Abstract
Although some morphological investigations on aged human sublingual glands (HSG) found eventual phenomena identified as autolysis and mucous extravasation, the exact meaning of these findings has not been elucidated.
Objective
The aim of this work is to investigate whether acinar autolysis and mucous extravasation are related to the aging process in human sublingual glands. We also speculate if autolytic changes may assist forensic pathologists in determining time of death.
Material and Methods
186 cadavers’ glands were allocated to age groups: I (0–30 years); II (31–60), and III (61–90). Time and mode of death were also recorded. Acinar autolysis and mucous extravasation were classified as present or absent. Ultrastructural analysis was performed using transmission electron microscopy (TEM). Data were compared using Mann-Whitney U, Spearman’s correlation coefficient, Kruskal-Wallis, and Dunn tests (p<0.05).
Results
There was correlation between age and acinar autolysis (r=0.38; p=0.0001). However, there was no correlation between autolysis and time of death. No differences were observed between genders. TEM showed mucous and serous cells presenting nuclear and membrane alterations and mucous cells were more susceptible to autolysis.
Conclusion
Acinar autolysis occurred in all age groups and increased with age while mucous extravasation was rarely found. Both findings are independent. Autolysis degrees in HSG could not be used to determine time of death.
Keywords: Aging, Salivary glands, Histology, Autolysis, Forensic Dentistry
INTRODUCTION
Age-related microscopic changes have been reported for the major 2 , 5 , 6 , 8 , 11 , 19 and minor 15 , 18 , 21 , 22 salivary glands of humans 2 , 5 , 8 , 14 , 15 , 18 , 19 , 21 , 22 and rats 6 , 11 . Main microscopic aspects described were: replacement of parenchyma by fat and connective tissue 2 , 8 , 15 , 21 , acinar atrophy and increase in the number of duct/duct-like structures 2 , 6 , 8 , 15 , 19 , 21 , focal and diffuse mononuclear infiltration 2 , oncocytosis 2 , 8 , 15 , and congested blood vessels 2 . In a previous study of age-related changes in human sublingual glands (HSG) 2 , in addition to the main microscopic aspects described, occasional phenomena such as acinar autolysis and mucous extravasation were also observed.
Mucous extravasation seems to be associated with acinar autolysis since they often appear together 2 . Autolysis is the enzymatic digestion of cells by the action of its own enzymes, and it mostly occurs in dying or dead cells 4 . An acinus is considered as autolyzed when it presents the minimum sign of loss of cell limit, regardless of the degree 11 . It is normally associated with autopsies and recognition of the phenomenon is very important to elucidate forensic cases 23 . Nery, et al. 11 (2010) studied sublingual glands (SG) of rats and demonstrated temporal progressive signs of acinar autolysis. Postmortem autolytic changes have also been described in heart 9 , 20 , pancreas 16 , 20 , kidneys 20 , liver 20 , skeletal muscle 20 , temporal bones 10 , blood cells 13 , blood vessels 12 , sweat glands 3 , and heart muscle 1 .
Although some previous investigations on HSG found contingent phenomena identified as autolysis and/or mucous extravasation 2 , 5 , 8 , 11 , the exact meaning of these findings has not been elucidated yet. Information is scarce concerning postmortem autolytic changes in the acini of SG, especially to distinguish these from pathological causes before death or others artifacts. According to some authors, postmortem autolysis depends on various factors and the most important is postmortem interval 9 , 10 , 16 , 17 . However, some of these postmortem autolytic changes are similar to alterations also described as apoptosis 20 and inadequate/delayed fixation 7 , 10 , 12 . It explains why these occasional findings can be misdiagnosed as fixation failures. Additionally, it is speculated if these phenomena may be a result of surgical dissection trauma during glandular removal. It seems evident that these contingent microscopic alterations may theoretically represent a potential problem for pathologists and surgeons 4 . Therefore, the aim of the present study is to document age-related changes of HSG of cadavers with regard to acinar autolysis and mucous extravasation. Elucidation of these alterations could assist researchers and pathologists in recognizing the phenomena and discard pathological areas and other artifacts. Moreover, autolytic changes may assist forensic pathologists in determining time of death.
MATERIAL AND METHODS
The Human Research Ethics Committee of the Bauru School of Dentistry – University of São Paulo (Process No. 059/2010) approved this study, which followed the guidelines of the Helsinki Declaration.
A total of 186 HSG were obtained bilaterally from 93 cadavers during necropsies at the São Paulo Death Verification Service (School of Medicine, University of São Paulo) using the methods and the exclusion criteria of a previous study 2 . Glands were dissected intact via a wide neck flap. Following the inferior cortical of the body of the mandible, soft tissues were dissected and the lower edge of the sublingual gland was accessed. Dissection extended into the floor of the mouth, without perforating it. Geniohyoid and genioglossus muscles were released with scissors and the tongue and suprahyoid muscle were pulled to the opposite side with an Allis forceps. Then, the gland was completely visible, attached to the tongue muscles, and was dissected with blunt scissors, from its inferior edge to the floor of the mouth, which was finally incised with a scalpel. Each piece carried a longitudinal strip of the floor of the mouth, approximately 1 cm wide. This strip worked as a reference for handling the sample. The same surgeon collected all glands and no differences or difficulties were found according to the postmortem interval. Glands with macroscopic autolysis were excluded 2 .
Cadavers (49 men/44 women) were divided into 9 decades of life, with age ranging from 7 months to 90 years. Considering that the present work is a continuation of a previous study 2 , the model of sample’s distribution is the same used before (Figure 1).
Figure 1. Distribution of individuals according to age (decade of life) and gender.
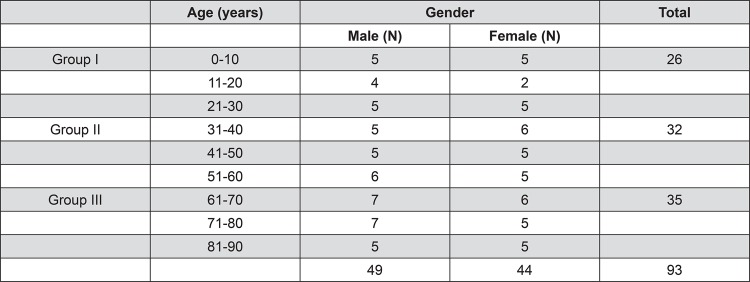
Approximately 5 cadavers of each gender per decade of life were studied. The sample was divided into 3 age groups: group I (≤30 years): 26 cadavers - 14 men (mean age: 14.8 years) and 12 women (mean age: 13.4 years); group II (31–60 years): 32 cadavers - 16 men (mean age: 47.0 years) and 16 women (mean age: 44.6 years); group III (≥ 61 years): 35 cadavers - 19 men (mean age: 74.5 years) and 16 women (mean age: 73.7 years). Data regarding cause of death were obtained from autopsy reports. According to internal rules of the Death Verification Service, no necropsies could be processed before 6 hours of arrival of the body. Interval between time of death and necropsy ranged from 6:05 to 92:55 h, with a mean of 16:46 h. The variable “time of death” was then considered for the purpose of elucidating the influence of postmortem interval on morphological appearance, which could reflect the original physiological state before death.
After fixation with phosphate-buffered formalin solution for 1 week at room temperature, specimens were cut into 5 mm thick slices. Regardless of the size of the gland, only 3 slices (anterior, middle, and posterior) were processed histologically. Alternate 5 µm thick sections were stained with hematoxylin and eosin (H.E.) and a single pathologist performed the microscopic examinations 2 .
Microscopic examination was performed in a light microscope Carl Zeiss MicroImaging GmbH (Carl Zeiss MicroImaging, Jena, Thuringia, Germany) using x5, x10, x40, and x100 sequential objectives, which resulted in original magnifications of x12.5, x25, x100, and x250, respectively. The whole microscopic slide was examined from the superficial portion of the gland section subjacent to the oral mucosa to the depth, and from anterior to posterior borders 2 .
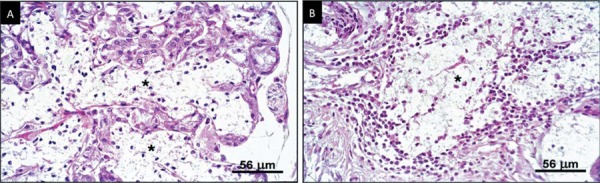
An acinus was counted as autolyzed when it presented the minimum sign of loss of cell limit, regardless of the degree 11 (Figure 2A). Mucous extravasation was sparsely distributed in the glandular parenchyma (Figure 2B).
Figure 2. A: Acinar autolysis (*), Male, 49 years old (H.E. Original magnification: x100); B: Mucous extravasation (*), Female, 63 years old (H.E. Original magnification: x100). H.E.=Hematoxylin & eosin.
Acinar autolysis and mucous extravasation were occasional findings classified as present or absent. When present, a degree of severity was attributed to them 15 . Microscopic findings (sign of loss of cell limits and/or presence of mucous sparsely distributed in the glandular parenchyma) were classified as discrete when observed in up to 1/3 of the section, moderate when involving 1/3–2/3 of the section, and intense when more than 2/3 of the section was affected. Scores were attributed to those microscopic aspects, ranging from 0 to 3, with 0: absent, 1: discrete, 2: moderate, and 3: intense.
As 3 initial scores were obtained for right gland and 3 initial scores for left gland, we had to obtain one intermediate score to represent the right gland and one intermediate score to represent the left one. Thus, the 3 initial scores of right gland were added, resulting in only one value (Intermediate score I), which varied from 0 (if all the initial scores were 0 - absent) to 9 (if the 3 initial scores were 3 - intense). This value was submitted to a conversion scale, so that, if it were 0, the intermediate score would be 0. If it were 1–3, the intermediate score would be 1; if it was 4–6, the intermediate score would be 2, and if it was 7–9, the intermediate score would be 3. The same steps were repeated for the left gland, and finally only two intermediate scores were obtained (Intermediate score II). When both of them were added, we obtained one value that varied from 0 (if both scores were absent) to 6 (if both scores were intense). Then, a second conversion scale was applied, in the same way as the latter, so that just one value (final score) could be obtained, representative of the degree of severity of each microscopic finding 2 . An example of this score’s conversion is illustrated in the scheme bellow.
Observer reproducibility was assured as follows: the slides of 24 HSG from 12 individuals were presented blindly and randomly to the same observer. After 2 months, microscopic analysis was re-initiated and the same slides were presented to the observer again. Kappa statistics measured observer reproducibility. Results obtained from each of the microscopic aspects were: acinar autolysis (K=0.72) and mucous extravasation (K=0.85).
One sublingual gland of a male cadaver (71 years) with advanced macroscopic autolysis was removed and processed for transmission electron microscopy (TEM). Guided by the light microscopy, the points of possible autolysis were captured for examination.
The correlation between acinar autolysis/mucous extravasation and chronological age was carried out by Spearman’s correlation coefficient. The microscopic aspects that showed a medium and low correlation (r<0.60) and were significant were submitted to Kruskal-Wallis and Dunn tests (for comparison between age groups I, II, and III). The Mann-Whitney U test was used to analyze the relationship between microscopic aspects and gender. Level of significance was set at 5% for all tests and the software Pacotico v.0.5, Microsoft Visual FoxPro, was used.
RESULTS
An acinus was counted as autolyzed when it presented the minimum sign of loss of cell limit, regardless of the degree 11 . The loss of integrity of the external limits was observed particularly in the mucous acini (Figure 3).
Figure 3. Acinar autolysis. Loss of acinar cell limits and disperse nuclei. Male, 85 years old (H.E. Original magnification: x400). H.E.=Hematoxylin & eosin.
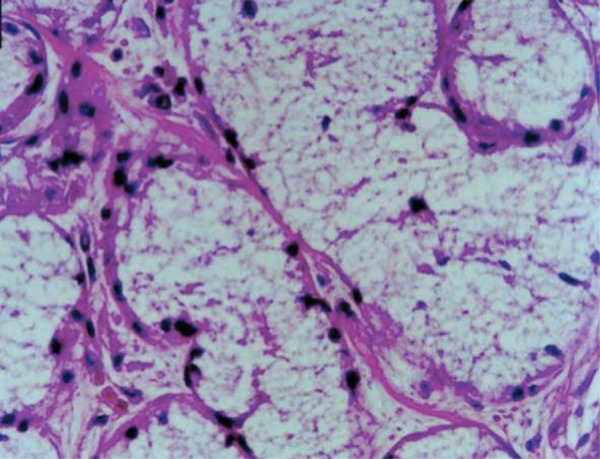
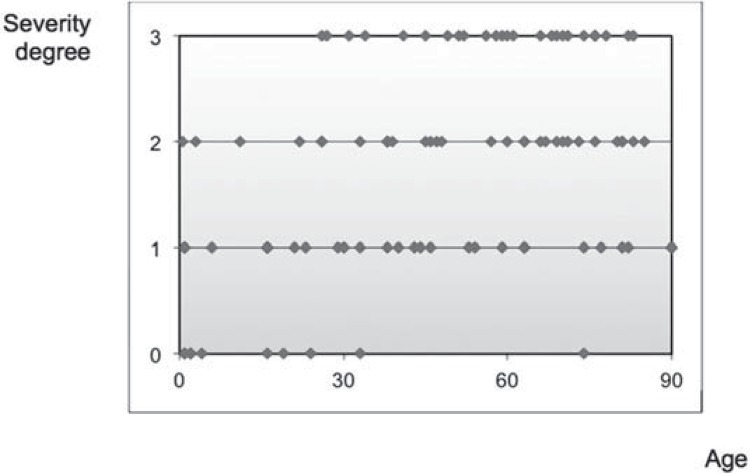
Acinar autolysis was observed on nearly the entire sample (Figure 3). The phenomenon was not found in only 10 cadavers (8 in Group I, 1 in Group II, and 1 in Group III) (Table 1). While, in Group I, 18 individuals (69.23%) presented acinar autolysis with degrees 0 or 1, in group III 26 individuals (74.29%) presented degrees 2 or 3. The correlation between age and the acinar autolysis was low, but significant (r=0.38; p=0.0001). There were significant difference between groups I and II, and between groups I and III (H=16.81; p=0.0002) (Table 1).
Table 1. Distribution of the different degrees of acinar autolysis according to the age group.
| Age group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||
| N | % | N | % | N | % | N | % | N | |
| Ia | 8 | 30.77 | 10 | 38.46 | 6 | 23.08 | 2 | 7.69 | 26 |
| IIb | 1 | 3.13 | 9 | 28.13 | 11 | 34.38 | 11 | 34.38 | 32 |
| IIIb | 1 | 2.86 | 8 | 22.86 | 14 | 40.00 | 12 | 34.29 | 35 |
| 10 | 27 | 31 | 25 | 93 | |||||
H=16.81; p=0.0002 * Groups followed by the same letter did not differ from one another.
Figure 4 shows the acinar autolysis phenomenon distribution and the different degrees of severity in the groups. It is important to emphasize the absence of autolysis (degree 0) and the low degrees of severity in the young individuals.
Figure 4. Distribution of the different degrees of acinar autolysis according to age.
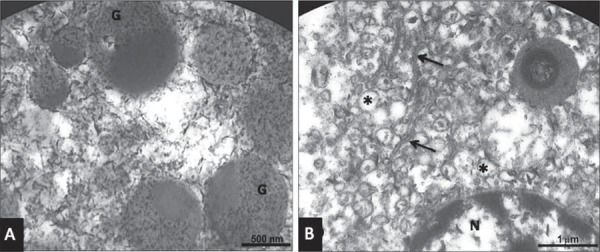
TEM examination showed mucous cells presenting granulations and loss of membrane integrity, with their contents partially degraded, and absence of nucleus and organelles (Figure 5). In the serous acini, secretion granules were partially preserved and cells still presented cytoplasmic organelles and nuclei integrity despite the loss of membrane integrity (Figure 6).
Figure 5. Transmission electron microscopy (TEM) of a human sublingual gland with advanced autolysis (male, 71 years old). A: Secretion granules (SG) of mucous cells with partially degraded content; B: Secretion granules (SG) with membrane rupture and degradation of content.

Figure 6. Transmission electron microscopy (TEM) of a human sublingual gland with advanced autolysis (male, 71 years old). A: Secretion granules of a serous cell (G) with partially preserved content and destruction of cytoplasmic organelles; B: Cisternae (arrow) and vesicles (*) of the rough endoplasmic reticulum and preserved nucleus (N) of a serous cell.
Despite there were cases of mucous extravasation, these phenomena were rarely found. They occurred mainly at degree 1 (Figure 2B), with the values dispersed in the different ages (Table 2). There was no significant correlation between age and mucous extravasation (p=0.6976).
Table 2. Distribution of the different degrees of diffuse mucous extravasation according to the aging.
| Age group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||
| N | % | N | % | N | % | N | % | N | |
| I | 9 | 34.62 | 10 | 38.46 | 6 | 23.08 | 1 | 3.85 | 26 |
| II | 4 | 12.50 | 17 | 53.13 | 8 | 25.00 | 3 | 9.38 | 32 |
| III | 11 | 31.43 | 15 | 42.86 | 9 | 25.71 | 0 | 0.00 | 35 |
| 24 | 42 | 23 | 4 | 93 | |||||
Variables acinar autolysis and mucous extravasation showed no significant differences between male and female genders (p>0.05). Considering the variable “time of death”, there was no significant correlation between autolysis and postmortem interval (p>0.05). Main causes of death included acute and chronic diseases, such as pulmonary edema, bronchopneumonia, acute myocardial infarction, cerebral vascular accident, ischemic heart disease, cerebral edema, and congestive heart failure.
DISCUSSION
When an individual dies, cells and their structures lose integrity, and endogenous enzymes begin to break down the body tissue by autolysis 17 . Postmortem autolytic changes have been described in many organs and tissues 3 , 9 , 10 , 12 , 13 , 16 , 20 . We did not find studies related to the acinar autolysis process in HSG. It is known that many microscopic changes in SG occur with the aging process 2 , 8 , 19 . Autolysis and mucous extravasation are only mentioned in some postmortem 2 , 8 , 11 and in vivo studies 5 in human 2 , 5 , 8 , 19 and animal 11 SG as occasional findings. However, these phenomena are not clarified or even described in the literature.
Shimizu, et al. 16 (1990) found a high correlation between autolysis and postmortem interval in the pancreas. According to the authors, in general, it is true that the longer the postmortem interval, the more extensive the autolysis 16 . Nevertheless, they agree that there were some cases in which the pancreas was unexpectedly well preserved irrespective of the postmortem interval (i.e., no autolysis at a postmortem interval of 40 h and, in some cases, poorly preserved at a postmortem interval of 2 h) 16 . In the present investigation, there was no significant correlation between autolysis and postmortem interval. It is known that autolysis depends on various factors 10 , 20 , such as body temperature and environmental temperature, as well as mode of death 16 . The study of Shimizu, et al. 16 (1990) suggests that mode of death, temperature at time of death, as well as various causes of death and clinical conditions (i.e., alcoholism, hypertension, smoking, allergy) need to be taken into account with respect to those cases of unexpectedly severe or mild postmortem autolysis, which can occur regardless of a short or long duration of the postmortem interval. In forensic practice, factors influencing postmortem degeneration are extremely diverse 20 . Further studies would be helpful to clarify the precise effects of various conditions before death, and of environmental conditions after death, on the time course of postmortem ultrastructural changes 20 .
Considering mode of death, individuals that die by chronic processes (as malignant neoplasias, cardiovascular and respiratory chronic diseases, diabetes mellitus, etc.) have less enzymes or less enzymatic activity than those who die due by acute processes 16 . Therefore, when death is acute, autolysis is severe, and when death is chronic, autolysis is moderated 16 . Our sample was mixed, with deaths caused by acute and chronic diseases, which may explain the differences in the degrees of autolysis in the different individuals (Table 1).
Regarding ultrastructural cellular alterations, our findings were similar to the changes described in SG of rats 11 and in other cell types 3 , 10 , 12 , 13 , 20 . In the present study, TEM examination was also conducted in order to give information regarding the autolytic process in both serous and mucous acini and whether the autolytic process was different between both types of acini. SG are mixed glands, composed by both serous and mucous acini. Maybe one type of acini would be more susceptible to the autolytic process than the other. TEM examination showed mucous cells presenting partial or complete loss of limits and a disorganized cytoplasm, with their contents partially degraded. The nuclei and organelles were absent (Figure 5). In the serous acini, the secretion granules were partially preserved and the cells still presented cytoplasmic organelles. Nuclei integrity was preserved, despite the loss of membrane integrity (Figure 6). In other words, we observed that a greater resistance to autolysis occurred in serous cells when compared to mucous cells, corroborating studies in both humans 2 , 8 , 21 and rats 11 . Contrary to previous reports, Tandler, et al. 18 (1969), who studied human labial glands that are pure mucus in nature, considered that serous acinar cells may represent immature mucous cells, while mucous cells are mature and less susceptible to morphological age-related changes.
Some of these postmortem autolytic changes are similar to alterations also described as apoptosis 20 and inadequate/delayed fixation 7 , 10 , 12 . As stated by Margarone, et al. 7 (1985), acini of minor salivary glands are especially susceptible to delay in fixation, therefore presenting artifacts similar to postmortem autolytic changes. Nuclear alterations similar to apoptosis 20 or fixation artefact 1 have been described for different organs of the body, including the SG 11 . It is worth pointing out that nuclear changes were also observed in the mucous acinar cells of the present study.
In our study, the SG were fixed in phosphate-buffered formalin solution for 1 week at room temperature respecting all technical principles of fixation. We do not believe that the occasional events of autolysis were caused by fixation due to the following reasons: 1 - autolysis should be greater in the center of the gland because penetration of the solution is centripetal. However, the areas of autolysis were scattered in the glandular parenchyma and did not obey any direction or arrangement; 2 - the SG of the elderly were smaller macroscopically. Thus, the solution penetrated the whole gland more easily, avoiding the autolysis process; 3- Autolysis can be prevented by fixation of the tissue with formalin because the enzymes in the cells are thus inactivated 16 . There was significant correlation between autolysis and chronological age of the cadavers. In addition, 3 glands remained in saline solution for 24, 48, and 72 h before fixing and processing them with the same criteria of the studied material. To our surprise, the gland preserved its histological architecture up to 48 h, allowing the histological study. At 72 h, the architecture was microscopically damaged. Thus, fixation fails were not responsible for the autolysis artifacts occasionally found.
We did not find studies that associate autolysis and/or mucous extravasation with age. In our investigation, there was significant correlation between acinar autolysis and the aging process (r=0.38; p=0.0001), despite some degree of autolysis being present in all ages. The high degrees of autolysis in our material were found in glands of adult and old individuals (Table 1). This is an unprecedented finding. We found no studies relating the autolysis process and the aging, even because this phenomenon is usually investigated in postmortem studies and not in works related to senile changes. It seems evident that the process of autolysis is not exclusively a postmortem event, since Sá, et al. 14 (2013) found this phenomenon in patients in vivo. However, association with the aging was not investigated by the authors 14 . We believe that autolysis integrates a set of changes already proven in the salivary glands of the elderly. However, the explanation for these changes has not been elucidated yet. We can infer that the reduction in the production of saliva with aging plays a role in the glandular physiology, resulting in various morphological changes. On the other hand, the opposite could also be considered. Extrapolating this data to the clinic, we understand that the junction of all age-related microscopic changes may explain the decrease in glandular function 19 .
There was no significant correlation between mucous extravasation and age (Table 2). Therefore, although mucous extravasation and acinar autolysis often appear together 2 , the contradictory results when both were correlated to age discards the speculation that mucous extravasation is a result of acinar autolysis. That is why we consider both as “distinct occasional findings”.
Despite autolysis increasing with age, young glands also presented this alteration, as well as mucous extravasation. We hypothesized that this phenomenon may be a result of surgical dissection trauma during glandular removal, even with the surgical principles being respected. We highlight the possible association of acinar autolysis and mucous extravasation with surgical dissection because, in microscopic studies of HSG in vivo 5 , these alterations were also found. In the study of Nery, et al. 11 (2010), isolated autolytic changes were found even in the 0 h group. Pallot, et al. 12 (1992) stated that some cells show autolytic changes within the few minutes required to complete the organ dissection. We agree with these authors and speculate that SG manipulation during dissection could promote the occurrence of this condition. An investigation between surgical trauma and postmortem interval should be conducted to highlight the phenomenon.
In addition to the other microscopic age-related changes in HSG 2 , 5 , 8 , acinar autolysis and mucous extravasation had no significant differences between males and females.
We agree with Hyunn, et al. 4 (2012) that autolysis (as well as all occasional microscopic alterations) may theoretically represent a potential problem for pathologists and surgeons. It is important to know that these phenomena are inherent to the aging process, but that they can also be a result of surgical manipulation. This knowledge avoids incorrect diagnosis. Surgeons and pathologists should communicate with each other for reliable diagnosis and decision.
CONCLUSION
Aged human sublingual glands are more susceptible to acinar autolysis. However, mucous extravasation is an age-independent and rare finding that can be a result of the surgical trauma when the glands are removed. This trauma may also contribute to autolysis, mainly when this phenomenon is sectorial. The autolysis degrees in HSG could not be used to determine time of death. Although postmortem interval is an important factor, autolysis depends on various factors.
REFERENCES
- 1.Armiger LC, Seelye RN, Carnell VM, Smith CU, Gavin JB, Herdson PB. Morphologic and biochemical changes in autolysing dog heart muscle. Lab Invest. 1976;34(4):357–362. [PubMed] [Google Scholar]
- 2.Azevedo LR, Damante JH, Lara VS, Lauris JR. Age-related changes in human sublingual glands: a post mortem study. Arch Oral Biol. 2005;50(6):565–574. doi: 10.1016/j.archoralbio.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Cingolani M, Osculati A, Tombolini A, Tagliabracci A, Ghimenton C, Ferrara SD. Morphology of sweat glands in determining time of death. Int J Legal Med. 1994;107(3):132–140. doi: 10.1007/BF01225600. [DOI] [PubMed] [Google Scholar]
- 4.Hyunn JJ, Chun HJ, Keum B, Seo YS, Kim YS, Jeen YT, et al. Autolysis: a plausible finding suggestive of long ESD procedure time. Surg Laparosc Endosc Percutan Tech. 2012;22(2):e115–e117. doi: 10.1097/SLE.0b013e318247c347. [DOI] [PubMed] [Google Scholar]
- 5.Iwaki L, Filho, Damante JH, Consolaro A, Bonachela WC, Damante CA. Mouth floor enlargements related to the sublingual glands in edentulous or partially edentulous patients: a microscopic study. J Appl Oral Sci. 2006;14(4):264–269. doi: 10.1590/S1678-77572006000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Denny PA, Denny P. The effect of ageing on parenchymal cell populations in adult female mouse submandibular gland. Arch Oral Biol. 2000;45(7):585–592. doi: 10.1016/s0003-9969(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 7.Margarone JE, Natiella JR, Vaughan CD. Artifacts in oral biopsy specimens. J Oral Maxillofac Surg. 1985;43(3):163–172. doi: 10.1016/0278-2391(85)90154-5. [DOI] [PubMed] [Google Scholar]
- 8.Moreira CR, Azevedo LR, Lauris JR, Taga R, Damante JH. Quantitative age-related differences in human sublingual gland. Arch Oral Biol. 2006;51(11):960–966. doi: 10.1016/j.archoralbio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz DR, Almeida M, Lopes EA, Iwamura ES. Potential definition of the time of death from autolytic myocardial cells: a morphometric study. Forensic Sci Int. 1999;104(2-3):81–89. doi: 10.1016/s0379-0738(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 10.Nadol JB, Jr, Burgess B. A study of postmortem autolysis in the human organ of Corti. J Comp Neurol. 1985;237(3):333–342. doi: 10.1002/cne.902370305. [DOI] [PubMed] [Google Scholar]
- 11.Nery LR, Moreira CR, Cestari TM, Taga R, Damante JH. Postmortem acinar autolysis in rat sublingual gland: a morphometric study. J Appl Oral Sci. 2010;18(5):509–514. doi: 10.1590/S1678-77572010000500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallot DJ, Seker M, Abramovici A. Post-mortem changes in the normal rat carotid body: possible implications for human histopathology. Virchows Arch A Pathol Anat Histopathol. 1992;420(1):31–35. doi: 10.1007/BF01605981. [DOI] [PubMed] [Google Scholar]
- 13.Penttilä A, Laiho K. Autolytic changes in blood cells of human cadavers. II. Morphological studies. Forensic Sci Int. 1981;17(2):121–132. doi: 10.1016/0379-0738(81)90004-9. [DOI] [PubMed] [Google Scholar]
- 14.Sá JC, Tolentino ES, Azevedo-Alanis LR, Iwaki L, Filho, Lara VS, Damante JH. Morphology and morphometry of the human sublingual glands in mouth floor enlargements of edentulous patients. J Appl Oral Sci. 2013;21(6):540–546. doi: 10.1590/1679-775720130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale. 1980;8(3):187–200. [PubMed] [Google Scholar]
- 16.Shimizu M, Hayashi T, Saitoh Y, Ohta K, Itoh H. Postmortem autolysis in the pancreas: multivariate statistical study. The influence of clinicopathological conditions. Pancreas. 1990;5(1):91–94. doi: 10.1097/00006676-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sterzik V, Belenkaia L, Liehr AW, Bohnert M. Spectrometric evaluation of post-mortem optical skin changes. Int J Legal Med. 2014;128(2):361–367. doi: 10.1007/s00414-013-0855-2. [DOI] [PubMed] [Google Scholar]
- 18.Tandler B, Denning CR, Mandel ID, Kutscher AH. Ultrastructure of human labial salivary glands. I. Acinar secretory cells. J Morphol. 1969;127(4):383–407. doi: 10.1002/jmor.1051270402. [DOI] [PubMed] [Google Scholar]
- 19.Tolentino ES, Soares CT, Honório HM, Damante JH. Phenotype and cell proliferation activity of duct-like structures in human sublingual glands: a histological and immunohistochemical study. J Appl Oral Sci. 2015 doi: 10.1590/1678-775720140349. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita Y, Nihira M, Ohno Y, Sato S. Ultrastructural changes during in situ early postmortem autolysis in kidney, pancreas, liver, heart and skeletal muscle of rats. Legal Med. 2004;6(1):25–31. doi: 10.1016/j.legalmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Vered M, Buchner A, Boldon P, Dayan D. Age-related histomorphometric changes in labial salivary glands with special reference to the acinar component. Exp Gerodontol. 2000;35(8):1075–1084. doi: 10.1016/s0531-5565(00)00129-7. [DOI] [PubMed] [Google Scholar]
- 22.Vered M, Buchner A, Haimovici E, Hiss Y, Dayan D. Focal lymphocytic infiltration in aging human palatal salivary glands: a comparative study with labial salivary glands. J Oral Pathol Med. 2001;30(1):7–11. doi: 10.1034/j.1600-0714.2001.300102.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Yamamoto Y, Matsumoto H, Hayase T, Ojima K, Matsubayashi K, et al. Unusual post-mortem autolytic change in the liver: wavy transformation of hepatocytes. Med Sci Law. 1997;37(3) doi: 10.1177/002580249703700312. [DOI] [PubMed] [Google Scholar]