Variables
| BMI | VFA/SFA ratio | PNF, mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p Value |
<30 (n = 57) |
≥30 (n = 39) |
p Value |
Mean | SD | p Value |
V/S <0.4 (n = 38) |
V/S ≥0.4 (n = 61) |
p Value |
Mean | SD | p Value |
|
| Age, y (mean) | 63 (13.2) | 57 (11.4) | 0.03 | 56.1 (14.5) | 63.4 (11.2) | 0.006 | |||||||||
| Sex | |||||||||||||||
| Male | 29.7 | 5.6 | 0.13 | 29 (51.8) | 25 (64.1) | 0.29 | 0.67 | 0.32 | <0.001 | 10 (26.3) | 47 (77.1) | <0.001 | 18.0 | 9.0 | <0.001 |
| Female | 27.6 | 7.6 | 27 (48.2) | 14 (35.9) | 0.37 | 0.18 | 28 (73.7) | 14 (22.9) | 7.1 | 6.3 | |||||
| Comorbidities | |||||||||||||||
| Hypertension | 30.0 | 6.8 | 0.13 | 20 (35.7) | 19 (48.7) | 0.29 | 0.66 | 0.31 | 0.002 | 10 (26.3) | 30 (50) | 0.02 | 16.2 | 8.6 | 0.01 |
| None | 27.9 | 6.4 | 36 (67.3) | 20 (51.3) | 0.47 | 0.28 | 28 (73.7) | 30 (50) | 11.2 | 9.7 | |||||
| Dyslipidemia | 28.8 | 7.3 | 0.99 | 10 (17.9) | 8 (20.5) | 0.79 | 0.67 | 0.36 | 0.05 | 3 (7.9) | 16 (26.7) | 0.03 | 16.6 | 12.3 | 0.08 |
| None | 28.8 | 6.5 | 46 (82.1) | 31 (79.5) | 0.51 | 0.29 | 35 (92.1) | 44 (73.3) | 12.4 | 8.7 | |||||
| Diabetes | 32.0 | 7.9 | 0.03 | 8 (14.0) | 8 (20.5) | 0.58 | 0.66 | 0.38 | 0.09 | 3 (7.9) | 13 (21.7) | 0.09 | 19.1 | 12.2 | 0.04 |
| None | 28.1 | 6.1 | 48 (85.7) | 31 (79.5) | 0.52 | 0.29 | 35 (92.1) | 47 (78.3) | 12.1 | 8.6 | |||||
| Preoperative stage | |||||||||||||||
| Stage II | 28.8 | 6.3 | 0.62 | 23 (46.9) | 14 (38.9) | 0.51 | 0.59 | 0.29 | 0.31 | 12 (35.3) | 26 (48.2) | 0.27 | 13.6 | 7.7 | 0.85 |
| Stage III | 29.5 | 6.9 | 26 (53.1) | 22 (61.1) | 0.52 | 0.33 | 22 (64.7) | 28 (51.9) | 14.0 | 11.1 | |||||
| Pathologic response to neoadjuvant treatment | |||||||||||||||
| None/partial | 28.4 | 6.7 | 0.29 | 47 (84.0) | 24 (61.5) | 0.02 | 0.56 | 0.31 | 0.52 | 26 (68.4) | 48 (78.7) | 0.34 | 13.0 | 9.5 | 0.56 |
| Complete | 30.3 | 6.2 | 9 (16.0) | 15 (38.5) | 0.51 | 0.32 | 12 (32.6) | 13 (21.3) | 14.3 | 10.0 | |||||
| Histologic grade after neoadjuvant | |||||||||||||||
| Well/moderate* | 28.9 | 6.6 | 0.42 | 51 (96.2) | 35 (89.7) | 0.40 | 0.55 | 0.31 | 0.26 | 35 (94.6) | 55 (93.2) | 1.0 | 13.2 | 9.7 | 0.46 |
| Poorly/undifferentiated | 31.1 | 6.0 | 2 (3.8) | 4 (10.3) | 0.40 | 0.17 | 2 (5.4) | 4 (6.8) | 16.2 | 9.1 | |||||
| Surgical site infection | |||||||||||||||
| No | 28.1 | 6.2 | 0.06 | 48 (85.7) | 28 (71.8) | 0.10 | 0.55 | 0.30 | 0.86 | 29 (78.4) | 50 (82.0) | 0.66 | 12.6 | 8.5 | 0.18 |
| Yes | 31.3 | 7.7 | 8 (14.3) | 11 (28.2) | 0.54 | 0.36 | 8 (21.6) | 11 (18.0) | 16.9 | 13.1 | |||||
| Postoperative complication | |||||||||||||||
| No | 29.3 | 6.5 | 0.43 | 29 (51.8) | 24 (61.5) | 0.35 | 0.56 | 0.31 | 0.74 | 22 (59.5) | 34 (55.7) | 0.72 | 14.0 | 8.8 | 0.50 |
| Yes | 28.2 | 6.7 | 27 (48.2) | 15 (38.5) | 0.54 | 0.31 | 15 (40.5) | 27 (44.3) | 12.7 | 10.7 | |||||
Adiposity variables, BMI, V/S, and PNF. Differences in adiposity by factors determined by one-way ANOVA or chi-square Fisher’s exact test.
Well to moderate category includes 2 patients that had complete response to neoadjuvant treatment and no tumor remaining at surgery.
BMI, body mass index; PNF, perinephric fat thickness; SFA, subcutaneous fat area; VFA, visceral fat area; V/S, visceral fat to subcutaneous fat ratio.
Adiposity and rectal cancer outcomes
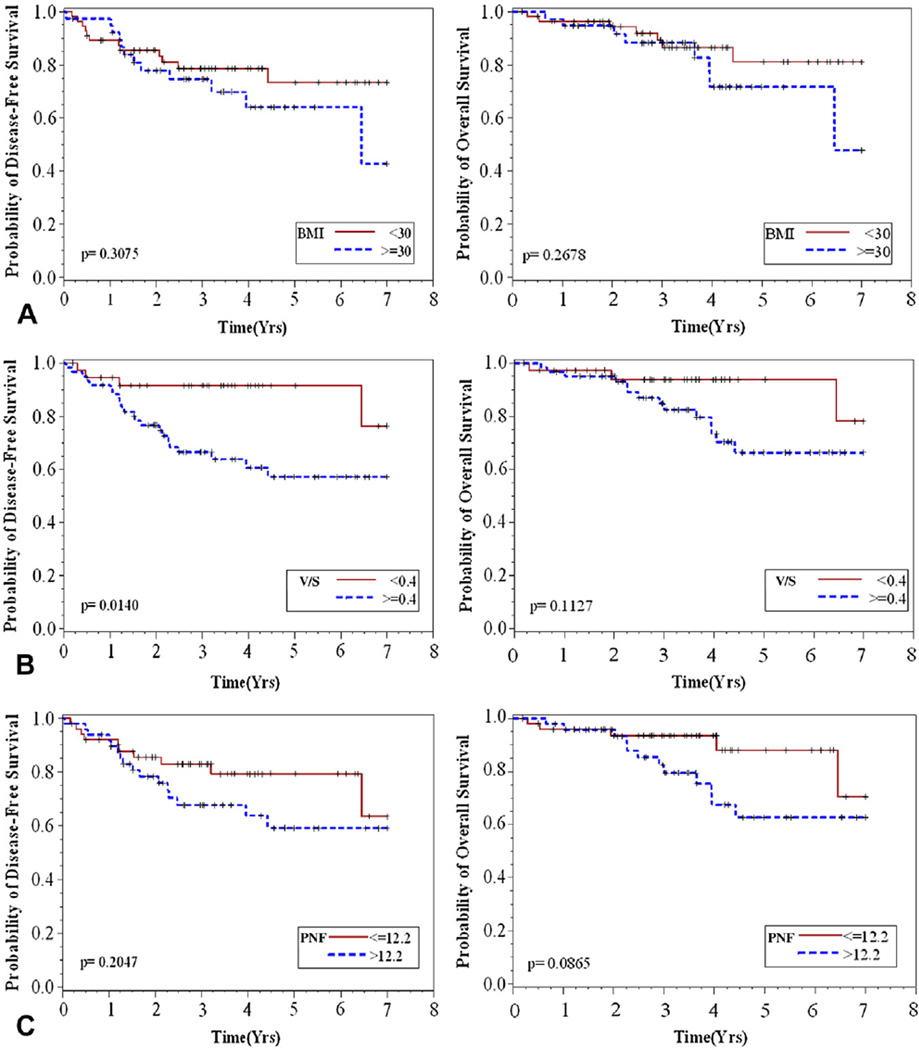
After resection, 5-year OS rate was 83.84% and DFS rate was 74.75%. Twenty-six patients had recurrent disease or died during follow-up. Figure 2 presents Kaplan-Meier estimates of DFS and OS time by obesity measures (BMI, V/S, and PNF). Disease-free survival and OS did not differ by obesity when defined by BMI (Fig. 2A). Viscerally obese patients (V/S ≥0.4) had shorter DFS (p = 0.02, Fig. 2B) and a higher number of documented recurrences (22 vs 4) than patients that were not viscerally obese. A trend toward worse OS was observed among those with V/S ≥0.4 (Fig. 2B; p = 0.14). Perinephric fat thickness higher than the median (12 mm) was associated with worse OS (p = 0.047) but not DFS (Fig. 2C; p = 0.18).
Figure 2.
Kaplan-Meier estimates of disease-free and overall survival by obesity measures, defined by (A) body mass index (BMI) (≥30), (B) visceral to subcutaneous fat area ratio (V/S) (≥0.4), or (C) perinephric fat thickness (PNF) (>12.2 mm).
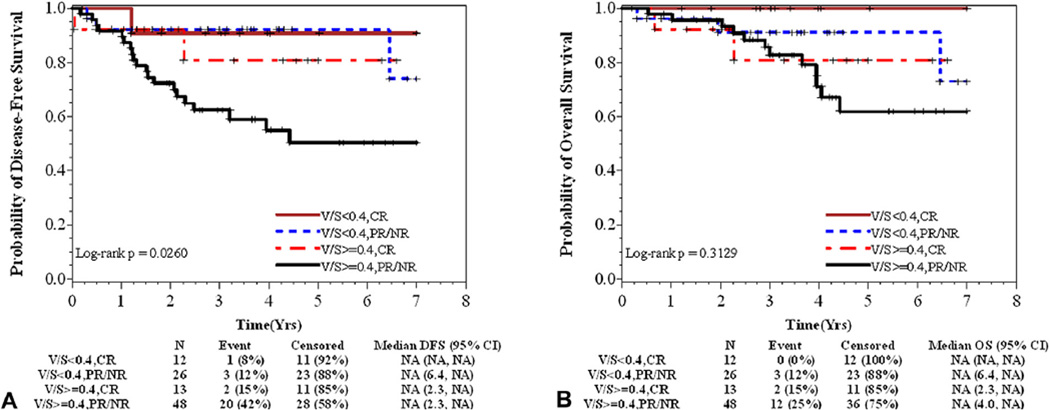
Table 4 presents the associations among BMI, V/S, and PNF and rectal cancer outcomes. In crude analysis, patients with elevated V/S had a significantly elevated risk of shorter DFS (HR = 3.50; 95% CI, 1.21 –10.17). When adjusted for grade and pathologic response to treatment (a highly correlated measure of clinical response with additional clinical accuracy), the association became marginally significant (AHR = 2.5; 95% CI, 0.9–7.5; p = 0.09). In the stratified analysis, patients with well to moderately differentiated tumors and high visceral obesity (V/S ≥0.4) were more likely to have shorter DFS (AHR = 4.99; 95% CI, 1.2–21.6), after adjusting for response to treatment. Figure 3 presents the survival probability when combining treatment response and visceral adiposity. Patients with partial/no pathological response to neoadjuvant therapy with high visceral adiposity had the poorest DFS compared with patients with compete pathological response or those with partial/no response and low V/S (p = 0.02). Results were unchanged in multivariate models when controlling for comorbid conditions (eg, diabetes, hypertension, and hypercholesterolemia) or age (data not shown). There were no differences in DFS or OS by BMI, overall or among patients with well to moderately differentiated tumors. Increasing PNF appeared to be associated with worse OS in unadjusted models (HR = 1.06; 95% CI, 1.01 – 1.11), but significance was not maintained when adjusting for grade and treatment response (AHR = 1.04; 95% CI, 0.99–1.09; Table 4) or stratified by grade (AHR = 1.05; 95% CI, 0.99–1.11).
Table 4.
Multivariate Cox Regression Modeling: Adiposity Variable and Rectal Cancer Outcomes
| Disease-free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR | Adjusted HR* | 95% CI | p Value | Crude HR | Adjusted HR | 95% CI | p Value | |
| Overall (n = 96) | ||||||||
| BMI | ||||||||
| <30 | 1.00 | 1.00 | Ref | 0.36 | 1.00 | 1.00 | Ref | 0.31 |
| ≥30 | 1.51 | 1.52 | 0.63–3.67 | 1.77 | 1.77 | 0.59–5.32 | ||
| V/S | ||||||||
| <0.4 | 1.00 | 1.00 | Ref | 0.09 | 1.00 | 1.00 | Ref | 0.28 |
| ≥0.4 | 3.50 | 2.53 | 0.85–7.49 | 2.64 | 2.03 | 0.57–7.20 | ||
| PNF, mm | 1.03 | 1.01 | 0.97–1.06 | 0.52 | 1.06 | 1.04 | 0.99–1.09 | 0.16 |
| Well-moderately differentiated tumors (n = 90) | ||||||||
| BMI | ||||||||
| <30 | 1.00 | 1.00 | Ref | 0.16 | 1.00 | 1.00 | Ref | 0.23 |
| ≥30 | 1.75 | 1.97 | 0.76–5.10 | 1.98 | 2.09 | 0.64–6.86 | ||
| V/S | ||||||||
| <0.4 | 1.00 | 1.00 | Ref | 0.03 | 1.00 | 1.00 | Ref | 0.17 |
| ≥0.4 | 5.45 | 4.99 | 1.15–21.63 | 3.14 | 2.89 | 0.64–13.10 | ||
| PNF, mm | 1.03 | 1.03 | 0.99–1.07 | 0.22 | 1.05 | 1.05 | 0.99–1.11 | 0.09 |
Adjusted for grade (poorly differentiated vs well differentiated/moderate) and pathologic response (none/partial vs complete).
BMI, body mass index; HR, hazard ratio; PNF, perinephric fat thickness; SFA, subcutaneous fat area; VFA, visceral fat area; V/S, visceral fat to subcutaneous fat ratio.
Figure 3.
Probability of (A) disease-free and (B) overall survival by response to neoadjuvant therapy and visceral to subcutaneous fat area ratio (V/S). (A) Patients with partial/no response (PR/NR) to neoadjuvant therapy with high V/S had the poorest disease-free survival compared with patients with compete response (CR) or those with partial/no response (PR/NR) that had low V/S (p = 0.03). (B) Similarly, PR/NR patients with high V/S appeared to have the poorest overall survival compared with others, but the differences among groups were not statistically significant (p = 0.32).
DISCUSSION
This is the first report to apply multiple quantitative visceral fat measurements (ie, SFA, VFA, and PNF) in addition to BMI to examine the associations between obesity and nonmetastatic rectal cancer patient outcomes after neoadjuvant chemoradiation treatment. We evaluated 2 quantitative measures of visceral adiposity, V/S, and the thickness of the PNF. Interestingly, in our set of rectal cancer patients, 62% of patients met criteria for obesity by V/S and only 41% of patients had BMI ≥30. The 2 different quantitative measures of visceral adiposity (V/S and PNF) were strongly associated with key preoperative metabolic comorbidities, and BMI was not. Our study suggests that elevated visceral adiposity is associated with an increased risk of recurrence, which was most evident among patients with well to moderately differentiated tumors and those with incomplete pathological response to neoadjuvant chemoradiation treatment. Given the disparity in reported data about obesity and CRC outcomes, this report helps to elucidate the relationship between rectal cancer outcomes and obesity.
The correlations between visceral adiposity and metabolic abnormalities among rectal cancer patients observed in this study are consistent with what has been shown among patients with cardiovascular disease and visceral fat.31 Balentine and colleagues have shown that quantitative measures of visceral obesity, such as VFA and SFA, were superior to BMI in correlating with comorbid conditions associated with obesity, such as diabetes and hypertension.28 Similarly, we observed that both high V/S and PNF had significant associations with the conditions that contribute to metabolic syndrome, including diabetes, hypertension, and hypercholesterolemia, and BMI did not. Males were more likely to be viscerally obese by V/S in our study. This is consistent with the observation that central obesity, although a less precise surrogate measure of visceral adiposity, is more common in males.32
A unique contribution of this study is that visceral obesity was studied in the select population of patients with rectal cancer that underwent neoadjuvant therapy. There have been few studies evaluating adiposity and rectal cancer outcomes.3,9,18,34 Meyerhardt and colleagues reported that among male patients with rectal cancer, increased BMI was associated with an increased risk of local recurrence (HR =1.61; 95% CI, 1.00–2.59); andnoassociations were found among women or for OS in either sex.9 However, conclusions remain inconsistent with other conflicting studies reporting no difference or superior outcomes among obese rectal cancer patients.3,18,34 Similarly, this study found no significant associations with obesity defined by BMI and rectal cancer survival.
Our findings of elevated risk of shorter DFS with visceral adiposity (eg, V/S) are consistent with findings reported within the few studies that have examined radiologic measures of adiposity. Using similar measures to those reported here, Moon and colleagues demonstrated that CRC patients with high V/S had a significantly worse DFS compared with patients with a low V/S (AHR = 1.98; 95% CI, 1.02–3.87), and BMI had no influence on outcomes.27 Kang and colleagues, who defined visceral adiposity by VFA alone (obesity VFA >130), reported a trend toward worse DFS among viscerally obese rectal cancer patients (3-year DFS of 82.5% vs 74.4%, respectively), but these differences were not statistically significant.34 In addition, among metastatic colon cancer patients, higher VFA independently predicted poorer outcomes in patients treated with targeted bevaci-zumab therapy, but not standard chemotherapy treatment.35 The associations between PNF and OS in univariate models are consistent with the poorer outcomes observed with elevated PNF in hepatobiliary,29 pancreatic,36 and prostate37 cancers. Notably, very few studies have examined whether obesity is an independent prognostic factor in multivariable models, as thoroughly conducted in this study. In addition, we found that a higher V/S among patients with well to moderately differentiated tumors were associated with worse DFS. We identified a high-risk group of patients with the combination of an incomplete pathological response to neoadjuvant therapy and high visceral adiposity who had far worse DFS compared with patients with complete pathological response or those with incomplete response that had low V/S. These findings suggest that after controlling for tumor grade and response to treatment (strong prognostic factors),38 visceral obesity has a detrimental effect on outcomes for patients with rectal cancer. A larger study using radiologic measures of visceral adiposity is needed to confirm our findings.
The fundamental biological basis for the association between adiposity and CRC outcomes remains largely unelucidated. Adipose tissue, which was historically considered merely an energy storage depot, is now increasingly recognized as a complex secretory organ that produces pro- and anti-inflammatory adipokines (eg, adiponectin, leptin, and resistin) and cytokines (eg, tumor necrosis factor–α, interleukin-6, interleukin-1, and monocyte chemoattractant protein-1)23,39 that can play a role locally, peripherally, or centrally in a variety of physiological processes.23 Visceral adipose tissue has been associated with a greater degree of obesity-related metabolic derangements than subcutaneous fat,21–23 which is the rationale behind evaluating V/S.27,31 These metabolic derangements include insulin resistance, aberrant insulin-like growth factor–axis signaling, 40 alterations in adipokine levels and low-level chronic inflammation,24 all of which have been associated with increase cell growth, tumor proliferation, and progression.23,41 Given the intimate association and proximity of visceral adipose tissue to rectal cancers, it is biologically plausible that visceral adiposity in endocrine and/or paracrine fashion can promote poor outcomes among patients with rectal cancer.
It could be postulated that survival differences in rectal cancer attributed to visceral obesity might be a result of the technical challenges associated with operations on obese patients as opposed to normal-weight individuals. In particular, pelvic dissection with optimal total mesorectal excision can be particularly difficult in viscerally obese patients. Although obesity (BMI) has been associated with worse short-term and perioperative outcomes; oncologic parameters among obese patients with rectal cancer are similar to those in nonobese patients.3,18,42,43 In fact, a recent series of almost 600 patients with rectal cancer reported no difference in 5-year DFS or OS in obese vs nonobese patients undergoing radical resection.18 In this current study, there were no differences in radial margin status or number of lymph nodes removed between obese and nonobese patients. We are confident that the differences observed in DFS and OS by visceral obesity (V/S or PNF) are not explained by the technical differences in surgical procedures. It could also be hypothesized that visceral adiposity can impact response to neoadjuvant chemoradiation, as evidenced by our observation of less T down-staging in viscerally obese patients. The current dosing of adjuvant and neoadjuvant therapy is often based on standard parameters such as body surface area and BMI and can potentially be better formulated with consideration of more precise measures of visceral adiposity.6 Finally, we observed that visceral obesity was more associated with pre-existing metabolic abnormalities than BMI, emphasizing the physiologic relevance of visceral obesity and supporting its link with cancer outcomes. It is possible that the accurate determination of visceral obesity can represent a surrogate for the multifactorial influence of surgical and medical treatment delivery and biology that underlie potentially worse outcomes in patients with rectal cancer.
There are limitations of this study. Body mass index and V/S were obtained at the time of diagnosis from medical records, which might not reflect changes in BMI that occurred before diagnosis. Weight loss is a major symptom of advanced disease; however, it is not widely prevalent among stage II and III patients and should not have impacted the results of this study. In addition, we had a relatively small sample size that limited our power to detect or rule out (eg, BMI) significant associations in some analyses and inhibited stratified analyses by factors such as sex. However, unlike previous studies that have reported on radiologically determined adiposity, this study includes a homogeneous population of nonmetastatic rectal cancer patients that were all treated with neoadjuvant chemoradiation followed by radical resection (low anterior or abdominoperineal resection). Although small, this study was strengthened by the focus on a homogeneous patient population that reduced the possibility of outcomes differences due to stage or treatment differences. A larger sample size in future studies will help to confirm our findings. Finally, the study population was primarily non-Hispanic white patients and does not provide information on minority populations. We comprehensively analyzed the association between adiposity measures and comorbid conditions as well as outcomes using systematic statistical analyses. Overall, we have demonstrated that there is a significant relationship between visceral adiposity and oncologic outcomes (eg, survival), including superior DFS with lower V/S. Even when subject to multivariate analysis, V/S but not BMI was a predictor of DFS among this group of rectal cancer patients.
CONCLUSIONS
Given the epidemic of obesity in the United States and performance-based health care reform, more studies are needed to precisely delineate the relationship between obesity and cancer. It is becoming increasingly apparent that BMI alone is inadequate as a consistent prognostic indicator. As the focus on outcomes and comparative effectiveness research increases, the development of accurate and reliable risk and prognosis stratification strategies are clearly necessary to better guide patient treatment decisions, such as the use of behavioral interventions and more aggressive or accurately dosed adjuvant therapy regimens. Radiologic visceral fat quantification with its use of existing diagnostic imaging might ultimately represent an easily performed and more cost-effective, precise, and reproducible instrument of clinical prognostication. Although it remains unclear which of these quantitative measures, such as V/S or PNF, is the most optimal measure, our study represents an important proof of concept that visceral adiposity is an important determinant of outcomes in patients with rectal cancer undergoing neoadjuvant chemoradiation and radical resection. Additional studies that elucidate the physiologic and biologic mechanisms (eg, endocrine/adipokine activity of adipose tissue, hyperinsulinemia, insulin-like growth factor signaling) mediating the adiposity–CRC link are also clearly needed. The incorporation of quantitative measures of visceral obesity and corresponding correlative molecular studies into prospective oncologic therapeutic and epidemiologic studies is warranted.
Acknowledgment
The authors wish to acknowledge the assistance in manuscript preparation provided by Suellen Sachariat and Melissa Quintana.
This study was funded in part by the Moffitt Cancer Center TJF Colorectal Cancer Research Fund.
Abbreviations and Acronyms
- AHR
adjusted hazard ratio
- BMI
body mass index
- CRC
colorectal cancer
- DFS
disease-free survival
- OS
overall survival
- PNF
perinephric fat thickness
- SFA
subcutaneous fat area
- VFA
visceral fat area
- V/S
visceral fat to subcutaneous fat ratio
Footnotes
Disclosure Information: Nothing to disclose.
Abstract presented at the 97th Annual Clinical Congress of the American College of Surgeons, Surgical Forum, San Francisco CA, October 2011.
Author Contributions
Study conception and design: Clark, Siegel, Parsons, Hernandez, Choi, Shibata
Acquisition of data: Clark, Parsons, Weber, Thareja, Choi
Analysis and interpretation of data: Clark, Siegel, Chen, Zhao, Parsons, Hernandez, Weber, Choi, Shibata
Drafting of manuscript: Clark, Siegel, Chen, Zhao, Parsons, Hernandez, Weber, Thareja, Choi, Shibata
Critical revision: Clark, Siegel, Chen, Zhao, Parsons, Hernandez, Weber, Thareja, Choi, Shibata
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Hjartaker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 3.Ballian N, Yamane B, Leverson G, et al. Body mass index does not affect postoperative morbidity and oncologic outcomes of total mesorectal excision for rectal adenocarcinoma. Ann Surg Oncol. 2010;17:1606–1613. doi: 10.1245/s10434-010-0908-4. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Tamakoshi K, Wakai K, Kojima M, et al. A prospective study of body size and colon cancer mortality in Japan: the JACC Study. Int J Obes Relat Metab Disord. 2004;28:551–558. doi: 10.1038/sj.ijo.0802603. [DOI] [PubMed] [Google Scholar]
- 6.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 8.Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 10.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropo-metric characteristics: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison DS, Batty GD, Kivimaki M, et al. Risk factors for colonic and rectal cancer mortality: evidence from 40 years’ follow-up in the Whitehall I study. J Epidemiol Community Health. 2011;65:1053–1058. doi: 10.1136/jech.2010.127555. [DOI] [PubMed] [Google Scholar]
- 15.Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colangelo LA, Gapstur SM, Gann PH, et al. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 17.Trevisan M, Liu J, Muti P, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001;10:937–941. [PubMed] [Google Scholar]
- 18.Chern H, Chou J, Donkor C, et al. Effects of obesity in rectal cancer surgery. J Am Coll Surg. 2010;211:55–60. doi: 10.1016/j.jamcollsurg.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Hryniuk WM, Figueredo A, Goodyear M. Applications of dose intensity to problems in chemotherapy of breast and colo-rectal cancer. Semin Oncol. 1987;14:3–11. [PubMed] [Google Scholar]
- 20.Appelt AL, Ploen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemo-radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74–80. doi: 10.1016/j.ijrobp.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karelis AD, St-Pierre DH, Conus F, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 23.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 24.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 25.Gradmark AM, Rydh A, Renstrom F, et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray ab-sorptiometry and anthropometry. Br J Nutr. 2010;104:582–588. doi: 10.1017/S0007114510000796. [DOI] [PubMed] [Google Scholar]
- 26.Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 2008;15:1918–1922. doi: 10.1245/s10434-008-9891-4. [DOI] [PubMed] [Google Scholar]
- 28.Balentine CJ, Marshall C, Robinson C, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res. 2010;164:18–22. doi: 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 29.Morris K, Tuorto S, Gonen M, et al. Simple measurement of intra-abdominal fat for abdominal surgery outcome prediction. Arch Surg. 2010;145:1069–1073. doi: 10.1001/archsurg.2010.222. [DOI] [PubMed] [Google Scholar]
- 30.Cecchini S, Cavazzini E, Marchesi F, et al. Computed tomography volumetric fat parameters versus body mass index for predicting short-term outcomes of colon surgery. World J Surg. 2011;35:415–423. doi: 10.1007/s00268-010-0888-3. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3:645S–647S. doi: 10.1002/j.1550-8528.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 32.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 33.Chen YA, Almeida JS, Richards AJ, et al. A nonparametric approach to detect nonlinear correlation in gene expression. J Comput Graph Stat. 2010;19:552–568. doi: 10.1198/jcgs.2010.08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang J, Baek SE, Kim T, et al. Impact of fat obesity on lapa-roscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis. 2012;27:497–505. doi: 10.1007/s00384-011-1333-2. [DOI] [PubMed] [Google Scholar]
- 35.Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 36.House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 37.von Hafe P, Pina F, Perez A, et al. Visceral fat accumulation as a risk factor for prostate cancer. Obes Res. 2004;12:1930–1935. doi: 10.1038/oby.2004.242. [DOI] [PubMed] [Google Scholar]
- 38.McDermott FT, Hughes ES, Pihl EA, et al. Influence of tumour differentiation on survival after resection for rectal cancer in a series of 1296 patients. Aust N Z J Surg. 1984;54:53–58. doi: 10.1111/j.1445-2197.1984.tb06685.x. [DOI] [PubMed] [Google Scholar]
- 39.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 40.Sridhar SS, Goodwin PJ. Insulin-insulin-like growth factor axis and colon cancer. J Clin Oncol. 2009;27:165–167. doi: 10.1200/JCO.2008.19.8937. [DOI] [PubMed] [Google Scholar]
- 41.Siegel EM, Ulrich CM, Poole EM, et al. The effects of obesity and obesity-related conditions on colorectal cancer prognosis. Cancer Control. 2010;17:52–57. doi: 10.1177/107327481001700107. [DOI] [PubMed] [Google Scholar]
- 42.Bege T, Lelong B, Francon D, et al. Impact of obesity on short-term results of laparoscopic rectal cancer resection. Surg Endosc. 2009;23:1460–1464. doi: 10.1007/s00464-008-0266-5. [DOI] [PubMed] [Google Scholar]
- 43.Healy LA, Ryan AM, Sutton E, et al. Impact of obesity on surgical and oncological outcomes in the management of colo-rectal cancer. Int J Colorectal. 2010;25:1293–1299. doi: 10.1007/s00384-010-0963-0. [DOI] [PubMed] [Google Scholar]