Abstract
Background
Clostridium difficile infection (CDI) has become a global epidemiological problem for both hospitalized patients and outpatients. The most commonly used drugs to treat CDI are metronidazole and vancomycin. The aim of this study was to compare the efficacy and safety of metronidazole monotherapy with vancomycin monotherapy and combination therapy in CDI patients.
Methods
A comprehensive search without publication status or other restrictions was conducted. Studies comparing metronidazole monotherapy with vancomycin monotherapy or combination therapy in patients with CDI were considered eligible. Meta-analysis was performed using the Mantel-Haenszel fixed-effects model, and odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated and reported.
Results
Of the 1910 records identified, seventeen studies from thirteen articles (n = 2501 patients) were included. No statistically significant difference in the rate of clinical cure was found between metronidazole and vancomycin for mild CDI (OR = 0.67, 95% CI (0.45, 1.00), p = 0.05) or between either monotherapy and combination therapy for CDI (OR = 1.07, 95% CI (0.58, 1.96), p = 0.83); however, the rate of clinical cure was lower for metronidazole than for vancomycin for severe CDI (OR = 0.46, 95% CI (0.26, 0.80), p = 0.006). No statistically significant difference in the rate of CDI recurrence was found between metronidazole and vancomycin for mild CDI (OR = 0.99, 95% CI (0.40, 2.45), p = 0.98) or severe CDI (OR = 0.98, 95% CI (0.63, 1.53), p = 0.94) or between either monotherapy and combination therapy for CDI (OR = 0.91, 95% CI (0.66, 1.26), p = 0.56). In addition, there was no significant difference in the rate of adverse events (AEs) between metronidazole and vancomycin (OR = 1.18, 95% CI (0.80, 1.74), p = 0.41). In contrast, the rate of AEs was significantly lower for either monotherapy than for combination therapy (OR = 0.30, 95% CI (0.17, 0.51), p<0.0001).
Conclusions
Metronidazole and vancomycin are equally effective for the treatment of mild CDI, but vancomycin is superior for the treatment of severe CDI. Combination therapy is not superior to monotherapy because it appears to be associated with an increase in the rate of AEs.
Introduction
Clostridium difficile is an anaerobic, gram-positive, spore-forming bacillus[1]. The incidence and severity of C. difficile infection (CDI) appear to be on the rise[2], and CDI has become an epidemiological problem for both hospitalized patients and outpatients worldwide[2].
Oral metronidazole is recommended for patients undergoing initial therapy and for those with mild infections, whereas oral vancomycin is recommended for seriously ill patients. Furthermore, the combination of oral vancomycin and intravenous metronidazole is suggested for the treatment of severe or complicated infections[3,4]. As risk of the development of vancomycin-resistant enterococci (VRE) associated with both metronidazole and vancomycin treatment has emerged[5], studies examining fidaxomicin[6,7], rifaximin[8,9], rifampin[10], nitazoxanide[11,12], tolevamer[13], and teicoplanin[14] therapies have been performed. Furthermore, in patients with recurrent CDI, infusion of donor feces has resulted in improved treatment outcomes. In particular, patients with multiple relapses of CDI have benefited from this approach[15]. Unfortunately, prospective clinical trials of most of the above-mentioned antibiotics evaluating their applicability for general use have not yet been performed[4], and several questions regarding fecal transplantation remain unanswered; for example, the optimal protocol for donor feces infusion is unknown[15]. Therefore, the treatment of CDI has relied primarily on metronidazole and vancomycin[16].
Although the Healthcare Epidemiology of America (SHEA)/Infectious Diseases Society of America (IDSA) guidelines recommend the use of oral vancomycin for severe CDI, this recommendation is based on the results of few clinical trials[17,18]. In addition, despite the frequent use of combination therapy in clinical settings, it is unclear whether combination therapy is more effective than monotherapy because, as emphasized by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID)[4], few randomized controlled trials (RCTs) have been performed to compare them. Moreover, the many associated adverse events (AEs)[16] necessitate safety analyses of these two drugs. To address this knowledge gap, we performed a systematic review and meta-analysis to compare the efficacy and safety of metronidazole monotherapy with vancomycin monotherapy and combination therapy for the treatment of patients with CDI.
Methods
Our study protocol and analysis were planned in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[19], and no ethical approval was needed.
Search strategy
A systematic literature search of the following electronic databases was conducted to identify relevant literature published in English or Chinese before November 2014: PubMed, Embase, Web of Science, and Cochrane Library. Three Chinese language databases—China National Knowledge Infrastructure (CNKI; available at www.cnki.net), Chinese Scientific Journals Database (VIP; available at www.cqvip.com), and WANFANG DATA (available at www.wanfangdata.com.cn)—were also searched. The search strategy included the following medical subject headings and key words: “pseudomembranous enterocolitis,” “antibiotic-associated diarrhea,” “antibiotic-associated colitis,” “Clostridium difficile,” “metronidazole,” and “vancomycin.” These search terms were combined using Boolean logic as follows: both the medical subject headings and the terms related to the patient population of interest (Clostridium difficile, pseudomembranous colitis, antibiotic-associated diarrhea, and antibiotic-associated colitis) were combined with those describing interventions (metronidazole OR vancomycin). The complete search strategy used to search each database is described in S1 Table. Furthermore, the references in the initially identified articles, including relevant reviews, were manually searched and reviewed. Academic dissertations and abstracts presented at scientific conferences were also searched using ProQuest Dissertation & Theses Database and The Conference and Academic Dissertation Database of CNKI, respectively, to ensure that no relevant study was missed (up to May 2015).
Study selection
Two reviewers (RL and LCL) assessed all potentially relevant studies and reached a consensus on all items. They then independently searched the literature and examined relevant studies to obtain data on the rates of clinical cure, CDI recurrence and AEs. In the case of disagreement between the two reviewers, the senior coauthor (XL) was consulted, and the disagreement was resolved by consensus. Studies were included if they met the following criteria: (1) contained original data, (2) contained data regarding the use of metronidazole or vancomycin for the treatment of CDI, (3) contained data regarding clinical therapeutic outcomes or AEs, and (4) reported on RCTs or case-control studies. Studies were excluded if they (1) were unrelated to CDI, (2) were duplicate reports, (3) were not written in English or Chinese, or (4) were not case-control studies.
Data extraction
Two reviewers (RL and LCL) independently abstracted data from each eligible study. Each investigator was blinded to the other investigator’s extracted data. A standardized form was used to record the following information: (1) first author; (2) year of publication; (3) disease severity (although there were four classifications according to disease severity, including mild CDI, severe CDI, complicated CDI, and recurrent CDI[3,4], none of the included studies evaluated complicated CDI, and fecal microbiota transplantation has been performed for recurrent CDI. Additionally, one included study referred to “mild-moderate” because the treatment of mild CDI and moderate CDI is identical[20], and we categorized this classification of CDI into the mild CDI group; thus, this systematic review and meta-analysis was primarily focused on the treatment of mild or severe CDI); (4) mean age; (5) male (%); (6) follow-up duration; (7) number of enrolled patients; (8) drug regimen; (9) rate of clinical cure; (10) rate of CDI recurrence; (11) AEs related to the study medications; (12) case definitions; (13) quality of the evidence; and (14) overall risk of bias. The extracted data (and specifically, the rates of clinical cure and CDI recurrence) were analyzed based on the intention-to-treat population.
Quality appraisal
The included studies were independently appraised for methodological quality by two authors (RL and LCL), without blinding to the source journal or authorship. Discrepancies were resolved by discussion or consultation with the third reviewer (XL) if required. The quality of each included study was evaluated according to the modified Jadad score[21]. The overall risk of bias of each included study was also evaluated[22]. Potential publication bias was assessed by visual inspection of asymmetry in Begg’s funnel plots, and Egger’s test was then used to provide statistical evidence of funnel plot symmetry (p<0.05 indicating bias and p>0.05 not indicating bias)[23,24]. We also performed sensitivity analyses on subgroups from the studies according to explicit or inexplicit classification of severity and follow-up duration to explore underlying sources of heterogeneity. All of these analyses, except for publication bias analysis (which was performed using STATA software: version 12.0, StataCorp, College Station, TX, USA), were performed using Review Manager software (RevMan, version 5.1, Oxford, UK; The Cochrane Collaboration, 2008).
Outcomes analyzed
Regarding the outcome measures used to assess efficacy, in this meta-analysis, the rate of clinical cure was used as the primary outcome measure, and the rate of CDI recurrence was used as the secondary outcome measure. We present the following two comparisons based on the rates of clinical cure and CDI recurrence: a comparison between metronidazole and vancomycin monotherapy for the treatment of patients with mild or severe CDI and a comparison of vancomycin or metronidazole monotherapy with combined vancomycin and metronidazole therapy or either drug combined with another antibiotic for the treatment of patients with CDI. For the latter comparison, we performed subgroup analysis according to the therapeutic intervention. AEs were used as the primary safety outcome measure in this meta-analysis. Here, we also present the following two comparisons based on AEs: metronidazole vs. vancomycin and monotherapy vs. combination therapy. We performed subgroup analysis according to AEs for both comparisons.
Data analysis and statistical methods
Statistical analysis was accomplished using Review Manager version 5.1 software. Both I2 and Q statistics were considered. In our analyses, we used the I2 statistic to quantitatively describe heterogeneity across studies. An I2 value of greater than 50% indicated high heterogeneity; a value ranging from 25% to 50% indicated moderate heterogeneity; and a value of less than 25% signified low heterogeneity[22]. Pooled odds ratios (ORs; calculated by adding 0.5 to each cell of the 2×2 table for the trial when one arm of the study contained no events[25]) and 95% confidence intervals (CIs; if 95% CIs did not include the null value, the results were considered to be significantly different) were also used in meta-analysis. The Mantel-Haenszel fixed-effects model was used for analysis of the total groups because the I2 values in our subgroup meta-analysis ranged from 0% to 6%.
Results
Search results
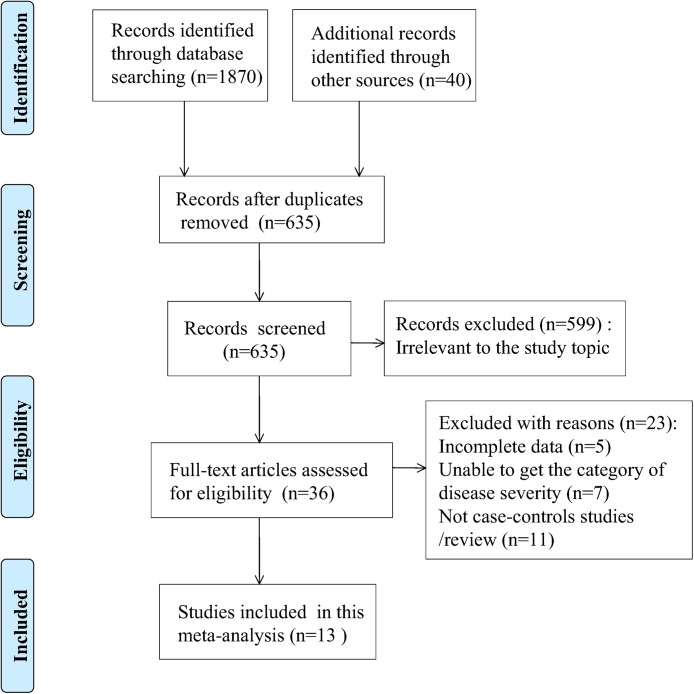
A total of 1910 records were identified (Fig 1). After excluding duplicates and screening the titles of the studies, 635 articles were reviewed. After screening the abstracts of these potentially relevant articles, 36 were selected for full-text review based on relevance to the study topic (Fig 1). Thirteen articles containing seventeen studies (one article included three separate studies and two articles each included two separate studies) and 2501 patients were included[10,13,20,26–35]. In addition, we attempted to contact the corresponding authors of five potentially relevant studies via e-mail. The authors of four studies did not respond to our e-mail communication[36–39], and the corresponding author of the fifth study communicated that we would require approval by their institutional review board for additional individual patient data[40]. Therefore, all five studies had to be excluded from our final set of included studies. Fig 1 shows the selection process for the studies included in the meta-analysis.
Fig 1. Flow chart depicting the selection process for the studies included in the meta-analysis.
Study quality assessment and risk of bias assessment
Using the modified Jadad score (S2 Table), the results of the quality assessment of each included study indicated that five studies were of high quality[10,13,26,27,33] and that the remaining eight studies were of moderate quality[20,28–32,34,35] (Table 1, S2 Table). The results of the assessment of the overall risk of bias for each included study indicated that three reports exhibited a low risk of bias[10,13,26] and that the remaining ten reports exhibited an unclear risk of bias[20,27–35] (Table 1, S1 Fig). The main characteristics of the included studies are shown in Table 1.
Table 1. Main characteristics of the studies included in the meta-analysis.
| Study | Disease Severity | MeanAge | Male(%) | Follow-up(weeks) | Enrolled Patients | Drug Regimen | Case Definitions | AssessmentIndex | EvidenceQuality | Risk of Bias | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||||||
| Met vs. Van | ||||||||||||
| Stuart,2014[13] | Mild/Severe | 65.0 | 47.0 | 4 | 278 | 258 | Met | Van | Method 1 | (1)(3) | High | Low |
| Fred,2007[26] | Mild/Severe | 58.5 | 54.7 | 3 | 90 | 82 | Met | Van | Method 1 | (1)(2)(3) | High | Low |
| Wafa,2008[27] | Mild | 71.0 | NA | 12 | 34 | 18 | Met | Van | Method 1 | (2)(3) | High | Unclear |
| Frank,2012[20] | Mild/Severe | 60.5 | 49.0 | 12 | 128 | 16 | Met | Van | Method 1 | (1)(2)(3) | Moderate | Unclear |
| Jacques-a,2006[28] | Severe | NA | 44.1 | 8 | 115 | 171 | Met | Van | Method 2 | (2)(3) | Moderate | Unclear |
| Enrico,2010[29] | Severe | 53.0 | 50.0 | 4 | 19 | 7 | Met | Van | Method 1 | (1)(2)(3) | Moderate | Unclear |
| Wenisch,1996[30] | NA | 41.0 | 53.2 | 4 | 31 | 31 | Met | Van | Method 1 | (3) | Moderate | Unclear |
| Ethan,2011[31] | Mild | 12.1 | 48.7 | 8 | 37 | 37 | Met | Van | Method 1 | (1) | Moderate | Unclear |
| Sahil,2013[32] | Mild | 2.3 | 54.3 | 12 | 69 | 6 | Met | Van | Method 1 | (1)(2) | Moderate | Unclear |
| Mono vs. Combi | ||||||||||||
| Danny,2006[10] | NA | 69.0 | 41.0 | 4 | 20 | 19 | Met | Met+Rif | Method 1 | (1)(2)(3) | High | Low |
| Bass,2013[33] | Severe | 65.8 | NA | 4 | 35 | 43 | Van | Met+Van | Method 1 | (1)(2)(3) | High | Unclear |
| Jacques-b,2006[28] | Severe | NA | 44.1 | 8 | 115 | 36 | Met | Met+Van | Method 2 | (2)(3) | Moderate | Unclear |
| Jacques-c,2006[28] | Severe | NA | 44.1 | 8 | 171 | 36 | Van | Met+Van | Method 2 | (2)(3) | Moderate | Unclear |
| Mihaela-a,2013[34] | NA | 67.1 | 41.7 | 8 | 132 | 98 | Met | Met+Van | Method 1 | (2) | Moderate | Unclear |
| Mihaela-b,2013[34] | NA | 67.1 | 41.7 | 8 | 76 | 98 | Van | Met+Van | Method 1 | (2) | Moderate | Unclear |
| Sapna-a,2014[35] | NA | 60.0 | 57.5 | 6 | 54 | 13 | Met | Met+Van | Method 1 | (1)(2) | Moderate | Unclear |
| Sapna-b,2014[35] | NA | 60.0 | 57.5 | 6 | 6 | 13 | Van | Met+Van | Method 1 | (1)(2) | Moderate | Unclear |
Abbreviations: T: Treatment (Met or Mono); C: Control (Van or Combi); Met: Metronidazole; Van: Vancomycin; Rif: Rifampin; Mono: Monotherapy group; Combi: Combination therapy group; NA: Not available. Method 1: C. difficile toxin assay and/or a clinical diagnosis; Method 2: C. difficile toxin assay; (1): Rate of clinical cure; (2): Rate of CDI recurrence; (3): AEs.
Efficacy outcomes
Rate of clinical cure
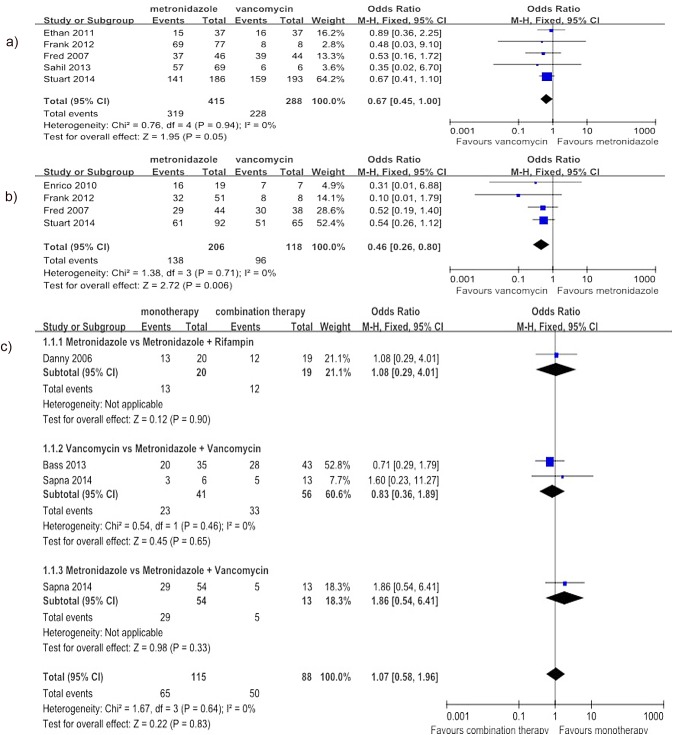
Regarding the comparison of metronidazole with vancomycin, the meta-analysis results suggested that there was no significant difference in the rate of clinical cure for the treatment of mild CDI (5 studies[13,20,26,31,32], 703 patients, OR = 0.67, 95% CI (0.45, 1.00), p = 0.05, I2 = 0%) (Fig 2A). However, the rate of clinical cure was lower for metronidazole than for vancomycin for the treatment of severe CDI (4 studies[13,20,26,29], 324 patients, OR = 0.46, 95% CI (0.26, 0.80), p = 0.006, I2 = 0%) (Fig 2B). Regarding the comparison of monotherapy with combination therapy, three articles were included[10,33,35], and the cases were divided into three subgroups according to therapeutic intervention used. The meta-analysis results did not show a significant difference in the rate of clinical cure between monotherapy and combination therapy (4 studies (one article included two separate studies[35]), 190 patients, OR = 1.07, 95% CI (0.58, 1.96), p = 0.83, I2 = 0%) (Fig 2C).
Fig 2. Forest plot of the rate of clinical cure (a: metronidazole vs. vancomycin for mild CDI; b: metronidazole vs. vancomycin for severe CDI; c: monotherapy vs. combination therapy).
The vertical line indicates no difference between the groups. ORs are represented by diamond shapes, and 95% CIs are depicted by horizontal lines. Squares indicate point estimates, and the size of each square indicates the weight of the given study in the meta-analysis. M-H, Mantel-Haenszel fixed-effects model.
Rate of CDI recurrence
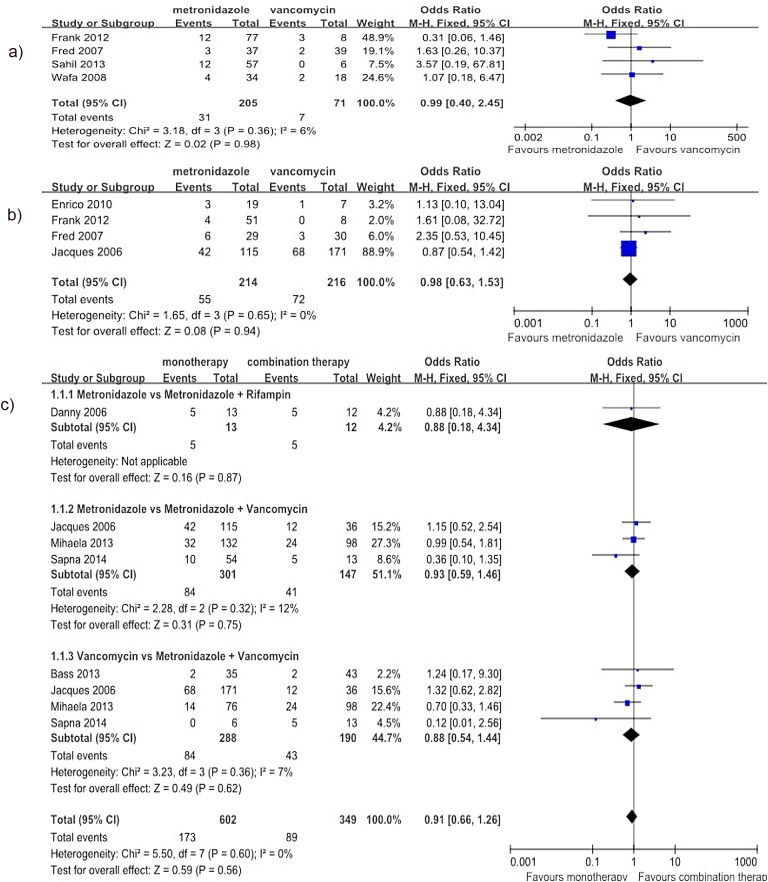
Regarding the comparison of metronidazole with vancomycin, the meta-analysis results did not show any significant difference in the rate of CDI recurrence for the treatment of mild CDI (4 studies[20,26,27,32], 276 patients, OR = 0.99, 95% CI (0.40, 2.45), p = 0.98, I2 = 6%) (Fig 3A) or severe CDI (4 studies[20,26,28,29], 430 patients, OR = 0.98, 95% CI (0.63, 1.53), p = 0.94, I2 = 0%) (Fig 3B). Regarding the comparison of monotherapy with combination therapy, five articles were included[10,28,33–35], and the cases were divided into three subgroups according to the therapeutic intervention. The meta-analysis results did not show any significant difference in the rate of CDI recurrence between monotherapy and combination therapy (8 studies (three articles each included two separate studies[28,34,35]), 804 patients, OR = 0.91, 95% CI (0.66, 1.26), p = 0.56, I2 = 0%) (Fig 3C).
Fig 3. Forest plot of the rate of CDI recurrence (a: metronidazole vs. vancomycin for mild CDI; b: metronidazole vs. vancomycin for severe CDI; c: monotherapy vs. combination therapy).
The vertical line indicates no difference between the groups. ORs are represented by diamond shapes, and 95% CIs are depicted by horizontal lines. Squares indicate point estimates, and the size of each square indicates the weight ofthe given study in the meta-analysis. M-H, Mantel-Haenszel fixed-effects model.
Safety outcomes
AEs
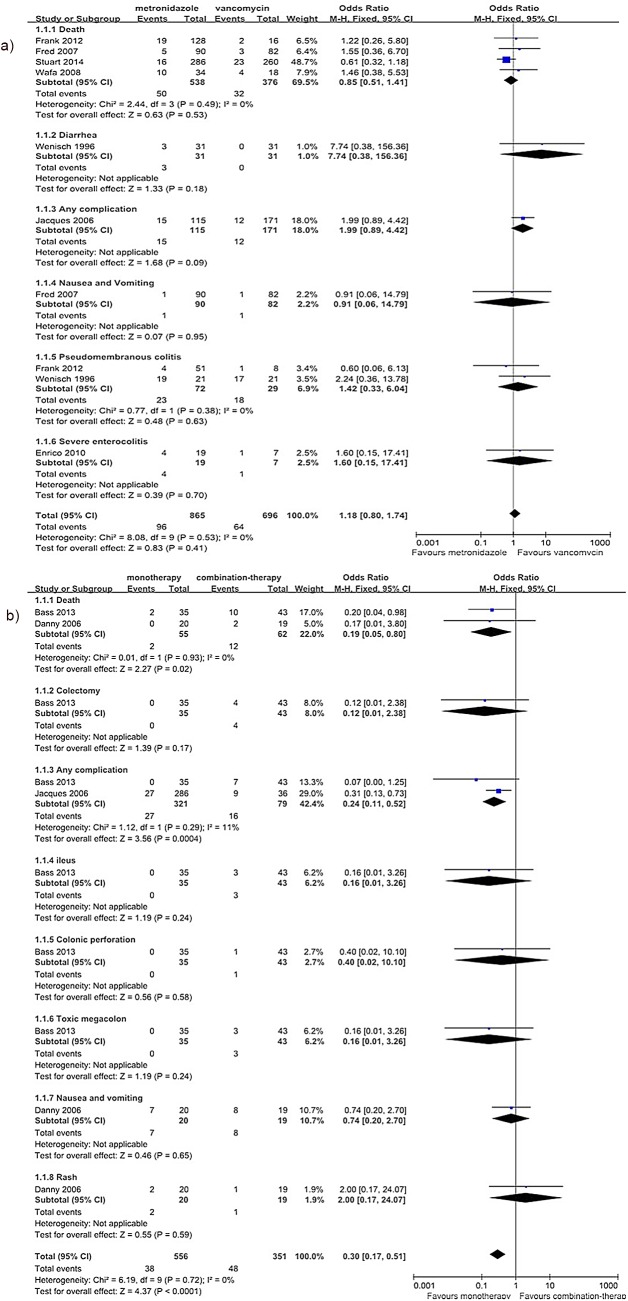
The reported AEs from the included studies consisted of death, colectomy, diarrhea, any complication, ileus, colonic perforation, nausea and vomiting, pseudomembranous colitis, toxic megacolon, rash and severe enterocolitis. We performed subgroup analysis according to the AEs. The meta-analysis results did not show any significant difference in the rate of AEs between metronidazole and vancomycin (the results from 7 studies were separated into six subgroups[13,20,26–30], 1330 patients, OR = 1.18, 95% CI (0.80, 1.74), p = 0.41, I2 = 0%) (Fig 4A). However, the rate of AEs was significantly lower for monotherapy than for combination therapy (the results from 3 studies were separated into eight subgroups [10,28,33], 439 patients, OR = 0.30, 95% CI (0.17, 0.51), p<0.0001, I2 = 0%) (Fig 4B); in fact, this rate was more than 4-fold higher for combination therapy than for monotherapy (46.9% vs.11.1%).
Fig 4. Forest plot of the rate of AEs (a: metronidazole vs. vancomycin; b: monotherapy vs. combination therapy).
The vertical line indicates no difference between the groups. ORs are represented by diamond shapes, and 95% CIs are depicted by horizontal lines. Squares indicate point estimates, and the size of each square indicates the weight of the given study in the meta-analysis. M-H, Mantel-Haenszel fixed-effects model.
Publication bias and sensitivity analyses
The shape of Begg’s funnel plot and the Egger’s test results (all p>0.05) did not demonstrate any evidence of publication bias (S1 File). Considering that meta-analysis showed little heterogeneity (I2 = 6%) between the studies for the comparison of the rate of CDI recurrence between metronidazole and vancomycin. Thus, we conducted sensitivity analyses of the subgroups from these studies according to explicit or inexplicit classifications of severity and follow-up duration to explore underlying sources of heterogeneity. These analyses showed that the primary results were not influenced by the inexplicit classification of severity or by the follow-up durations (S2 File).
Discussion
The aim of this study was to compare the efficacy and safety of metronidazole monotherapy with vancomycin monotherapy and combination therapy in CDI patients. Based on the analyses according to disease severity and the treatment methods used, this study has revealed that metronidazole and vancomycin are equally effective for the treatment of mild CDI but that vancomycin is superior for severe CDI. Additionally, these findings suggest that combination therapy is not superior to monotherapy.
Regarding efficacy, the results of this meta-analysis demonstrated that the treatments were equivalent for mild disease but not for severe disease. Moreover, regarding the cost of the medications, metronidazole is less expensive than vancomycin[41], and oral vancomycin may be more likely to promote the generation and overgrowth of VRE than metronidazole[5]. As a result, metronidazole is recommended for treating mild CDI, and vancomycin is recommended for treating severe CDI. This meta-analysis also demonstrated that combination therapy might not be more effective than monotherapy. Regarding safety, although there was no statistically significant difference in the rate of AEs between metronidazole and vancomycin, there was an increase in the rate of AEs in association with combination therapy, and the combination therapy group experienced more fatal AEs, such as death and complications. Therefore, monotherapy appears to be safe and well tolerated. Nonetheless, it is worth mentioning that because of the limited number of included studies, we did not compare monotherapy with combination therapy for all four categories of disease severity. Moreover, our results may have been influenced by the medical conditions or treatment (e.g., nasogastric feeds[10]) of the patients receiving combination therapy, who may have suffered from more serious illnesses than those receiving monotherapy; therefore, these influences may have been associated with the increased risk of experiencing AEs or developing complications among those receiving combination therapy.
Our study has several limitations. First, although the quality of the majority of the studies was high or medium and although the total sample size was sufficient, the sample size of each subgroup was relatively small and was thus susceptible to false-positive or false-negative results. Second, although sensitivity analyses indicated that the primary results were not influenced by the inexplicit classification of severity or by the follow-up duration, we roughly determined disease severity based on the proportion of patients, which potentially resulted in bias. For example, if 77% of the patients had mild CDI[27], we categorized all of the patients into the mild CDI group. Moreover, the follow-up duration for CDI treatment varied greatly (from 3–12 weeks) among the different studies, which may have resulted in bias. Third, because very few articles presented research on combination therapy, we could not compare the subgroups according to disease severity between monotherapy and combination therapy; therefore, the general patient conditions may have caused prescription bias. Fourth, only studies published in English were included, which might have rendered the results vulnerable to bias related to language and ethnicity. Fifth, additional data on combination therapy using other novel antibiotics are needed.
In conclusion, these findings have important clinical implications and support the recommendations of the current CDI treatment guidelines[3,4]. However, validation of the routine use of combination therapy may require additional evidence. To compensate for the shortcomings of our study, further large-scale clinical trials and well-designed research studies are needed to identify more effective therapies for patients with CDI.
Supporting Information
(DOC)
Review about each risk of bias item for each included study.
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We would like to thank Jennie L. for editing this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Paredes SD, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 2014; 22(7): 406–416. 10.1016/j.tim.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matteo B, Giovanni V, Davide P, Alessandra A, Mark W. Epidemiology, diagnosis and treatment of Clostridium difficile infection. Expert Rev Anti Infect ther. 2012; 10(12): 1405–1423. 10.1586/eri.12.135 [DOI] [PubMed] [Google Scholar]
- 3. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31(5): 431–455. 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 4. Bauer MP, Kuijper EJ, Dissel JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect. 2009; 15(12): 1067–1079. 10.1111/j.1469-0691.2009.03099.x [DOI] [PubMed] [Google Scholar]
- 5. Wafa NA, Ajay KS, Yue JL, Michael JP, Michelle MR, Curtis JD. Both Oral Metronidazole and Oral Vancomycin Promote Persistent Overgrowth of Vancomycin-Resistant Enterococci during Treatment of Clostridium difficile-Associated Disease. Antimicrob Agents Ch. 2008; 52(7): 2403–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tonya C, Emily H, Larry D. Fidaxomicin: A novel macrocyclic antibiotic for the treatment of Clostridium difficile infection. Am J Health-Syst Pharm. 2012; 69(11): 933–943. 10.2146/ajhp110371 [DOI] [PubMed] [Google Scholar]
- 7. Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012; 12(4): 281–289. 10.1016/S1473-3099(11)70374-7 [DOI] [PubMed] [Google Scholar]
- 8. Garey KW, Jiang ZD, Bellard A, Dupont HL. Rifaximin in treatment of recurrent Clostridium difficile-associated diarrhea:an uncontrolled pilot study. J Clin Gastroenterol. 2009; 43(1): 91–93. 10.1097/MCG.0b013e31814a4e97 [DOI] [PubMed] [Google Scholar]
- 9. Johnson S, Schriever C, Patel U, Patel T, Hecht DW, Gerding DN. Rifaximin redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe. 2009; 15(6): 290–291. 10.1016/j.anaerobe.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 10. Danny L, Serena H, Marek S, Fiona S, Christine L. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of clostridium difficile-associated diarrhea. Clin Infect Dis. 2006; 43(5): 547–552. [DOI] [PubMed] [Google Scholar]
- 11. Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006; 43(4): 421–427. [DOI] [PubMed] [Google Scholar]
- 12. Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009; 48(4): 41–46. [DOI] [PubMed] [Google Scholar]
- 13. Stuart J, Thomas JL, Gerding DN, Cornely OA, Chasan TS, Fitts D, et al. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results From Two Multinational, Randomized, Controlled Trials. Clin Infect Dis. 2014; 59(3): 345–354. 10.1093/cid/ciu313 [DOI] [PubMed] [Google Scholar]
- 14. Herpers BL, Vlaminckx B, Burkhardt O, Blom H, Biemond HS, Hornef M, et al. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin Infect Dis. 2009; 48(12): 1732–1735. 10.1086/599224 [DOI] [PubMed] [Google Scholar]
- 15. Van NE, Anne V, Max N, Susana F, Erwin GZ, Willem MV, et al. Duodenal Infusion of Donor Feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368(5): 407–415. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 16. Serina BT. The Role of Vancomycin and Metronidazole for the Treatment of Clostridium difficile–Associated Diarrhea. J Pharm Pract. 2013; 26(5): 488–490. 10.1177/0897190013499525 [DOI] [PubMed] [Google Scholar]
- 17.Louie T, Gerdsom M, Grimard D. Results of a phase III trial comparing tolevamer, vancomycin, and metronidazole in patients with Clostridium difficile associated diarrhea (CDI). Abstract presented at the 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, 17–20 September 2007.
- 18. Horn SD, Sharkey PD, Bertram DA. Measuring severity of illness: homogeneous case mix groups. Med Care. 1983; 21(1): 14–30. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009; 62(10):1–34. [DOI] [PubMed] [Google Scholar]
- 20. Frank L, Vaneet A, Palmer HR, Dhara NS, Miguel S, Hannah R, et al. A Real-World Evaluation of Oral Vancomycin for Severe Clostridium difficile Infection: Implications for Antibiotic Stewardship Programs. Pharmacotherapy. 2012; 32(2): 129–134. 10.1002/PHAR.1002 [DOI] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized Clinical trials: is blinding necessary. Control Clin Trials. 1996; 17(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. Available: http://www.cochrane-handbook.org/ [December 2014]. [Google Scholar]
- 23. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. Brit Med J. 2001; 323(7304): 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jan OF, Neill KJ, Joseph B. Inclusion of zero total event trials in meta-analysesmaintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007; 7(5): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zar FA, Bakkanagari SR, Moorthi KM, Melinda BD. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007; 45(3): 302–307. [DOI] [PubMed] [Google Scholar]
- 27. Wafa NA, Ajay KS, Michelle MN, Greg SB, Robin LP, Curtis JD. Comparison of clinical and microbiological response to treatment of Clostridium difficile–associated disease with metronidazole and vancomycin. Clin Infect Dis. 2008; 47(1): 56–62. 10.1086/588293 [DOI] [PubMed] [Google Scholar]
- 28. Jacques P, Sophie R, Sandra G, Isabel B. Management and Outcomes of a First Recurrence of Clostridium difficile–Associated Disease in Quebec, Canada. Clin Infect Dis. 2006; 42(6): 758–764. [DOI] [PubMed] [Google Scholar]
- 29. Enrico S, Ulrich RM, Brigitte K, Katrin S, Martin M. Clostridium difficile associated diarrhoea, a frequent complication in patients with acute myeloid leukaemia. Ann Hematol. 2010; 89(1): 9–14. 10.1007/s00277-009-0772-0 [DOI] [PubMed] [Google Scholar]
- 30. Wenisch C, Parschalk B, Hasenhiindl M, Hirschi AM, Graninger W. Comparison ofVancomycin, Teicoplanin, Metronidazole, and Fusidic Acid for the Treatment of Clostridium difficile-Associated Diarrhea. Clin Infect Dis. 1996; 22(5): 813–818. [DOI] [PubMed] [Google Scholar]
- 31. Ethan M, Elizabeth AM, Kim WH, Christopher JL, Mitchell BC. Clostridium difficile Infection and Treatment in the Pediatric Inflammatory Bowel Disease Population. J Pediatr Gastr Nutr. 2011; 52(4): 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sahil K, Larry MB, Charles HW, Patricia PK, William AF, Alan RZ, et al. The Epidemiology of Clostridium difficile Infection in Children: A Population-Based Study. Clin Infect Dis. 2013; 56(10): 1401–1406. 10.1093/cid/cit075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bass SN, Bauer SR, Neuner EA, Lam SW. Comparison of treatment outcomes with vancomycin alone versus combination therapy in severe Clostridium difficile infection. J Hosp Infect. 2013; 85(1): 22–27. 10.1016/j.jhin.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 34. Mihaela L, Mirela F, Andreea C, Irina F, Nicolae T. Predictors of First Recurrence in Clostridium diffcile-Associated Disease. A Study of 306 Patients Hospitalized in a Romanian Tertiary Referral Center. J Gastrointestin Liver Dis. 2013; 22(4): 397–403. [PubMed] [Google Scholar]
- 35. Sapna RP, Valkal B, Jie Y, Qiao Z, Michael S. A retrospective review of metronidazole and vancomycin in the management of Clostridium difficile infection in patients with hematologic malignancies. J Oncol Pharm Pract. 2014; 20(3): 172–182. 10.1177/1078155213490004 [DOI] [PubMed] [Google Scholar]
- 36. Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile–associated diarrhea and colitis. Lancet. 1983; 2: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 37. Morinville V, McDonald J. Clostridium difficile–associated diarrhea in 200 Canadian children. Can J Gastroenterol. 2005; 19(8): 497–501. [DOI] [PubMed] [Google Scholar]
- 38. Anilrudh AV, Kathleen R, Shilpa MP, Susanna S, Houssein J, Sharon V, et al. Lack of association of outcomes with treatment duration and microbiologic susceptibility data in Clostridium difficile infections in a non-NAP1/BI/027 setting. Scand J Infect Dis. 2012; 44: 243–249. 10.3109/00365548.2011.631029 [DOI] [PubMed] [Google Scholar]
- 39. Jardin CG, Palmer HR, Shah DN, Le F, Beyda ND, Jiang Z, et al. Assessment of treatment patterns and patient outcomes before vs after implementation of a severitybased Clostridium difficile infection treatment policy. J Hosp Infect. 2013; 85: 28–32. 10.1016/j.jhin.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 40. Yi CL, Jann TW, An CC, Wang HS, Shan CC, Yee CC. Changing incidence and clinical manifestations of Clostridium difficile-associated diarrhea detected by combination of glutamate dehydrogenase and toxin assay in Northern Taiwan. J Microbiol Immunol. 2012; 45: 287–295. [DOI] [PubMed] [Google Scholar]
- 41. Christine P, Eva T, Sarah N, John C, Louis V, Kelly F. Vancomycin or Metronidazole for Treatment of Clostridium difficile Infection: Clinical and Economic Analyses. CADTH Technology Report. 2011; 1(136): 1–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Review about each risk of bias item for each included study.
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.