Abstract
Living cells sense absolute temperature and temporal changes in temperature using biological thermosensors such as ion channels. Here, we reveal, to our knowledge, a novel mechanism of sensing spatial temperature gradients within single cells. Spherical mitotic cells form directional membrane extensions (polar blebs) under sharp temperature gradients (≥∼0.065°C μm−1; 1.3°C temperature difference within a cell), which are created by local heating with a focused 1455-nm laser beam under an optical microscope. On the other hand, multiple nondirectional blebs are formed under gradual temperature gradients or uniform heating. During heating, the distribution of actomyosin complexes becomes inhomogeneous due to a break in the symmetry of its contractile force, highlighting the role of the actomyosin complex as a sensor of local temperature gradients.
Introduction
Temperature-sensitive Ca2+ signaling is used by single cells to respond to temporal temperature changes. Transient receptor potential channels are representative biological thermosensors in cells (1), but other Ca2+ channels such as ryanodine receptor (2), IP3 receptor (3,4), and Orai1-STIM1 complex (5) also sense temporal temperature changes. This thermosensing function of ion channels is used to remotely control neural activity with radiofrequency magnetic field local heating (6,7). Local heating itself can also induce local gene expression (8), membrane depolarization (9,10), and muscle contraction (11–13). These local heating techniques have a strong potential for the spatial control of cell functions. However, except for thermotaxis (14–17), biological responses to local temperature gradients remain unclear. In vitro studies have revealed that temperature gradients affect cytoskeletal polymerization (18,19), molecular motor activities (20–22), and the distribution of biomolecules including DNA and RNA (23,24), but few studies have observed these biomolecular dynamics in living cells under temperature gradients (25,26). To elucidate the system for sensing temperature gradients, we applied a local temperature gradient to single living cells and observed the directional morphological response coupled with asymmetric protein dynamics.
Materials and Methods
Cell culture
HeLa cells were cultured in flasks (AGC Techno Glass, Shizuoka, Japan) in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin at 37°C with 5% CO2. DMEM, FBS, penicillin, and streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA). 1–2 days before the experiments, cells were seeded on a glass dish (AGC Techno Glass).
Experimental solutions
Cells on the glass dish were rinsed twice with 1 mL Leibovitz’s L-15 medium (Thermo Fisher Scientific) containing 10% FBS and incubated in 2 mL L-15 medium containing 10% FBS under the microscope for 15 min at room temperature (25 ± 1°C), at 35 ± 0.5°C, or at a physiological temperature (37 ± 0.5°C) adjusted by a thermostatically controlled incubator on the sample stage (INUG2-ONICS; Tokai Hit, Shizuoka, Japan).
Drug treatments
Cytoskeleton (actin filament and microtubule) and motor protein (myosin II) inhibitors were mixed into L-15 medium containing 10% FBS 15 min before observation. Actin polymerization was inhibited by 500 nM (final concentration) latrunculin B (Calbiochem, Merck KGaA, Darmstadt, Germany) and 10 μM cytochalasin D (Wako Pure Chemical Industries, Osaka, Japan). Actin polymerization was promoted by 50 nM jasplakinolide (Calbiochem). Microtubules were depolymerized with 10 μM nocodazole (Merck KGaA) or stabilized with 20 μM paclitaxel (taxol) (Sigma-Aldrich, St. Louis, MO). Myosin II ATPase was inhibited with 100 μM blebbistatin (Toronto Research Chemicals, Ontario, Canada). Rho-kinase (ROCK) and the myosin light chain kinase inhibitors were 10 μM Y-27632 (Wako Pure Chemical Industries) and 20 or 50 μM ML-7 (Sigma-Aldrich), respectively. The stock solutions of latrunculin B, cytochalasin D, jasplakinolide, nocodazole, taxol, blebbistatin, Y-27632, and ML-7 were 500 μM, 10 mM, 50 μM, 10 mM, 20 mM, 100 mM, 10 mM, and 20 mM, respectively. Y-27632 was dissolved in sterile water, and the others were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). The stock solutions were stored at −20°C. Spherical interphase cells were prepared by rinsing twice with 1 mL phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4) containing 0.2 g L−1 EDTA and 0.025% trypsin (Thermo Fisher Scientific) and incubated for 1 min at 25°C, and the solution was replaced by 2 mL L-15 medium containing 10% FBS for observation.
Optical setup
The microscope with local heating systems was previously described (11). Confocal fluorescence images were captured with a confocal unit (CSU-X1, Yokogawa Electric, Tokyo, Japan), an electron multiplying charge-coupled device camera (iXon-Ultra, Andor Technologies plc, Belfast, Northern Ireland), and an objective lens (PlanApo N 60×/1.45 Oil, Olympus, Tokyo, Japan) attached to the inverted microscope (IX-70, Olympus). The excitation light sources were 488 nm (Sapphire, Coherent, Santa Clara, CA) and 561 nm (Sapphire, Coherent). The confocal unit was set up with a YOKO-T405/488/561 dichroic mirror (Semrock, Rochester, NY) and YOKO-FF01-520/32 and YOKO-FF01-617/73 emission filters (Semrock). The excitation and emission light passed through an FF409-Di03 dichroic mirror (Semrock) in the microscope. Europium (III) thenoyltrifluoroacetonate trihydrate (Eu-TTA), fluorescent beads, blebbistatin, calcein, and ethidium homodimer-1 (EthD-1) were excited by a mercury lamp (Olympus) with excitation filters for Eu-TTA (BP360–370, Olympus) or others (BP470–490, Olympus). Fluo-4 was excited by a SPECTRA Light Engine (485/20 nm, Lumencor, Beaverton, OR). For epifluorescence microscopy of Eu-TTA, an FF409-Di03 dichroic mirror (Semrock) was used. For epifluorescence microscopy of fluorescent beads, blebbistatin, fluo-4, calcein, and EthD-1, a DM505 dichroic mirror (Olympus) and a BA515IF excitation filter (Olympus) were included in the microscope, and fluorescence was observed by epifluorescence microscopy with an electron multiplying charge-coupled device camera (iXon EM+ 897, Andor Technologies plc) and the same objective lens used for confocal microscopy. Images were captured with iQ (Andor Technologies plc) or in-house software with LabVIEW (National Instruments, Austin, TX).
Temperature measurement generated by infrared laser
The local temperature around the cell was increased by an infrared (IR) laser (λ = 1455 nm, KPS-STD-BT-RFL-1455-02-CO, Keopsys, Lannion, France). The duration of irradiation was controlled by a mechanical shutter (SSH-C4B, Sigma Koki, Tokyo, Japan). Laser power through the objective lens at the specimen position was measured with a thermal disk sensor and a power meter (LM-3 and FieldMaster, Coherent). Local temperature changes were calculated from thermal quenching of tetramethylrhodamine (TMR-dextran) or Alexa Fluor 555 (Alexa Fluor 555-dextran) conjugated to 10 kDa dextran (Thermo Fisher Scientific). 25 μg mL−1 TMR-dextran or 10 μg mL−1 Alexa Fluor 555-dextran in L-15 medium containing 10% FBS was excited with the 561 nm laser and observed by confocal microscopy. Local temperature changes were also measured with Eu-TTA (Acros Organics, Pittsburgh, PA) in a glass capillary (27). A thin-walled glass capillary (G-100, Narishige, Tokyo, Japan) was pulled by a PC-10 puller (Narishige), and the tip was closed by a microforge (MF-900, Narishige). 500 μM Eu-TTA in DMSO was loaded into the glass capillary by a microloader (Eppendorf AG, Hamburg, Germany). The glass capillary was manipulated by a three-axis motorized micromanipulator (EMM-3NV, Narishige).
The temperature dependence of the fluorescence intensity was measured by a fluorescence spectrophotometer (F-4500, Hitachi High-Technologies, Tokyo, Japan) at various temperatures. The temperature in the cuvette was controlled by a precision thermostatic circulator (AB-1600, ATTO, Tokyo, Japan) and measured by a digital thermometer (ASF-250T, AS ONE, Osaka, Japan). The excitation and emission wavelengths were respectively 555 nm and 578–580 nm for TMR-dextran, 540 nm and 565–568 nm for Alexa Fluor 555-dextran, and 365 nm and 612–618 nm for Eu-TTA. The relative fluorescence intensity of TMR-dextran normalized at 25.1°C was fitted with the quadratic function y = 1.31 × 10−4 x2 – 2.47 × 10−2 x + 1.53, where x and y are the temperature [°C] and normalized fluorescence intensity, respectively (Fig. S1 A in the Supporting Material). The fluorescence intensity of Alexa Fluor 555-dextran normalized at 24.9°C was fitted with y = 2.36 × 10−4 x2 – 3.66 × 10−2 x + 1.76 (Fig. S1 A), and that of Eu-TTA normalized at 25.1°C was fitted with y = 3.33 × 10−4 x2 – 5.17 × 10−2 x + 2.09 (Fig. S1 A). The spatial temperature gradients were fitted with the logarithmic function y = −a ln (x/x0) + b, where x, x0, and y were the distance from the heat source [μm], 1 [μm] as a reference, and temperature [°C], respectively (4). The positive numbers a and b were determined with Microsoft Excel. This fitting is based on a model that steady-state temperature distribution T(r) [K] around a cylindrical heat source is described in T(x1) = T(x2) – Q/(2πlk) × ln(x1/x2), where x1 [m] and x2 [m] are the distances from a center of heat source, Q [W] is a derived power, l [m] is a height of a cylindrical heat source, and k [W m−1 K−1] is thermal conductivity of medium. T(x) around a spherical heat source, T(x1) = T(x2) + Q/(4πk) × (1/x1 – 1/x2), was not well fitted with the spatial temperature gradients measured in this study.
Visualization of actin, myosin, and the cell membrane
Actin-red fluorescent protein (RFP) and tubulin-green fluorescent protein (GFP) were expressed by a baculovirus expression system with CellLights (Thermo Fisher Scientific) 1 day before observation. F-actin was visualized by Lifeact-RFP (28) or Lifeact-GFP. Myosin II was visualized by mCherry-human myosin, with light chain 2 fluorescently modified (mCherry-MRLC). The plasmids containing Lifeact-RFP, Lifeact-GFP, or mCherry-MRLC were transfected with Lipofectamine LTX (Thermo Fisher Scientific) 1 day before the observation. The plasma membrane (PM) was stained with 2.5 μg mL−1 CellMask Orange (Thermo Fisher Scientific) in 2 mL L-15 medium containing 10% FBS for 20 min at 37°C.
Live/dead assay
To confirm whether bleb-forming cells were alive, we used the LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher Scientific). Cells were heated by an IR laser in HEPES-buffered saline (HBS) (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM Na2HPO4, 10 mM HEPES, 2 mM CaCl2, 5 mM D(+)-glucose, pH 7.4). To prepare dead cells, cells were incubated in L-15 medium containing 70% methanol for 30 min at room temperature. Both live cells (with or without heating) and dead cells were incubated in HBS containing 2 μM calcein-AM or 4 μM EthD-1 for 15 min at room temperature. The cultures were rinsed twice with 1 mL HBS and incubated in 2 mL HBS, and the fluorescence intensity was measured 1 h after heating.
Visualization of convection flow
The flow of extracellular solution was visualized with 0.2-μm diameter FluoSpheres Carboxylate-Modified Microspheres (Thermo Fisher Scientific) (3). Convection-like water flow was generated by negative pressure produced inside an open-tipped glass capillary placed near a cell. The negative pressure was manually controlled with a syringe pump.
Ca2+ imaging
Fluo-4-AM (Thermo Fisher Scientific) was used to observe the intracellular Ca2+ dynamics. The cells were incubated in HBS containing 1 μM fluo-4-AM for 30 min at room temperature. The solution was exchanged with HBS for observation. To chelate intracellular Ca2+, cells were incubated in HBS containing 30 μM EGTA-AM (Thermo Fisher Scientific) or 50 μM BAPTA-AM (Dojindo Laboratories, Kumamoto, Japan) and 1 μM fluo-4-AM for 30 min at room temperature, and then observed in Ca2+-free HBS [140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM Na2HPO4, 10 mM HEPES, 5 mM D(+)-glucose, 500 μM EGTA, pH 7.4]. Fluo-4-AM (1 mM), EGTA-AM (30 mM), and BAPTA-AM (50 mM) stocks in DMSO were stored at −20°C. The cells were incubated under the microscope for 15 min before observation to stabilize the temperature. The experiments were completed within 45 min.
Data analysis
A directional membrane extension was termed a polar bleb. When organelles inside cells became motionless (see Fig. 1 F; Movie S7), the state was termed Motionless. ImageJ (National Institutes of Health, Bethesda, MD) was used for measuring the size of polar blebs. We defined the length and angle of cellular membrane expansion as described below (see also Fig. S2 A). We modeled each cell and bleb as a sphere, and defined the circular cell surface before heating (C0) and the bleb at its maximal extension (Cbleb) (see dashed circles in Fig. S2 A). A line passing through the centers of C0 and Cbleb was defined as LC, and the intersections between LC and the circular surfaces of the bleb and the cell were respectively denoted A and B. The extension length of the membrane, i.e., the size of a polar bleb, was defined as the length of AB. Another line segment connecting the laser heat source with the center of C0 was defined as LL. The expansion angle was defined as the angle between LC and LL. A distance from the heat source was defined as the difference between the length of LL and the radius of C0.
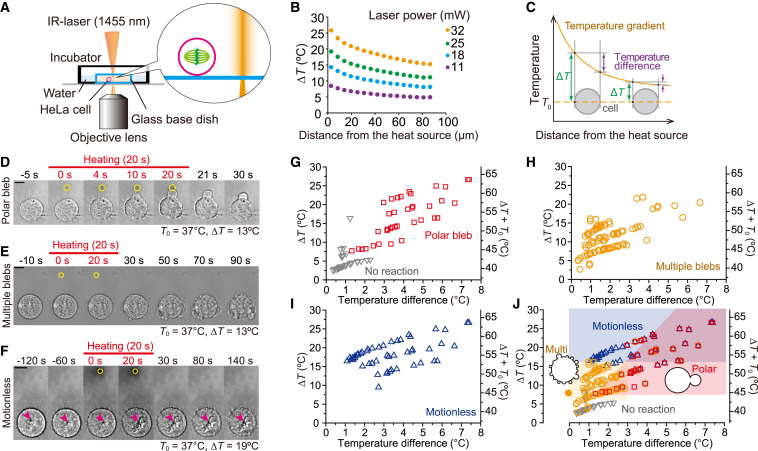
Figure 1.
Directional blebs formed under temperature gradient in mitotic HeLa cells. (A) Schematic illustration of a local heating system. (B) Various temperature gradients generated by different laser powers. The heat source indicates the position the IR laser was focused. The gradients were measured with TMR-dextran. T0 = 25°C. (C) Schematic illustration of the definition of temperature increase (ΔT) and temperature difference between both sides of a cell. ΔT is defined as the maximum temperature increase in a cell, i.e., the temperature increase at the side of cells near the heat source. (D) Sequence of bright-field images showing polar bleb formation induced by local heating (ΔT = 13°C) at T0 = 37°C (from Movie S1). (E) Sequence of bright-field images showing multiple blebs induced by local heating (ΔT = 13°C) at T0 = 37°C (from Movie S5). (F) Sequence of bright-field images showing Motionless induced by strong heating (ΔT = 19°C) at T0 = 37°C (from Movie S7). Arrowheads indicate the positions of a representative granule. The movement of the granule stopped after heating. Scale bars in (D)–(F), 10 μm. Yellow circles indicate the positions of the heat source. Heating continued for 20 s starting at t = 0 s (red bars). (G–J) The relationship between cell reaction and temperature difference between the sides of a cell. T0 = 37°C. A closed yellow circle in (J) indicates the formation of multiple blebs induced by uniform heating (Fig. S5 D).
The actin, myosin, and PM distributions were measured as the fluorescence intensity of a region of interest (ROI) with ImageJ (see Figs. 3 and S12). The change in fluorescence, ΔF, (see Figs. 3 B and S12) was determined as the difference between the fluorescence intensity 1.7 s after termination of heating and the intensity 0.5 s before heating, F0. The time course of changes in actin and PM localization were shown as a series of kymographs (see Figs. 4 B and S13 B). The normalized fluorescence intensities of Lifeact and CellMask (see Figs. 4 C and S13 C) were measured at the black bars shown in the kymographs (see Figs. 4 B and S13 B). The intensity was normalized to the average intensity over 4 s before heating. To observe the dynamics of the cortex, fluorescence images of the cortex were transformed to polar coordinates (see Figs. 4 D and S11). The profiles start at one intersection of the dashed line through the laser position and the center of the cell (Fig. S11 A, gray dashed line) with the cell membrane and then follow the cell contour (Fig. S11 A, gray circle) in the cortex region in a clockwise direction. The cell contour was subdivided into 360 points. Image sequence (Fig. S11 B) was then obtained from a ROI surrounding the cortex (Fig. S11 A, gray rectangle).
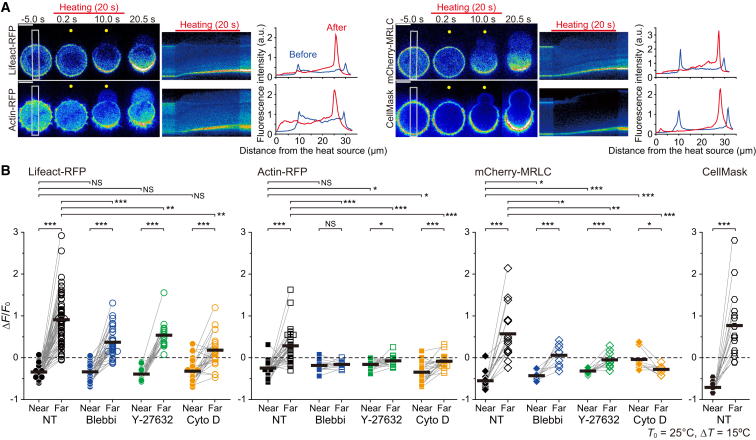
Figure 3.
Temperature gradient causes symmetry breaking of the actomyosin cortex. (A) Left: image sequences of Lifeact-RFP, actin-RFP, mCherry-MRLC, and CellMask in different cells (created from the original images in Movies S10, S11, S12, and S16). Yellow circles indicate the positions of the heat sources. Middle: kymographs from the white rectangles in left images. Red bars, the periods of heating. Right: line profiles of fluorescence intensities averaged along white rectangles in the left images before (blue) and after heating (red). Scale bars, 10 μm. (B) Relative changes in the maximum fluorescence intensity after heating at the near and the far sides of cells from the heat source. Thick horizontal bars indicate the mean. Statistical significance between the near and far sides for each treatment is represented by grouped bars at the top and reported using a paired t-test, and the fluorescence intensity at each side is compared to non-treated (NT) cells using a t-test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; NS, not significant). ΔT = 15°C, T0 = 25°C. Blebbi, 100 μM blebbistatin. Y-27632, 10 μM Y-27632. Cyto D, 10 μM cytochalasin D.
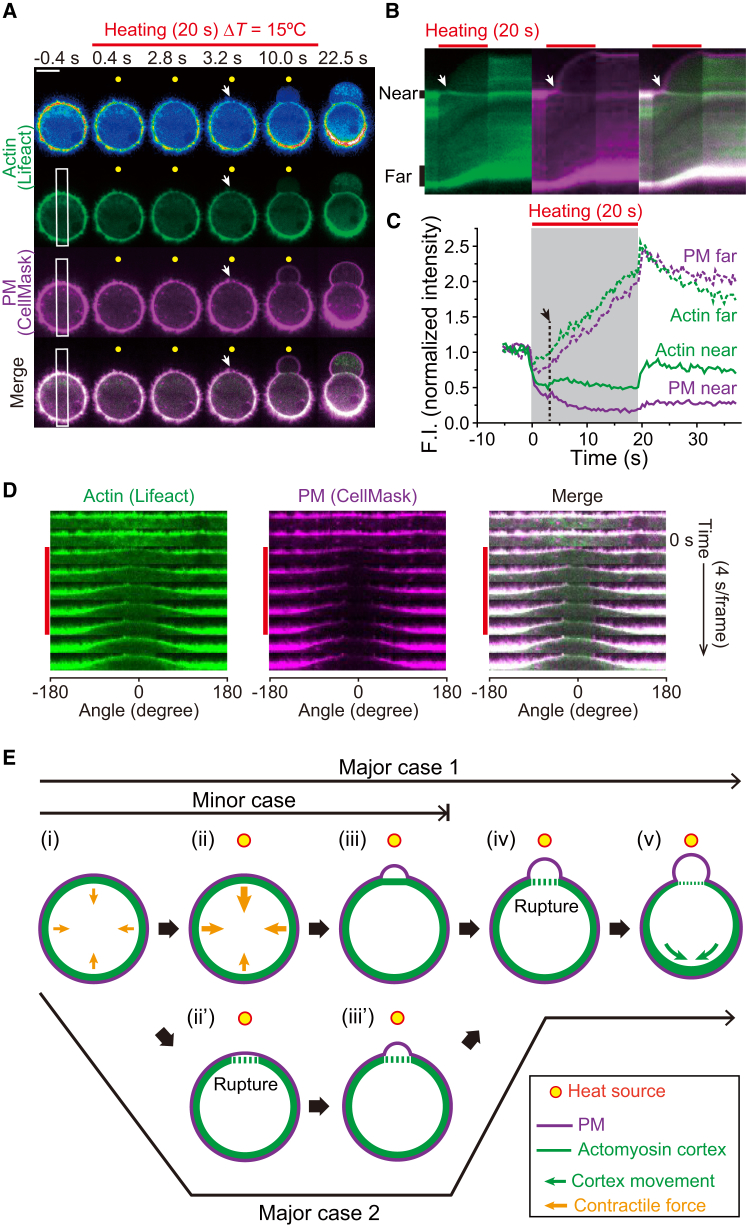
Figure 4.
Asymmetric distribution of the actin cortex and PM in the initiation of a directional polar bleb under temperature gradient. (A) Image sequences of fluorescently labeled F-actin (Lifeact-GFP) and PM (CellMask) in the same cell (created from the original images of Movie S17). ΔT = 15°C at T0 = 25°C. Top images are in pseudocolor to highlight the dynamics of Lifeact-GFP. Scale bar, 10 μm. (B) Kymographs of Lifeact-GFP (green) and CellMask (magenta), and their merged images from the white rectangles in (A). (C) Time courses of fluorescence intensities measured at vertical black bars in (B). Arrows and red bars in (A)–(C) indicate the timing of initiation of bleb formation and the period of heating, respectively. The gray area in (C) also indicates the period of heating. The rapid fluorescence decrease at the onset of heating and increase at the end of heating are due to the effect of thermal quenching of fluorophores. (D) In-phase movement of the actin cortex and PM to the cooler side. These sequences were reconstructed from the polar transformed images of (A). Vertical red bars indicate the period of heating. Angle is defined as relative to the heat source (see Fig. S2: the position at 0° is the nearest to the heat source), and negative value is defined as the counterclockwise direction. See also Fig. S11. (E) Schematic model on the initiation of directional bleb formation by local heating. A possible Major case 1: (i and ii) local heating increases the contractile forces (orange arrows) more at the warmer side. (iii) The increased force at the warmer side detaches the cortex from the PM and the bleb formation begins. (iv) Although the cortex is ruptured at the warmer side, the bleb continues to extend. (v) The cortex rupture results in the movement of the cortex and PM toward the cooler side (green arrows). A possible Major case 2: cortex ruptures at the warmer side (ii’), initiating the bleb formation (iii’). A possible Minor case: in a small number of cases, the process stops at stage (iii), where cortex rupture is not observed (Fig. S13; Movie S18). Yellow circles indicate the positions of the heat source.
The fluorescence intensity of fluo-4 was normalized to the average of intensities for 1 s before heating (F0). The ROI was set inside the cell to accommodate cell movement due to bleb formation. Fmax/F0 for a polar bleb was defined as the maximum fluorescence intensity during the first 35 s of heating. Fmax/F0 for multiple blebs and Motionless were defined as the maximum fluorescence intensity during the first 50 s of heating.
Statistical analysis
Extension lengths were compared using the Wilcoxon/Mann-Whitney test. The probabilities of forming multiple blebs were compared using Fisher’s exact test. The changes in fluorescence intensity of Lifeact-RFP, actin-RFP, and mCherry-MRLC at each side of the cell in the presence of chemical agents were compared to those in the absence of the agent by the following order. First, the variances were compared with the F-test. If the variance was equal (p ≥ 0.05), Student’s t-test was used. If the difference of variance was statistically significant (p < 0.05), Welch’s t-test was used. To compare the changes in fluorescence intensity between sides, a paired t-test was used. Wilcoxon/Mann-Whitney test and Fisher’s exact test were performed in OriginPro 9.1 (OriginLab, Northampton, MA). All t- and F-tests were performed in Microsoft Excel. SE are shown for data with statistical analyses; otherwise, SD are stated.
Results
Polar bleb formation induced by temperature gradient
We found that a spherical mitotic HeLa cell formed a directional membrane extension (bleb), termed a polar bleb, under a local temperature gradient created by a focused IR laser beam (λ = 1455 nm) (Figs. 1, A–D and S1; Movie S1). Bleb formation initiated a few seconds after heating began and continued to expand toward the heat source with strong directionality (Fig. S2). When heating was terminated, expansion stopped, and the bleb slowly shrunk. Upon local heating, 88% (78/89) of spherical metaphase cells formed polar blebs, whereas only 29% (4/14) of interphase cells flattened on the glass surface formed significantly smaller blebs (Fig. S3; Movie S2). However, when the interphase cells were treated with trypsin to induce spherical morphology, 98% (42/43) formed polar blebs during heating (Fig. S3; Movie S3). These results indicate that spherical cells form a polar bleb independent of the cell cycle phase. We further confirmed that formation of the polar bleb was not due to convective flow of water (Fig. S4; Movie S4).
Temperature field to induce the polar bleb formation
Next, we examined various temperature increases (ΔT), at different ambient temperatures before heating (T0) and temperature differences between the proximal and distal ends of a cell, i.e., the temperature gradient within a cell, toward the heat source (Fig. 1 C) by varying the laser power and the distance from the heat source to the cell (Fig. 1 B). When cells were heated from 37°C to 42–50°C under a small temperature gradient within a cell (<1.3°C), 84% (59/70) of cells formed multiple sequential, nondirectional blebs after recooling (Figs. 1 E and S5; Movies S5 and S6). During and after heating above ∼50°C, organelles inside cells became motionless (Fig. 1 F; Movie S7). Hereafter, we call this state Motionless. Sharper temperature gradients induced polar bleb formation at the warmer side of cells with higher probability (Fig. 1, G–J). The minimum temperature difference between the ends of a cell required to form a polar bleb was as small as 1.3°C (Fig. 1 G), and the absolute temperature (ΔT + T0) required to induce these responses decreased as T0 decreased (Fig. S6). These results clearly showed that cell responses depend on ΔT and the spatial temperature gradient. A fluorescent cell viability assay showed that local heating for 20 s does not completely kill the cells within 1 h (100% alive after forming a polar bleb or multiple blebs, 83–86% alive after organelles became motionless), i.e., esterase activity and the PM are not impaired (Fig. S7). However, even a short pulse of local heating for 5 s increased the probability of normal cytokinesis inhibition for ΔT > 10°C at T0 = 37°C independent of bleb formation (Fig. S8).
Actomyosin interaction is essential for bleb formation
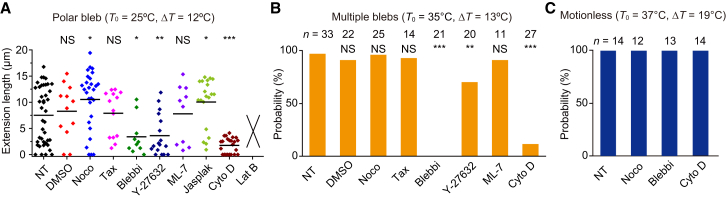
Without contacting surrounding cells, the shape of a spherical cell is precisely balanced at the cell periphery by the inward contractile force of cortical actomyosin and the outward hydrostatic pressure on the PM (29,30). A local imbalance immediately causes blebbing (31,32). Given these properties, what is the thermosensor for the polar bleb formation? Polar blebs were hardly formed in the presence of an actin polymerization inhibitor, i.e., latrunculin B or cytochalasin D (Fig. 2 A; Movie S8). In the presence of the actin polymerization-promoting drug, jasplakinolide, the length of extrusion was significantly increased. The inhibitor of myosin II ATPase, blebbistatin, also prevented the formation of polar blebs during heating (Figs. 2 A and S9), and Y-27632 (Rho-kinase inhibitor that indirectly inhibits myosin II) suppressed the formation of polar blebs (Fig. 2 A). However, the inhibitor of MLCK, ML-7, has no significant effect (p > 0.05) (Fig. 2 A). From these results except for ML-7, we conclude that actomyosin interaction is essential for the formation of polar blebs, which is similar to spontaneous blebbing during cytokinesis, apoptosis, and migration (31,32). We then focused on microtubule dynamics and found that nocodazole (microtubule-depolymerizing drug) significantly increased the length of extrusion, whereas taxol (microtubule stabilizer) did not increase the extension length (Fig. 2 A). These results suggest that microtubule-supported structures such as the mitotic spindle interfere with bleb extension. Multiple blebs were also suppressed by the inhibitors of actin polymerization and myosin II ATPase (Fig. 2 B). Motionless was not affected by these inhibitors (Fig. 2 C), suggesting that Motionless is probably attributable to thermal denaturation of proteins.
Figure 2.
Effect of inhibitors on bleb formation. (A) Extension length in the presence of chemical agents (from Movie S8). From left: Nontreated (NT) cells, 0.25% DMSO, 10 μM nocodazole (Noco), 20 μM taxol (Tax), 100 μM blebbistatin (Blebbi), 10 μM Y-27632, 50 μM ML-7, 50 nM jasplakinolide (Jasplak), 10 μM cytochalasin D (Cyto D), and 500 nM latrunculin B (Lat B). The extension length of chemically treated cells was compared to NT cells using the Wilcoxon/Mann-Whitney test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; NS, not significant). The extension length in the presence of latrunculin B could not be determined because the response was different from a typical polar bleb formation (see Movie S8). (B) The probability of forming multiple blebs in the presence of chemical agents. The concentrations of chemical agents were the same as in (A) except for 20 μM ML-7. The multiple blebs were again inhibited by blebbistatin and cytochalasin D, indicating that actomyosin is responsible for the initiation of multiple blebs as for polar bleb. The probability of chemically treated cells was compared to NT cells using Fisher’s exact test (∗∗p < 0.01, ∗∗∗p < 0.001; NS, not significant). (C) Probability of Motionless in the presence of chemical agents. Motionless occurred regardless of the presence of 10 μM nocodazole, 100 μM blebbistatin, or 10 μM cytochalasin D.
Temperature-sensitive Ca2+ dynamics is not essential for polar bleb formation
Next, we investigated the effects of the Ca2+ increase induced by local heating on bleb formation because Ca2+ dynamics is a major response to temperature changes and are expected to disturb the contractile force of actomyosin (Discussion). Under conditions that form polar blebs (T0 = 25°C, ΔT = 12°C) (Fig. S6), Ca2+ bursts were mainly observed immediately after recooling and occasionally during heating (Fig. S10, A and B; Movie S9). The Ca2+ burst induced by recooling is attributable to breaking the balance of the temperature sensitivities between IP3 receptors and sarco/endoplasmic reticulum Ca2+-ATPase in the endoplasmic reticulum (3,4). Under conditions that induce multiple blebs or Motionless, the Ca2+ bursts generally began during heating. Intracellular Ca2+ chelates (EGTA or BAPTA) prevented the Ca2+ burst (Fig. S10, B and C). However, polar bleb formation was not influenced in either EGTA- or BAPTA-loaded cells (Fig. S10 D), suggesting that the Ca2+ increase during heating was not essential for polar bleb formation (Fig. S10 A). However, the probability of multiple bleb formation was significantly decreased by the addition of EGTA or BAPTA, suggesting that Ca2+ bursts have some role in the initiation of multiple bleb formation (Fig. S10; Movie S9; see also Discussion).
Symmetry breaking of actomyosin cortex induced by temperature gradient
We further investigated the dynamics of the actomyosin cortex with Lifeact-RFP, which specifically binds to actin filaments (F-actin) (28), and actin-RFP expressed in the cell. Under the temperature gradient that induces polar bleb formation, the fluorescence intensities of both Lifeact-RFP and actin-RFP became asymmetric, decreasing and increasing at the near (warmer) and far (cooler) sides of the cell, respectively (Figs. 3, A and B, and S11; Movies S10 and S11). Fluorescence intensities of GFP and RFP decreased when the temperature was increased (6,8). Although the thermal quenching of RFP took place during heating, the quenched fluorescence immediately recovered upon recooling because it is a reversible process. Furthermore, in the presence of blebbistatin or Y-27632, the symmetry of actin-RFP was not broken (Fig. 3 B), showing that the temperature increase did not cause an irreversible decrease in the fluorescence intensity of proteins. These results show that the net decrease in the fluorescence intensity observed after heating is not attributable to the thermal quenching but to irreversible change in the actin cortex density. Larger ΔT values enhanced both the asymmetry and extension length of polar blebs (Fig. S12). The distribution pattern of mCherry-MRLC was similar to that of F-actin (Fig. 3, A and B; Movie S12). In the presence of blebbistatin, Y-27632, or cytochalasin D, both F-actin and myosin II accumulated less significantly to the cooler side than in nontreated cells, but the decrease in the amount of F-actin at the warmer side still occurred (Fig. 3 B; Movies S13, S14, and S15). From these results, we conclude that the accumulation of F-actin toward the cooler side is caused by the imbalance of the contractile force of the actomyosin II complex between the warmer and cooler sides (see Discussion below). The accumulations of the cortex and membrane toward the cooler side are coupled with polar bleb formation. CellMask-stained PMs showed inhomogeneous localization similar to Lifeact-RFP and actin-RFP (Fig. 3, A and B; Movie S16). Simultaneous observation of Lifeact-GFP and CellMask revealed that F-actin and the membrane were likely to move together to the cooler side until the bleb was formed (Fig. 4, A–D; Movie S17). During this rearrangement, a polar bleb began to appear ∼3 s after the start of heating (Fig. 4, A–C). In a small number of cases, we observed that smaller ΔT values also induced a polar bleb without noticeable inhomogeneous distribution of the cortex or with an increase in the cortex at the warmer side (Figs. S12 and S13; Movie S18).
Discussion
Spontaneous bleb formation is reportedly initiated by detachment of the PM from the cell cortex (31,32). This process is induced either by contractile force from actomyosin and/or by local rupture of the cortex (31,32). Our results suggest that both phenomena can explain the early stages of polar bleb formation induced by local heating; a temperature gradient increases the contractile force of actomyosin at the warmer side (Fig. 4 E, i–iii) or induces local rupture of the cortex (Fig. 4 E, i–iii’), both of which can trigger the detachment of cortex from the PM and initiate a polar bleb formation. Subsequently, at the warmer side, the actomyosin contractile force decreases and the cortex ruptures (Fig. 4 E, iv), immediately followed by the accumulation of cortex toward the cooler side and extension of the bleb at the warmer side (Fig. 4 E, v) (see Discussion below). Under small temperature gradients or uniform heating, the process as shown in (i)–(iii) or (i)–(iii’) occurs in many regions, resulting in the formation of multiple nondirectional blebs.
Increased contractility model for the initiation of polar bleb formation (Fig. 4 E, ii and iii) is expected by the following observations; after detachment of the PM from the cell cortex during heating, the shape of the cortex at the warmer side was straightened (10 s in Fig. 4 A and Movie S17) and then returned to an arched shape after recooling (22.5 s in Fig. 4 C and Movie S17). These changes in cortex shape can be explained by the increased contractile force at the warmer side during heating that is analogous to our previous reports on skeletal muscle myosin. We reported that the tension generated on an actin filament by skeletal muscle myosins increases at higher temperature (20) due to the increased number of crossbridges (33,34). In this line, it was reported that A549 cells become stiffer at higher temperature (35). Contrary to this report, other groups showed that stiffness and cortical tension decrease at higher temperature (36–40). We noted that the polar bleb was formed even when the cortex was stabilized by jasplakinolide (Fig. 2 A), suggesting the increased contractility model as a major route at least in the presence of this drug. Further investigation is desired for temperature dependence of force generation by cortical actomyosin in cells.
During blebbing, the rupture of cortex occurred at the warmer side in most cases (Fig. 4 E, iv or ii’), as observed by the decrease in cortex density (Figs. 3, A and B, and 4, A–C). This may occur either through actin network disassembly by the temperature-enhanced contractile force of myosin II (41–44) at the warmer side, or through decreased cortical tension following instantaneous enhancement by heating. Our following observation can be explained by the latter; the density of MRLC was decreased at the warmer side even in the presence of blebbistatin and Y-27632 (Fig. 3 B; Movies S12 and S15). This thermal dissociation of myosin II from actin filaments, or MRLC from myosin II molecules, may reduce the contractile force, and destabilize the cortex. This rupture could escalate bleb extension by eliminating the physical barrier for intracellular components (Fig. S14; Movie S19). The gap created in the cortex seems to be large, as a spindle can move into the polar bleb through the ruptured cortex (Fig. S14; Movie S19). After the polar bleb formation, F-actin reformed in the bleb (Fig. 4 A; Movie S17), which might have contributed to the retraction (Fig. S5 C) as in classical blebbing (31,32). In the polar bleb formation induced by temperature gradient, many kinds of other factors could be involved. Thermophoresis has a potential to generate asymmetric distribution of ATP and other molecules. Temperature gradient could break the symmetry of enzyme activities and kinetics of various proteins regulating actin polymerization/depolymerization, stability of cortex, adhesion of cortex-membrane, and force generation by myosin II. To rule in or out the involvement of these factors, the bottom-up approach using an artificial cell system (i.e., reconstructed actomyosin cortex in liposome) is desired in further studies.
Ca2+ increase may affect the structure of actomyosin complexes. For instance, high concentrations of Ca2+ induce the formation of dense F-actin networks (45). Ca2+/calmodulin-activated MLCK increases the activity of myosin II (46). To confirm whether Ca2+ bursts are involved in the bleb formation, we examined the significance of intracellular Ca2+ by applying Ca2+ chelators, EGTA-AM and BAPTA-AM (the Ca2+ binding rate of BAPTA is ∼40 times higher than that of EGTA (47)). Both intracellular EGTA and BAPTA prevented the Ca2+ burst during heating and also after recooling under conditions that induce polar blebs (T0 = 25°C, ΔT = 12°C) (Fig. S10, B and C). BAPTA-loaded cells then showed bleb extension similar to nontreated cells, whereas EGTA-loaded cells produced longer bleb extensions (Fig. S10 D). This difference between EGTA and BAPTA might be attributable to BAPTA that can depolymerize actin filaments (48), and probably reduce the cortex tension. Overall, these results suggest that Ca2+ burst during heating suppresses the extension of polar bleb. In accordance with these observations, the probability of forming multiple blebs decreased with both intracellular EGTA and BAPTA by about a half, probably because decreased intracellular Ca2+ concentration reduces force generation of the actomyosin cortex (40,49).
The different effects of Y-27632 (ROCK inhibitor) and ML-7 (MLCK inhibitor) on the formation of a polar bleb (Fig. 2 A) suggest that ROCK-mediated contractile force is mainly involved in the formation of polar blebs induced by local heating, but the contribution of MLCK-mediated force is limited. Y-27632 is known to inhibit osmotic stress-induced blebbing, but ML-7 does not suppress the blebbing (50). Our results are consistent with these observations. Further investigation into spatially distinct phosphorylation of MLC could reveal the more detailed mechanism for actomyosin-based cellular responses to temperature gradient.
Nocodazole (microtubule-depolymerizing drug) significantly increased the length of polar blebs induced by heating, whereas taxol (microtubule stabilizer) did not increase the extension length (Fig. 2 A). These results suggest that microtubule structures such as the mitotic spindle suppress the bleb extension. Supporting this result, in most cases, the spindle did not move into the bleb due to the physical cortex barrier (Fig. S14 A; Movie S19). In other cases, however, the spindle moved into the bleb through the gap where the actomyosin cortex was broken (Fig. S14 B; Movie S19), and this relocation of spindle led to large bleb formation (Fig. S14 C). In addition, the activation of RhoA by nocodazole through microtubule depolymerization (51,52) might also be related to increased extension of the polar bleb induced by heating.
The minimum temperature gradient producing polar blebbing is 1.3°C across the cell (diameter ∼20 μm: ≥∼0.065°C μm−1), equivalent to 50–100°C mm−1. To the best of our knowledge, such a steep temperature gradient has not been observed in a human body. On the other hand, local heating techniques attract attentions as novel noninvasive methods for regulating cell functions (6–13). Furthermore, in advanced thermal therapies of tumor tissues, magnetic or gold nanoparticles are currently used as heat sources in clinical trials (53–59). Considering that each micro- and nanosized heat source can increase the local temperature up to 46°C and generate a gradient as sharp as 1.3°C within each individual cell, blebs are expected to form in these thermal treatments. Furthermore, our results provide a safeguard for a bleb tension measurement with IR laser previously reported (60). With the estimated temperature increase of 0.02°C during tension measurement (60), IR laser is considered to have a limited effect, if any.
Although local heating for 20 s does not completely kill the cells within 1 h (Fig. S7), even short-pulsed local heating for only 5 s inhibited normal cytokinesis above 46°C (Fig. S8 A). Interestingly, trisection was observed in both unheated and heated cells. The cell size before trisection or multisection appeared to be larger than that of divided cells (Fig. S8 B), suggesting that these larger cells have a tendency to trisect independent of heating (61). However, even normal-sized cells (33%; 2/6) trisected due to heating (Fig. S8 B), indicating that local heating has a potential to induce trisection. We also observed that the spindle appeared disorganized due to mechanical stress at the interface between the cell body and the polar bleb (Fig. S14; Movie S19). These effects from local heating and bleb formation might contribute to the efficiency of thermal therapies.
Author Contributions
K.O., T.A., A.I., H.I., M.S., and S.I. designed the research. K.O., M.M., and M.S. developed the optical setup. K.O., T.A., A.I., T.S., and Y.S. performed experiments and analyzed the data under the guidance of M.S. and S.I. Y.S., T.I., and T.O. constructed the plasmids. K.O., T.A., A.I., M.S., and S.I. wrote the article. All authors discussed the results and commented on the article.
Acknowledgments
This research was supported by Research Fellowship for Young Scientists (to K.O.) from Japan Society for the Promotion of Science (JSPS), the Grants-in-Aid for Young Scientist (A) (to K.O. and M.S.), Scientific Research on Innovative Areas “Nanomedicine Molecular Science” (No. 2306) (to K.O. and M.S.), Specially Promoted Research (to S.I.), and Scientific Research (S) (to S.I.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. H.I. was supported by the A∗STAR Research Attachment Programme (ARAP) from Singapore’s Agency for Science, Technology and Research (A∗STAR).
Footnotes
Kotaro Oyama, Tomomi Arai, and Akira Isaka contributed equally to this work.
Kotaro Oyama’s and Tomomi Arai’s present address is Department of Cell Physiology, The Jikei University School of Medicine, 3-25-8 Nishi-shinbashi, Minato-ku, Tokyo 105-8461, Japan.
Supporting Discussion, 14 figures, and 19 movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00598-6.
Contributor Information
Kotaro Oyama, Email: kou_oyama@akane.waseda.jp.
Madoka Suzuki, Email: suzu_mado@aoni.waseda.jp.
Shin'ichi Ishiwata, Email: ishiwata@waseda.jp.
Supporting Citations
References (62–69) appear in the Supporting Material.
Supporting Material
T0 = 37°C, ΔT = 13°C. The yellow circle indicates the position of the heat source. Heating starts at t = 0 s. Scale bar, 10 μm.
The yellow circle indicates the position of the heat source. T0 = 25°C, ΔT = 12°C. Scale bar, 10 μm.
The yellow circle indicates the position of the heat source. T0 = 25°C, ΔT = 12°C. Scale bar, 10 μm.
Small particles are fluorescent beads. The medium was aspirated by a glass pipette, and convection flow was induced (0–20 s), but no bleb was formed. Local heating (T0 = 25°C, ΔT = 12°C) induced a polar bleb in the same cell. The yellow circle indicates the position of the heat source. Scale bar, 10 μm.
Cells were heated for 20 s (left), 40 s (middle), or 60 s (right). Each cell formed multiple blebs after heating. Yellow circles indicate the position of the heat source. T0 = 37°C, ΔT = 11–13°C. Scale bars, 10 μm.
Multiple blebs formed by the first heating period shrank, and larger multiple blebs formed after the second heating period. The yellow circle indicates the position of the heat source during heating. T0 = 37°C, ΔT = 9°C. Scale bar, 10 μm.
Organelles that were moving before heating stopped their motions after heating. The yellow circle indicates the position of the heat source during heating. T0 = 37°C, ΔT = 19°C. Scale bar, 10 μm.
Nontreated (NT) cells and cells in the presence of 10 μM nocodazole (Noco), 20 μM taxol (Tax), or 50 nM jasplakinolide (Jasplak) formed a polar bleb. The formation of polar blebs was suppressed by 100 μM blebbistatin (Blebbi), 10 μM Y-27632, 10 μM cytochalasin D (Cyto D), or 500 nM latrunculin B (Lat B). Blebbistatin aggregates were observed. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 12°C. Scale bar, 10 μm.
Intracellular Ca2+ was visualized with fluo-4. T0 = 25°C, △T = 12°C (left, a polar bleb). T0 = 37°C, △T = 13°C (middle, multiple blebs). T0 = 37°C, △T = 19°C (right, Motionless). Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing Lifeact-RFP. Lifeact shifted to the far side from the laser position during heating. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing actin-RFP. Actin-RFP shows basically the same distribution as Lifeact-RFP (Movie S10). The yellow circle indicates the position of the heat source during heating. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing mCherry-MRLC. Myosin II showed basically the same distribution as Lifeact-RFP and actin-RFP. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing Lifeact-RFP were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing actin-RFP were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing mCherry-MRLC were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source during heating. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a CellMask-stained cell. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Left: Lifeact-GFP (green), middle: CellMask (magenta), right: merge. F-actin and the cell membrane generally move together to the cooler side before bleb formation. After detachment of the cortex from the cell membrane, the cortex at the warmer side straightened during heating (10 s) and curved after recooling. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Left: Lifeact-GFP (green), middle: CellMask (magenta), right: merged images. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 12°C. Scale bar, 10 μm.
Membrane and spindles were visualized with CellMask (magenta, left) and tubulin-GFP (green, middle), respectively. Right: merged images. (Upper) The spindle appears to be pressed against the cortex after 14 s. T0 = 25°C, △T = 14°C. (Lower) The spindle passed through the ruptured cortex into a bleb that is as large as the spindle. The spindle appeared to be disorganized due to mechanical stress when it passed through the ruptured cortex. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
References
- 1.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Sakai T. Rapid cooling contracture. Jpn. J. Physiol. 1986;36:423–431. doi: 10.2170/jjphysiol.36.423. [DOI] [PubMed] [Google Scholar]
- 3.Tseeb V., Suzuki M., Ishiwata S. Highly thermosensitive Ca dynamics in a HeLa cell through IP3 receptors. HFSP J. 2009;3:117–123. doi: 10.2976/1.3073779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh H., Oyama K., Ishiwata S. Microscopic heat pulse-induced calcium dynamics in single WI-38 fibroblasts. Biophysics. 2014;10:109–119. doi: 10.2142/biophysics.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao B., Coste B., Patapoutian A. Temperature-dependent STIM1 activation induces Ca²+ influx and modulates gene expression. Nat. Chem. Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H., Delikanli S., Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010;5:602–606. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- 7.Chen R., Romero G., Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–1480. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 8.Kamei Y., Suzuki M., Yuba S. Infrared laser-mediated gene induction in targeted single cells in vivo. Nat. Methods. 2009;6:79–81. doi: 10.1038/nmeth.1278. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro M.G., Homma K., Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q., Frerck M.J., Rabbitt R.D. Exciting cell membranes with a blustering heat shock. Biophys. J. 2014;106:1570–1577. doi: 10.1016/j.bpj.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyama K., Mizuno A., Ishiwata S. Microscopic heat pulses induce contraction of cardiomyocytes without calcium transients. Biochem. Biophys. Res. Commun. 2012;417:607–612. doi: 10.1016/j.bbrc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Miyako E., Russier J., Bianco A. Photofunctional nanomodulators for bioexcitation. Angew. Chem. Int. Ed. Engl. 2014;53:13121–13125. doi: 10.1002/anie.201407169. [DOI] [PubMed] [Google Scholar]
- 13.Shintani S.A., Oyama K., Ishiwata S. High-frequency sarcomeric auto-oscillations induced by heating in living neonatal cardiomyocytes of the rat. Biochem. Biophys. Res. Commun. 2015;457:165–170. doi: 10.1016/j.bbrc.2014.12.077. [DOI] [PubMed] [Google Scholar]
- 14.Bonner J.T., Clarke W.W., Jr., Slifkin M.K. The orientation to light and the extremely sensitive orientation to temperature gradients in the slime mold Dictyostelium discoideum. J. Cell. Physiol. 1950;36:149–158. doi: 10.1002/jcp.1030360203. [DOI] [PubMed] [Google Scholar]
- 15.Maeda K., Imae Y., Oosawa F. Effect of temperature on motility and chemotaxis of Escherichia coli. J. Bacteriol. 1976;127:1039–1046. doi: 10.1128/jb.127.3.1039-1046.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahat A., Tur-Kaspa I., Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med. 2003;9:149–150. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- 17.Eisenbach M., Giojalas L.C. Sperm guidance in mammals - an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 18.Pomerance A., Matthews J., Losert W. Actin polymerization in a thermal gradient. Macromol. Symp. 2005;227:231–242. [Google Scholar]
- 19.Kakugo A., Tamura Y., Gong J.P. Formation of well-oriented microtubules with preferential polarity in a confined space under a temperature gradient. J. Am. Chem. Soc. 2009;131:18089–18095. doi: 10.1021/ja901538n. [DOI] [PubMed] [Google Scholar]
- 20.Kato H., Nishizaka T., Ishiwata S. Imaging of thermal activation of actomyosin motors. Proc. Natl. Acad. Sci. USA. 1999;96:9602–9606. doi: 10.1073/pnas.96.17.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi K., Ishiwata S. Thermal activation of single kinesin molecules with temperature pulse microscopy. Cell Motil. Cytoskeleton. 2001;49:41–47. doi: 10.1002/cm.1019. [DOI] [PubMed] [Google Scholar]
- 22.Iwaki M., Iwane A.H., Yanagida T. Local heat activation of single myosins based on optical trapping of gold nanoparticles. Nano Lett. 2015;15:2456–2461. doi: 10.1021/nl5049059. [DOI] [PubMed] [Google Scholar]
- 23.Duhr S., Braun D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA. 2006;103:19678–19682. doi: 10.1073/pnas.0603873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda Y.T., Buguin A., Libchaber A. Thermal separation: interplay between the Soret effect and entropic force gradient. Phys. Rev. Lett. 2011;107:038301-1–038301-4. doi: 10.1103/PhysRevLett.107.038301. [DOI] [PubMed] [Google Scholar]
- 25.Oyama K., Takabayashi M., Suzuki M. Walking nanothermometers: spatiotemporal temperature measurement of transported acidic organelles in single living cells. Lab Chip. 2012;12:1591–1593. doi: 10.1039/c2lc00014h. [DOI] [PubMed] [Google Scholar]
- 26.Reichl M.R., Braun D. Thermophoretic manipulation of molecules inside living cells. J. Am. Chem. Soc. 2014;136:15955–15960. doi: 10.1021/ja506169b. [DOI] [PubMed] [Google Scholar]
- 27.Zeeb V., Suzuki M., Ishiwata S. A novel method of thermal activation and temperature measurement in the microscopic region around single living cells. J. Neurosci. Methods. 2004;139:69–77. doi: 10.1016/j.jneumeth.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Riedl J., Crevenna A.H., Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat. Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecuit T., Lenne P.F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 30.Clark A.G., Paluch E. Mechanics and regulation of cell shape during the cell cycle. Results Probl. Cell Differ. 2011;53:31–73. doi: 10.1007/978-3-642-19065-0_3. [DOI] [PubMed] [Google Scholar]
- 31.Charras G., Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 32.Paluch E.K., Raz E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 2013;25:582–590. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai M., Kawaguchi K., Ishiwata S. Temperature change does not affect force between single actin filaments and HMM from rabbit muscles. Biophys. J. 2000;78:3112–3119. doi: 10.1016/S0006-3495(00)76848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai M., Kido T., Ishiwata S. Temperature change does not affect force between regulated actin filaments and heavy meromyosin in single-molecule experiments. J. Physiol. 2006;574:877–887. doi: 10.1113/jphysiol.2006.111708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunyer R., Trepat X., Navajas D. The temperature dependence of cell mechanics measured by atomic force microscopy. Phys. Biol. 2009;6:025009. doi: 10.1088/1478-3975/6/2/025009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson J.R., Shanahan M.O., Hochmuth R.M. The influence of temperature on red cell deformability. Blood. 1975;46:611–624. [PubMed] [Google Scholar]
- 37.Petersen N.O., McConnaughey W.B., Elson E.L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc. Natl. Acad. Sci. USA. 1982;79:5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B., Goergen C.J., Shao J.-Y. Effect of temperature on tether extraction, surface protrusion, and cortical tension of human neutrophils. Biophys. J. 2007;93:2923–2933. doi: 10.1529/biophysj.107.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rico F., Chu C., Moy V.T. Temperature modulation of integrin-mediated cell adhesion. Biophys. J. 2010;99:1387–1396. doi: 10.1016/j.bpj.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan C.J., Whyte G., Guck J. Impact of heating on passive and active biomechanics of suspended cells. Interface Focus. 2014;4:20130069. doi: 10.1098/rsfs.2013.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haviv L., Gillo D., Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J. Mol. Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 42.Wilson C.A., Tsuchida M.A., Theriot J.A. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murrell M.P., Gardel M.L. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl. Acad. Sci. USA. 2012;109:20820–20825. doi: 10.1073/pnas.1214753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reymann A.-C., Boujemaa-Paterski R., Blanchoin L. Actin network architecture can determine myosin motor activity. Science. 2012;336:1310–1314. doi: 10.1126/science.1221708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung S.-R., Kim M.-H., Koh D.-S. Control of granule mobility and exocytosis by Ca2+ -dependent formation of F-actin in pancreatic duct epithelial cells. Traffic. 2009;10:392–410. doi: 10.1111/j.1600-0854.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Eggermann E., Bucurenciu I., Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saoudi Y., Rousseau B., Job D. Calcium-independent cytoskeleton disassembly induced by BAPTA. Eur. J. Biochem. 2004;271:3255–3264. doi: 10.1111/j.1432-1033.2004.04259.x. [DOI] [PubMed] [Google Scholar]
- 49.Gyger M., Stange R., Käs J.A. Active contractions in single suspended epithelial cells. Eur. Biophys. J. 2014;43:11–23. doi: 10.1007/s00249-013-0935-8. [DOI] [PubMed] [Google Scholar]
- 50.Barfod E.T., Moore A.L., Lidofsky S.D. Myosin light chain kinase and Src control membrane dynamics in volume recovery from cell swelling. Mol. Biol. Cell. 2011;22:634–650. doi: 10.1091/mbc.E10-06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren X.D., Kiosses W.B., Schwartz M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norman L., Sengupta K., Aranda-Espinoza H. Blebbing dynamics during endothelial cell spreading. Eur. J. Cell Biol. 2011;90:37–48. doi: 10.1016/j.ejcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Johannsen M., Gneveckow U., Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int. J. Hyperthermia. 2005;21:637–647. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- 54.Johannsen M., Gneveckow U., Loening S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int. J. Hyperthermia. 2007;23:315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 55.Johannsen M., Gneveckow U., Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007;52:1653–1661. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 56.Maier-Hauff K., Ulrich F., Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wust P., Gneveckow U., Jordan A. Magnetic nanoparticles for interstitial thermotherapy—feasibility, tolerance and achieved temperatures. Int. J. Hyperthermia. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 58.van Landeghem F.K.H., Maier-Hauff K., von Deimling A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 59.Weintraub K. Biomedicine: the new gold standard. Nature. 2013;495:S14–S16. doi: 10.1038/495S14a. [DOI] [PubMed] [Google Scholar]
- 60.Peukes J., Betz T. Direct measurement of the cortical tension during the growth of membrane blebs. Biophys. J. 2014;107:1810–1820. doi: 10.1016/j.bpj.2014.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiraly G., Nagy G., Banfalvi G. Tumor cell fusion and multipolar trivision. J. Cancer Res. Ther. Oncol. 2014;2:1–5. [Google Scholar]
- 62.Kapiszewska M., Hopwood L.E. Changes in bleb formation following hyperthermia treatment of Chinese hamster ovary cells. Radiat. Res. 1986;105:405–412. [PubMed] [Google Scholar]
- 63.Sakamoto T., Limouze J., Sellers J.R. Blebbistatin, a myosin II inhibitor, is photoinactivated by blue light. Biochemistry. 2005;44:584–588. doi: 10.1021/bi0483357. [DOI] [PubMed] [Google Scholar]
- 64.Andersen T., Bahadori A., Bendix P.M. Nanoscale phase behavior on flat and curved membranes. Nanotechnology. 2014;25:505101. doi: 10.1088/0957-4484/25/50/505101. [DOI] [PubMed] [Google Scholar]
- 65.Johnson S.A., Stinson B.M., Baumgart T. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim. Biophys. Acta. 2010;1798:1427–1435. doi: 10.1016/j.bbamem.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hazel J.R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- 67.Jackson W.M., Kostyla J., Brandts J.F. Calorimetric study of protein transitions in human erythrocyte ghosts. Biochemistry. 1973;12:3662–3667. doi: 10.1021/bi00743a014. [DOI] [PubMed] [Google Scholar]
- 68.Brandts J.F., Erickson L., Taverna R.D. Calorimetric studies of the structural transitions of the human erythrocyte membrane. The involvement of spectrin in the A transition. Biochemistry. 1977;16:3450–3454. doi: 10.1021/bi00634a024. [DOI] [PubMed] [Google Scholar]
- 69.Brandts J.F., Taverna R.D., Lysko K.A. Calorimetric studies of the structural transitions of the human erythrocyte membrane. Studies of the B and C transitions. Biochim. Biophys. Acta. 1978;512:566–578. doi: 10.1016/0005-2736(78)90166-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T0 = 37°C, ΔT = 13°C. The yellow circle indicates the position of the heat source. Heating starts at t = 0 s. Scale bar, 10 μm.
The yellow circle indicates the position of the heat source. T0 = 25°C, ΔT = 12°C. Scale bar, 10 μm.
The yellow circle indicates the position of the heat source. T0 = 25°C, ΔT = 12°C. Scale bar, 10 μm.
Small particles are fluorescent beads. The medium was aspirated by a glass pipette, and convection flow was induced (0–20 s), but no bleb was formed. Local heating (T0 = 25°C, ΔT = 12°C) induced a polar bleb in the same cell. The yellow circle indicates the position of the heat source. Scale bar, 10 μm.
Cells were heated for 20 s (left), 40 s (middle), or 60 s (right). Each cell formed multiple blebs after heating. Yellow circles indicate the position of the heat source. T0 = 37°C, ΔT = 11–13°C. Scale bars, 10 μm.
Multiple blebs formed by the first heating period shrank, and larger multiple blebs formed after the second heating period. The yellow circle indicates the position of the heat source during heating. T0 = 37°C, ΔT = 9°C. Scale bar, 10 μm.
Organelles that were moving before heating stopped their motions after heating. The yellow circle indicates the position of the heat source during heating. T0 = 37°C, ΔT = 19°C. Scale bar, 10 μm.
Nontreated (NT) cells and cells in the presence of 10 μM nocodazole (Noco), 20 μM taxol (Tax), or 50 nM jasplakinolide (Jasplak) formed a polar bleb. The formation of polar blebs was suppressed by 100 μM blebbistatin (Blebbi), 10 μM Y-27632, 10 μM cytochalasin D (Cyto D), or 500 nM latrunculin B (Lat B). Blebbistatin aggregates were observed. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 12°C. Scale bar, 10 μm.
Intracellular Ca2+ was visualized with fluo-4. T0 = 25°C, △T = 12°C (left, a polar bleb). T0 = 37°C, △T = 13°C (middle, multiple blebs). T0 = 37°C, △T = 19°C (right, Motionless). Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing Lifeact-RFP. Lifeact shifted to the far side from the laser position during heating. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing actin-RFP. Actin-RFP shows basically the same distribution as Lifeact-RFP (Movie S10). The yellow circle indicates the position of the heat source during heating. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a cell expressing mCherry-MRLC. Myosin II showed basically the same distribution as Lifeact-RFP and actin-RFP. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing Lifeact-RFP were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing actin-RFP were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Cells expressing mCherry-MRLC were heated in the absence of inhibitors (NT), or in the presence of 100 μM blebbistatin (Blebbi), 10 μM Y-27632, or 10 μM cytochalasin D (Cyto D). Yellow circles indicate the position of the heat source during heating. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
A polar bleb was induced by local heating in a CellMask-stained cell. The yellow circle indicates the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Left: Lifeact-GFP (green), middle: CellMask (magenta), right: merge. F-actin and the cell membrane generally move together to the cooler side before bleb formation. After detachment of the cortex from the cell membrane, the cortex at the warmer side straightened during heating (10 s) and curved after recooling. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.
Left: Lifeact-GFP (green), middle: CellMask (magenta), right: merged images. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 12°C. Scale bar, 10 μm.
Membrane and spindles were visualized with CellMask (magenta, left) and tubulin-GFP (green, middle), respectively. Right: merged images. (Upper) The spindle appears to be pressed against the cortex after 14 s. T0 = 25°C, △T = 14°C. (Lower) The spindle passed through the ruptured cortex into a bleb that is as large as the spindle. The spindle appeared to be disorganized due to mechanical stress when it passed through the ruptured cortex. Yellow circles indicate the position of the heat source. T0 = 25°C, △T = 15°C. Scale bar, 10 μm.