Abstract
In Aggregatibacter actinomycetemcomitans, different serotypes have been described based on LPS antigenicity. Recently, our research group has reported a differential immunogenicity when T lymphocytes were stimulated with these different serotypes. In particular, it was demonstrated that the serotype b of A. actinomycetemcomitans has a stronger capacity to trigger Th1- and Th17-type cytokine production.
Objective
This study aimed to quantify the expression of different CC chemokines (CCLs) and receptors (CCRs) in T lymphocytes stimulated with the different A. actinomycetemcomitans serotypes. In addition, the expression of the transcription factors T-bet, GATA-3, RORC2, and Foxp3, master-switch genes implied in the Th1, Th2, Th17, and T-regulatory differentiation, respectively, was analysed in order to determine T-cell phenotype-specific patterns of CCL and CCR expression upon A. actinomycetemcomitans stimulation.
Material and Methods
Human naïve CD4+ T lymphocytes were obtained from healthy subjects and stimulated with autologous dendritic cells primed with the different A. actinomycetemcomitans serotypes. The expression levels for the chemokines CCL1, CCL2, CCL3, CCL5, CCL11, CCL17, CCL20, CCL21, CCL25, and CCL28, as well as the chemokine receptors CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 were quantified by qPCR. Similarly, the expression levels for the transcription factors T-bet, GATA-3, RORC2, and Foxp3 were quantified and correlated with the CCL and CCR expression levels.
Results
Higher expression levels of CCL2, CCL3, CCL5, CCL20, CCL21, CCL28, CCR1, CCR2, CCR5, CCR6, CCR7, and CCR9 were detected in T lymphocytes stimulated with the serotype b of A. actinomycetemcomitans compared with the other serotypes. In addition, these higher expression levels of CCLs and CCRs positively correlated with the increased levels of T-bet and RORC2 when T lymphocytes were stimulated with the serotype b.
Conclusion
A T-lymphocyte response biased towards a Th1- and Th17-pattern of CCL and CCR expression was detected under stimulation with the serotype b of A. actinomycetemcomitans.
Keywords: Aggregatibacter actinomycetemcomitans, Chemokines, Chemokine receptors, T-lymphocytes, Th1 cells, Th17 cells
INTRODUCTION
In humans, CC chemokines (CCLs) and their specific CC receptors (CCRs) play a central role in physiological and pathological recruitment of immune cells 9 , 24 . During infectious diseases, the expression of CCLs and CCRs produces a chemotactic gradient between regional lymph nodes and infected tissues where, depending on the pattern of CCLs and/or CCRs expressed, specific dendritic cells and T lymphocytes are chemoattracted. Thus, it is established a cellular pathway that goes both ways, in which 1) dendritic cells migrate toward lymphoid organs to present microbial antigens and 2) activated T helper (Th) lymphocytes migrate toward infected tissues to accomplish their specific immunological function 24 .
Th lymphocytes play a central role in the pathogenesis of periodontitis, and a Th1 and Th17-dominated immuno-inflammatory response has been associated with periodontal tissue destruction, alveolar bone resorption, and teeth loss. In this context, the pattern of CCLs and CCRs expressed by Th lymphocytes is crucial in the establishment of the local Th-pattern of immuno-inflammatory response and in the outcome of the disease 9 , 28 . In fact, greater levels of CCL3, CCL4, CCL5, CCL28, CCR1, CCR5, and CCR9 were detected in periodontal lesions of aggressive periodontitis patients, and increased levels of CCL2 and CCR4 were found in lesions of chronic periodontitis patients 8 , 23 . In addition, increased production of IFN-γ has been associated with both greater expression of CCR5 and differentiation of Th1 lymphocytes 16 . Similarly, increased production of IL-6 and IL-23 has been associated with both greater expression of CCL2, CCR6, and CCR7, and subsequent differentiation, migration, and activation of Th17 lymphocytes 13 , 15 . Conversely, increased production of IL-4 and IL-10 has been demonstrated to inhibit the production of CCR5 and to induce the expression of CCL11, CCR3, and CCR4, implied in the Th2 lymphocyte differentiation and function 18 .
Recently, our research group has reported a differential immunogenicity when dendritic cells and T lymphocytes were stimulated with the different serotypes of A. actinomycetemcomitans 2 , 3 . In particular, when T lymphocytes were stimulated with autologous monocyte-derived dendritic cells primed with the serotype b of A. actinomycetemcomitans, higher levels of Th1- and Th17-associated transcription factors and cytokines were detected compared with similar experiments with the other serotypes, demonstrating that serotype b strains of A. actinomycetemcomitans have a higher capacity of triggering Th1 and Th17 phenotype and function. It is, therefore, the aim of this investigation to elucidate whether the different serotypes of A. actinomycetemcomitans have a role on the differential expression of CCLs and CCRs. We hypothesized that the serotype b of A. actinomycetemcomitans, when used to stimulate T lymphocytes, induces higher Th1- and Th17-associated CCL and CCR expression when compared with the other A. actinomycetemcomitans serotypes.
MATERIAL AND METHODS
Experimental design
This experimental study consisted of cell cultures of peripheral naïve CD4+ T lymphocytes obtained from healthy humans and infected in vitro with A. actinomycetemcomitans. The protocol of the study was clearly explained to all the participants, who agreed to participate in it by signing an institutional review board-approved informed consent (Protocol 2010/14). The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000.
A. actinomycetemcomitans strains
The A. actinomycetemcomitans strains ATCC® 43717™ (serotype a), ATCC® 43718™ (serotype b), and ATCC® 43719™ (serotype c) were cultured on agar brain-heart infusion medium (Oxoid, Hampshire, UK) at 37°C and under capnophilic conditions (8% O2 and 12% CO2) using an appropriate microaerobic condition generator (CampyGen™; Oxoid, Hampshire, UK). Growth curves were obtained, and live bacteria, having their whole antigenic potentiality, were obtained at the exponential growth phase of the bacterial culture and used for in vitro cell stimulation.
Blood donors
Blood cells were obtained during platelet-apheresis process from healthy donors consecutively enrolled at the Blood Bank of the Hospital Del Salvador in the Eastern Metropolitan Health Service. The study group consisted of 12 adult individuals (seven males and five females, aged 21 to 38 years, mean age of 28.3±5.10 years) who did not have periodontal disease as determined by absence of gingival inflammation, clinical attachment loss, or increased probing depths (PD>3 mm). Further exclusion criteria were the positive test for HIV and hepatitis B or C virus, presence of manifest infections during the last month, fever, symptomatic allergies, abnormal blood cell counts, increased liver enzymes, or medication of any kind except vitamins and oral contraceptives.
Monocyte-derived dendritic cell generation
For each subject, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using standard procedures (Ficoll-Paque Plus; GE Healthcare, Uppsala, Sweden). For generating a purified population of immature dendritic cells, monocytes were purified from PBMCs by magnetic cell sorting separation (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, PBMCs were incubated with an anti-CD14 monoclonal antibody conjugated to magnetic beads for 15 min at 4°C, loaded onto LS columns and then separated in the magnetic field of a cell separator (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). The retained CD14+ cells, which correspond to monocytes, were then flushed out and washed twice in phosphate-buffered saline. Monocytes were then immediately differentiated to dendritic cell by culturing at 10 6 cells/mL in RPMI-1640 medium supplemented with 10% fetal calf serum (Gibco Invitrogen Corp., Grand Island, NY, USA) and 20 ng/mL rhGM-CSF and rhIL-4 (R&D Systems Inc., Minneapolis, MN, USA) for 6 d at 37°C.
T-lymphocyte purification
A purified population of naïve CD4+ T lymphocytes was obtained from PBMCs by magnetic cell sorting depletion (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, both non-T helper and memory T helper cells were depleted using a cocktail of biotin-conjugated monoclonal antibodies (CD8, CD14, CD15, CD16, CD19, CD25, CD34, CD36, CD45RO, CD56, CD123, TCRγ/δ, HLA-DR, and CD235a) and anti-biotin monoclonal antibodies conjugated to magnetic beads. The magnetically labelled cells were retained within LD columns in the magnetic field of a cell separator (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany), while the unlabelled naïve CD4+ T lymphocytes ran through.
Cell stimulation
Monocyte-derived dendritic cells were primed at a multiplicity of infection MOI=10 2 (bacteria/cells ratio) with different serotypes of A. actinomycetemcomitans, and then 10 6 T-lymphocytes/mL were activated with primed autologous dendritic cells (50:1) in culture with RPMI-1640 containing 10% foetal calf serum (Gibco Invitrogen Corp., Grand Island, NY, USA) for 5 d at 37°C. Previous to each cell co-culture, dendritic cells were washed twice with RPMI-1640 supplemented with 50 IU/mL penicillin and 50 μg/mL streptomycin (Sigma Chemical Co., St. Louis, MI, USA). T-lymphocyte cultures devoid of dendritic cells or exposed to non-induced autologous dendritic cells were used for comparisons. In each experimental step, dendritic cell and T-lymphocyte counting was performed with a hemocytometer and using a phase contrast microscopy (Axiovert 100; Zeiss Co., Göttingen, Germany), and cell viability equal to or higher than 95% was calculated by Trypan blue dye exclusion. For each individual, the experiment was performed separately.
Phenotypic cell analysis
T-lymphocyte purification and their subsequent activation were analyzed by flow cytometry (BD FACSCanto™; Becton Dickinson Immunocytometry Systems, San José, CA, USA). Cells were stained using the following monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE): anti-CD4 (CD4+ T-lymphocytes), anti-CD25α (activated CD4+ T-lymphocytes), anti-CD45RA (naïve CD4+ T-lymphocytes), and anti-CD45RO (memory CD4+ T-lymphocytes) following the manufacturer’s recommendations (BD Biosciences Pharmingen, San José, CA, USA). Isotype-matched control monoclonal antibodies were used to determine the negative cell populations.
Expression of CD25α, CCR, CCL, and transcription factor mRNAs
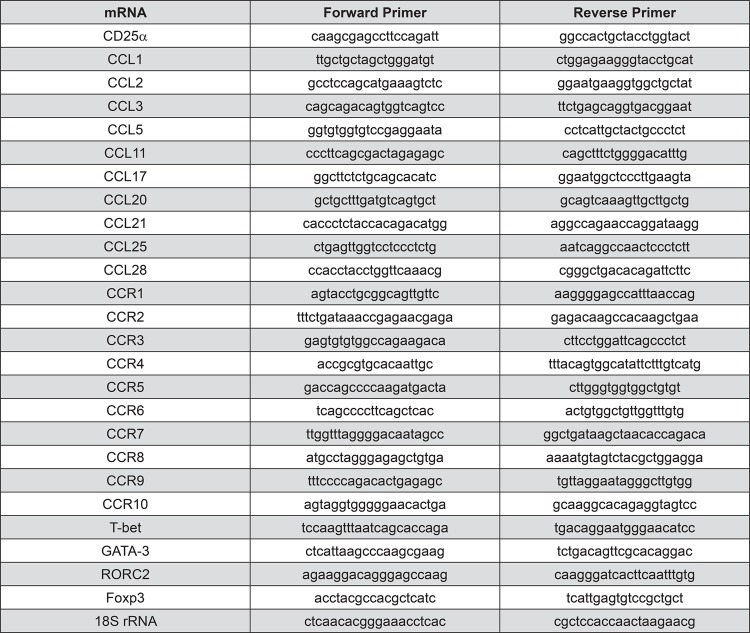
From activated T lymphocytes, total cytoplasmic RNA was isolated using 400 µl of ice-cold lysis buffer containing 0.5% Igepal® CA-630 (Sigma-Aldrich, Saint Louis, MO, USA), 50 mM Tris-HCl pH8, 100 mM NaCl, 5 mM MgCl2, and 10 mM VRC-40 (Gibco Invitrogen, Carlsbad, CA, USA). Isolated RNA was quantified using a spectrophotometer (Synergy HT; Bio-Tek Instrument Inc., Winooski, VT, USA), and the first-strand cDNA was synthesized using 5 µg of total RNA with a SuperScrip™III reverse transcription kit, following the manufacturer’s instructions (Invitrogen, Grand Island, NY, USA). The mRNA expression levels for the chemokines CCL1, CCL2, CCL3, CCL5, CCL11, CCL17, CCL20, CCL21, CCL25, and CCL28, the chemokine receptors CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 and the transcription factors T-bet (Th1), GATA-3 (Th2), RORC2 (Th17), and Foxp3 (T-regulatory), as well as for the activated T-lymphocyte marker CD25α, were quantified by qPCR using the appropriate primers (Figure 1). Briefly, 50 ng of cDNA were amplified using a KAPA™ SYBR® Fast qPCR reagent (KAPA Biosystems, Woburn, MA, USA) in a StepOnePlus® equipment (Applied Biosystems, Singapore) as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 3 s, and 60°C for 30 s, and finally a melt curve of 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s, for detection of non-specific product formation and false positive amplification. As an endogenous control, 18S rRNA expression levels were determined.
Figure 1. Forward and reverse primers used for CD25α, CCL, CCR, and transcription factor mRNA and 18S rRNA amplifications by qPCR.
Data analysis
The flow cytometry data were analyzed using the WinMDi 2.9 software (The Scripps Research Institute, La Jolla, CA, USA), represented as histograms, and expressed as the percentage of positive cells over the total. The qPCR data were analyzed using the StepOne Software 2.2.2 (Applied Biosystems, Singapore) and presented in fold-change of relative quantities by normalizing the CD25α, CCR, CCL, or transcription factor mRNA expression to 18S rRNA expression using the 2-ΔΔCt method. Data were statistically analyzed using the SPSS 15.0 software (Lead Technologies Inc., Charlotte, NC, USA). The normality of data distribution was determined using the Kolmogorov-Smirnov test. Differences regarding CD expression levels analyzed by flow cytometry were determined using the chi-square test. Differences among groups and within each group regarding the CD25α, CCR, CCL, and transcription factor mRNA expression were analyzed using the Kruskal-Wallis test or ANOVA and Tukey post-hoc tests. Correlation coefficients were obtained using the Pearson or Spearman tests. Asterisks were used to graphically indicate statistical significance. A value of p<0.05 was considered statistically significant.
RESULTS
T-lymphocyte purification and activation
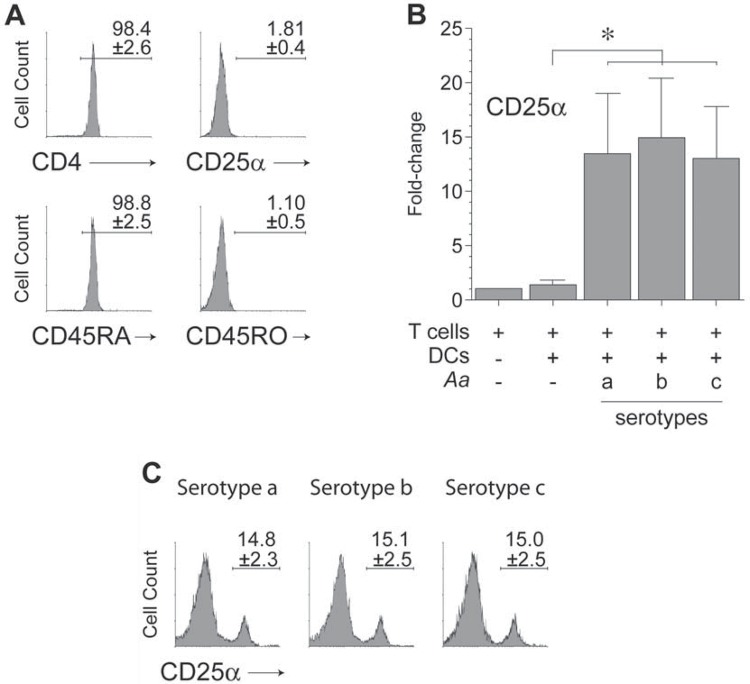
For this study, highly purified (>98%) populations of naïve CD4+ T lymphocytes (CD4+CD25α-CD45RA+CD45RO-), devoid of activated or memory CD4+ T lymphocytes, were isolated from peripheral blood of healthy donors (Figure 2A). These T lymphocytes activated at a similar extent upon stimulation with dendritic cells primed with the different serotypes of A. actinomycetemcomitans, as shown by the similar significant overexpression in CD25α mRNA levels (p<0.001) compared with T lymphocytes exposed to non-induced dendritic cells (Figure 2B). These similar levels of T lymphocyte activation were confirmed at a protein level when the cell-surface expression of CD25α was determined by flow cytometry. In fact, the frequency of CD25α expression (~15%) in T lymphocytes exposed to the different serotypes of A. actinomycetemcomitans did not differ significantly (Figure 2C).
Figure 2. T-lymphocyte purification and activation. A: T-lymphocyte purification. Flow cytometry analysis of CD4, CD25α, CD45RA, and CD45RO expression demonstrating the purity of naïve CD4+ T lymphocytes isolated from healthy donors. B: T-lymphocyte activation. The qPCR analysis for the CD25α mRNA expression in CD4+ T lymphocytes stimulated by autologous dendritic cells primed at a MOI=102 with the A. actinomycetemcomitans strains ATCC® 43717™ (serotype a), ATCC® 43718™ (serotype b), and ATCC® 43719™ (serotype c). C: T-lymphocyte activation. Flow cytometry analysis of the CD25α expression demonstrating the levels of activation of CD4+ T lymphocytes after 5-day stimulation under the same conditions described in Figure 2B. The flow cytometry data from each experiment were expressed as percentage of positive cells over the total, and shown as mean±SD from 4 independent experiments. For relative expression, the CD25α mRNA expression in T lymphocytes cultured in the absence of dendritic cells was considered as 1, as a reference for fold-change in expression. Data are represented as fold-change for 8 independent experiments. Each experiment was performed in duplicate. Comparisons were done versus T lymphocytes exposed to non-induced dendritic cells (*p<0.05). Aa, Aggregatibacter actinomycetemcomitans; CD, cluster of differentiation; DCs, dendritic cells.
Expression of CCLs by A. actinomycetemcomitans-induced T lymphocytes
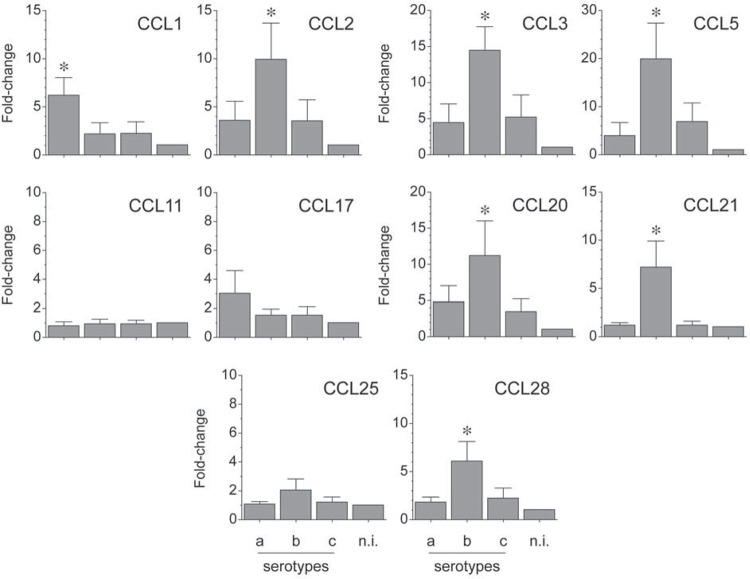
The mRNA expression for the analyzed chemokines was determined by qPCR and represented as fold-change for each condition (Figure 3). When the strain ATCC® 43718™ belonging to the serotype b of A. actinomycetemcomitans was used for T-lymphocyte activation, higher expression levels of CCL2 (p=0.025 and p=0.024), CCL3 (p=0.003 and p=0.005), CCL5 (p=0.004 and p=0.013), CCL20 (p=0.05 and p=0.02), CCL21 (p=0.001 and p=0.001), and CCL28 (p=0.004 and p=0.008) were detected, when compared with the strains ATCC® 43717™ and ATCC® 43719™ belonging to the serotypes a or c, respectively. Conversely, when the serotype a of A. actinomycetemcomitans was used for T-lymphocyte activation, higher expression levels of CCL1 (p=0.008 and p=0.009) and CCL17 (p>0.05 and p>0.05) were detected, when compared with the serotypes b or c, respectively. CCL11 and CCL25 were not over-expressed in any experimental condition.
Figure 3. CC chemokines (CCL) expression by A. actinomycetemcomitans-induced T lymphocytes. CCL mRNA expression in T lymphocytes activated by dendritic cells primed at a MOI=102 with the A. actinomycetemcomitans strains ATCC® 43717™ (serotype a), ATCC® 43718™ (serotype b), and ATCC® 43719™ (serotype c). For relative expression, the CCL mRNA expression in T lymphocytes exposed to non-induced dendritic cells was considered as 1, as a reference for fold-change in expression (n.i). Data are represented as fold-change for 8 independent experiments. Each experiment was performed in duplicate. Comparisons were done between the different A. actinomycetemcomitans serotypes (*p<0.05).
Expression CCRs by A. actinomycetemcomitans-induced T lymphocytes
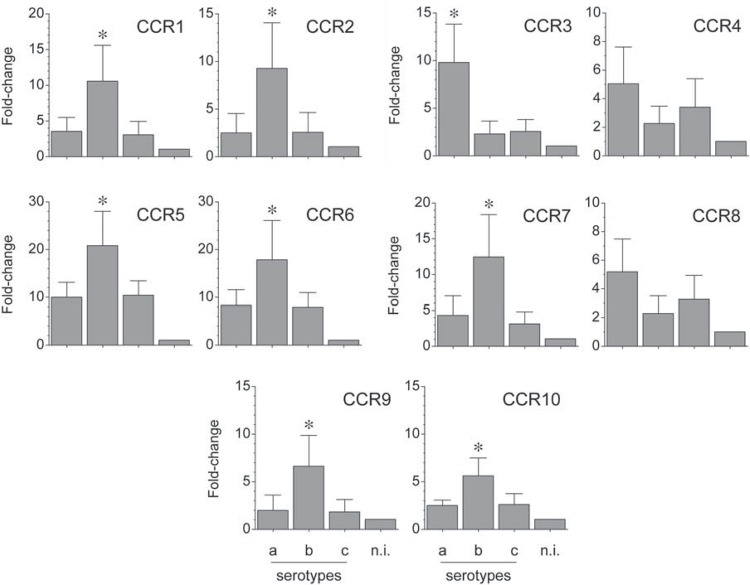
The mRNA expression for the analyzed chemokine receptors was determined by qPCR and represented as fold-change for each condition (Figure 4). When the serotype b of A. actinomycetemcomitans was used for T-lymphocyte activation, higher expression levels of CCR1 (p=0.036 and p=0.026), CCR2 (p=0.041 and p=0.042), CCR5 (p=0.029 and p=0.035), CCR6 (p=0.045 and p=0.044), CCR7 (p=0.039 and p=0.020), CCR9 (p=0.040 and p=0.035), and CCR10 (p=0.018 and p=0.022) were detected compared with the serotypes a or c, respectively. Conversely, when the serotype a of A. actinomycetemcomitans was used for T-lymphocyte activation, higher expression levels of CCR3 (p=0.006 and p=0.007), CCR4 (p>0.05 and p>0.05), and CCR8 (p>0.05 and p>0.05) were detected, when compared with the serotypes b or c, respectively.
Figure 4. CC receptors (CCR) expression by A. actinomycetemcomitans-induced T lymphocytes. CCR mRNA expression in T lymphocytes activated by dendritic cells primed at a MOI=102 with the A. actinomycetemcomitans strains ATCC® 43717™ (serotype a), ATCC® 43718™ (serotype b), and ATCC® 43719™ (serotype c). For relative expression, the CCR mRNA expression in T lymphocytes exposed to non-induced dendritic cells was considered as 1, as a reference for fold-change in expression (n.i). Data are represented as fold-change for 8 independent experiments. Each experiment was performed in duplicate. Comparisons were done between the different A. actinomycetemcomitans serotypes (*p<0.05).
Expression of T-bet, GATA-3, RORC2, and Foxp3 by A. actinomycetemcomitans-induced T lymphocytes
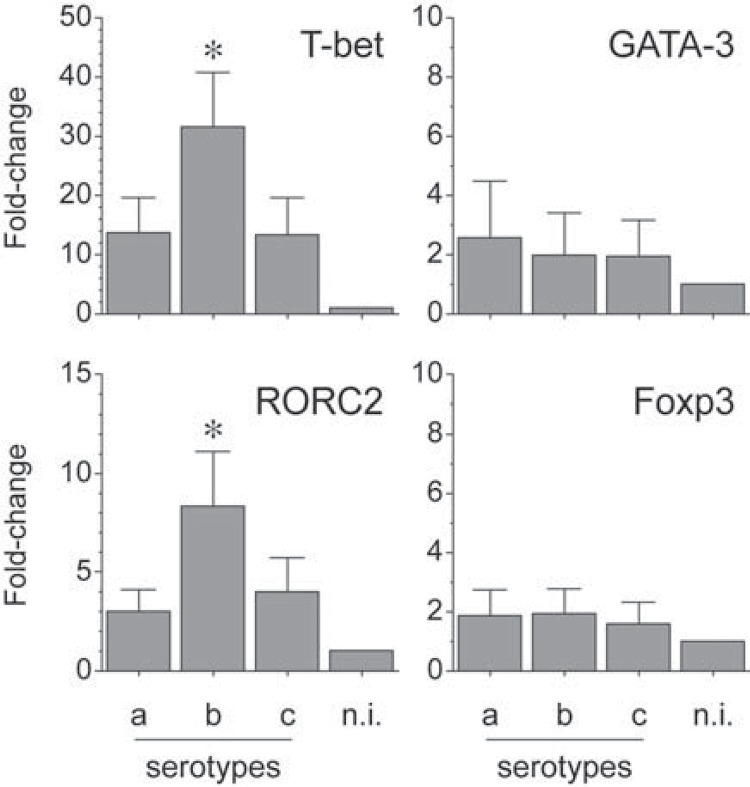
The mRNA expression for T-bet, GATA-3, RORC2, and Foxp3 was determined by qPCR in T lymphocytes stimulated by dendritic cells primed at a MOI=102 with the different serotypes of A. actinomycetemcomitans (Figure 5). Similarly to our previous experiments 2 , T lymphocytes stimulated with the serotype b showed a higher relative expression of T-bet (p<0.001 and p<0.001) and RORC2 (p<0.001 and p=0.001) mRNAs than the same cells stimulated with the serotypes a or c, respectively.
Figure 5. Transcription factor expression by A. actinomycetemcomitans-induced T lymphocytes. T-bet (Th1), GATA-3 (Th2), RORC2 (Th17), and Foxp3 (T-regulatory) mRNA expression in T lymphocytes stimulated by dendritic cells primed at a MOI=102 with the A. actinomycetemcomitans strains ATCC® 43717™ (serotype a), ATCC® 43718™ (serotype b), and ATCC® 43719™ (serotype c). For relative expression, the transcription factor mRNA expression in T lymphocytes exposed to non-induced dendritic cells was considered as 1, as a reference for fold-change in expression (n.i.). Data are represented as fold-change for 8 independent experiments. Each experiment was performed in duplicate. Comparisons were done between the different A. actinomycetemcomitans serotypes (*p<0.05).
Correlation analysis between T-bet, GATA-3, RORC2, and Foxp3 versus CCL and CCR expression levels
The correlation analyses between the expression of the transcription factors and the CCLs and CCRs on each activation condition tested (Table 1) yielded positive correlation between T-bet and CCL2, CCL3, CCL5, CCL25, CCR1, CCR2, CCR5, and CCR9, being statistically significant for CCL5 (p=0.049), CCR1 (p=0.040), and CCR2 (p=0.046), when T lymphocytes were stimulated with the serotype b of A. actinomycetemcomitans. Under the same condition, a positive correlation was also observed between RORC2 and CCL20, CCL21, CCR6, and CCR7, being statistically significant for CCL20 (p=0.041), CCL21 (p=0.004), and CCR7 (p=0.030). In contrast, GATA-3 showed positive correlation with CCL1, CCL17, CCR3, CCR4, and CCR8, being statistically significant for CCL1 (p=0.049), CCL17 (p=0.046), CCR4 (p=0.002), and CCR8 (p=0.048) when T lymphocytes were stimulated with the serotype a of A. actinomycetemcomitans and with CCR8 (p=0.044) when the same cells were stimulated with the serotype c. Foxp3 did not show positive correlation with any CCL or CCR (data not shown). Overall, a T-lymphocyte response biased towards a Th1- and Th17-pattern of CCL and CCR expression was detected under stimulation with the serotype b of A. actinomycetemcomitans.
Table 1. Correlation analysis of transcription factors versus CCL and CCR mRNA expressions. The Pearson’s correlation coefficient (r) between the transcription factors T-bet (Th1), GATA-3 (Th2), and RORC2 (Th17) and the Th1-, Th2-, or Th17-associated CCLs and CCRs were calculated using T lymphocytes stimulated by autologous dendritic cells primed at a MOI=102 with the different A. actinomycetemcomitans serotypes. *p<0.05.
| Aggregatibacter actinomycetemcomitans | |||||||
|---|---|---|---|---|---|---|---|
| Serotype a | Serotype b | Serotype c | |||||
| r | p-value | r | p-value | r | p-value | ||
| T-bet | CCL2 | -0.606 | 0.394 | 0.918 | 0.082 | -0.487 | 0.513 |
| CCL3 | -0.755 | 0.245 | 0.882 | 0.118 | -0.799 | 0.201 | |
| CCL5 | -0.485 | 0.515 | 0.931 | 0.049* | -0.682 | 0.318 | |
| CCL25 | 0.585 | 0.415 | 0.909 | 0.091 | 0.800 | 0.200 | |
| CCR1 | -0.250 | 0.750 | 0.956 | 0.040* | 0.522 | 0.478 | |
| CCR2 | -0.800 | 0.200 | 0.934 | 0.046* | -0.762 | 0.238 | |
| CCR5 | -0.745 | 0.255 | 0.882 | 0.118 | -0.966 | 0.004 | |
| CCR9 | -0.971 | 0.029 | 0.896 | 0.104 | -0.711 | 0.289 | |
| GATA-3 | CCL1 | 0.920 | 0.049* | 0.837 | 0.063 | 0.130 | 0.870 |
| CCL11 | -0.125 | 0.825 | 0.912 | 0.088 | -0.178 | 0.822 | |
| CCL17 | 0.934 | 0.046* | 0.519 | 0.481 | -0.072 | 0.928 | |
| CCL28 | -0.231 | 0.769 | 0.304 | 0.696 | 0.251 | 0.749 | |
| CCR3 | 0.880 | 0.120 | 0.628 | 0.372 | 0.620 | 0.380 | |
| CCR4 | 0.998 | 0.002* | -0.020 | 0.980 | 0.578 | 0.422 | |
| CCR8 | 0.932 | 0.048* | 0.940 | 0.060 | 0.956 | 0.044* | |
| CCR10 | 0.162 | 0.838 | -0.878 | 0.122 | 0.144 | 0.856 | |
| RORC2 | CCL20 | 0.897 | 0.103 | 0.939 | 0.041* | 0.357 | 0.643 |
| CCL21 | 0.403 | 0.597 | 0.996 | 0.004* | -0.229 | 0.771 | |
| CCR6 | -0.852 | 0.148 | 0.875 | 0.125 | -0.717 | 0.283 | |
| CCR7 | 0.998 | 0.002* | 0.970 | 0.030* | 0.909 | 0.091 | |
DISCUSSION
There is strong evidence suggesting that variations in the host immuno-inflammatory response, in particular, in the T lymphocyte phenotype and function, play an important role in the susceptibility, onset, and severity of periodontitis 6 , 10 . In particular, a Th1 and Th17-dominated immuno-inflammatory response has been associated with the pathogenesis of periodontitis, and an increased expression of Th1- and Th17-related transcription factors and pro-inflammatory mediators have been reported in active periodontal lesions, where alveolar bone resorption is occurring 6 , 17 . In this study, the expression levels of different CCLs and CCRs were analyzed in human naïve T lymphocytes, stimulated with dendritic cells primed with different serotypes of A. actinomycetemcomitans, demonstrating that the serotype b induced greater expression levels of Th1- and Th17-associated CCLs and CCRs, when compared with the other serotypes.
The association between the serotype b of A. actinomycetemcomitans and the pathogenesis of the periodontitis has been previously analyzed 2 , 3 , 27 , 30 , 32 . In fact, the serotype b of A. actinomycetemcomitans triggers a greater immunogenic and pathogenic response when in contact with different host cells compared with the other serotypes. For instance, the serotype b induces increased resistance to phagocytosis and to killing by macrophages and neutrophils 32 , higher expression of IL-8 and ICAM-1 in gingival epithelial cells 27 , greater production of IL-1β in macrophages 30 , and stronger induction of Th1- and Th17-type of response in dendritic cells and CD4+ T lymphocytes 2 , 3 .
To our knowledge, this is the first report associating the different serotypes of A. actinomycetemcomitans with a Th1- and Th17-pattern of immuno-inflammatory response by analyzing the CC chemokines and receptors involved in the selective chemo-attraction of Th lymphocytes. These findings are suggestive of the pathogenic role of A. actinomycetemcomitans in the aetiology of periodontitis, which may differ among serotypes and specifically let us propose that serotype b could play a role in the pathogenesis of the disease by the induction of a local inflammatory environment that favors the specific recruitment of Th1 and Th17 lymphocytes towards the infected periodontal tissues.
In rheumatoid arthritis, which is an inflammatory disease characterized by the development of a Th1 and Th17-dominated immuno-inflammatory response in the affected articular tissues 14 , it has been established that the chronicity of the inflammation is associated with a Th1- and Th17-pattern of CCL and CCR expression. In fact, in chronic inflamed joints, over-expressed levels of CCL2, CCL3, CCL5, CCR1, CCR2, and CCR5, related to a Th1-type response 26 , 29 , and CCL20, CCL21, CCR6, and CCR7, related to a Th17-type response 14 , 22 , have been detected compared with non-inflamed joints. In addition, increased expression of CCL20 has been associated with predominant Th17 lymphocyte infiltration in the affected articular tissues 11 and, when the chemokine receptors CCR1, CCR2, CCR5, and CCR6 were blocked, a decreased Th1 lymphocyte migration towards the inflamed joints was detected, promoting the resolution of the disease 11 , 26 .
The results of the present study are consistent with the available scientific evidence, and demonstrate that a Th1- and Th17-pattern of immuno-inflammatory response develops during the periodontal infection, at least during an in vitro mono-infection with A. actinomycetemcomitans. Thus, the Th1 and Th17-dominated immuno-inflammatory response described in periodontitis could be explained by the increased chemo-attraction of recently activated and differentiated Th1 and Th17 lymphocytes from regional lymph nodes and/or by the activation of naïve and memory Th1 and Th17 lymphocytes residing in the periodontal tissues 12 , 25 . In this context, the serotype b of A. actinomycetemcomitans could be associated with local periodontal tissue destruction by the over-production of Th1- and Th17-associated cytokines, such as IL1-β, IL-6, IL-17A, and TNF-α 2 , 3 , and the over-expression of Th1- and Th17-associated CCLs and CCRs, such as CCL2, CCL3, CCL5, CCR1, CCR2, CCR5, and CCR7, as demonstrated in the present study, which are involved in the differentiation and activation of osteoclasts and the resorption of tooth-supporting alveolar bone 6 - 9 , 28 . In fact, a more frequent and higher expression of CCL2 and its specific receptor CCR4 have been reported in chronic periodontitis, and a more prevalent and higher expression of CCL3 and its specific receptor CCR5 have been reported in aggressive periodontitis 9 . Thus, the A. actinomycetemcomitans serotype b-induced Th1 and/or Th17 lymphocytes could migrate towards infected tissues following the CCL2 and/or CCL3 chemotactic gradient, resulting in a Th1 and/or Th17-associated cytokine production at the periodontal lesion that locally promote the alveolar bone resorption.
The A. actinomycetemcomitans strains used in the present study are phenotypically smooth. In fact, these different serotypes correspond to structurally distinct O-polysaccharide components of their respective LPS that function as immuno-dominant antigens. The O-polysaccharide from the LPS produced by the serotype b of A. actinomycetemcomitans is structurally distinct from the O-polysaccharide produced by the other serotypes. In particular, the O-polysaccharide from serotype b consists of a repeating trisaccharide unit composed of α-D-fucose, α-L-rhamnose and β-D-N-acetyl-galactosamine residues, and the O-polysaccharide from serotypes a and c is composed of 6-deoxy-α-D-talose and 6-deoxy-α-L-talose, respectively 19 , 20 .
Interestingly, our data also show that serotype b of A. actinomycetemcomitans induces an increment in the CCR10 expression in stimulated T lymphocytes, a chemokine receptor associated with Th2 lymphocyte differentiation and function 31 . In this context, it has been demonstrated that CCR10 is also expressed by Th22 lymphocytes, a recently described T lymphocyte population that complies pro-inflammatory activities 5 ; however, this T-cell phenotype has not yet been described in periodontitis. Accordingly, it could be hypothesized that Th22 lymphocytes may be associated with the pathogenesis of periodontitis 1 , which would clarify, at least to a certain degree, the over-expressed levels of CCR10 and its specific chemokine ligand CCL28 detected in the present study. In fact, higher levels of CCL28 have been detected in the gingival crevicular fluid of chronic and aggressive periodontitis patients compared with gingivitis and healthy individuals 4 .
During periodontitis, antigen presentation may occur both in the regional lymph nodes that drain the periodontal tissues and locally in the infected periodontal tissues, in a periodontal site-specific manner. In fact, formation of periodontal lymphoid clusters in which dendritic cells present bacterial antigens to naïve or memory T lymphocytes has been reported, promoting the activation and selective differentiation of Th1 and Th17 lymphocytes 12 , 25 . In this scenario, CCR7 and its specific chemokine ligands CCL19 and CCL21 could play a role in the organization of these cellular clusters, as an ectopic lymphoid-like structure 21 , promoting the alveolar bone resorption characteristic of the periodontitis.
CONCLUSIONS
In T lymphocytes, the serotype b of A. actinomycetemcomitans induces higher expression levels of chemokines CCL2, CCL3, CCL5, CCL20, CCL21, and CCL28 and of chemokine receptors CCR1, CCR2, CCR5, CCR6, CCR7, and CCR9, when compared with the other serotypes. These increased levels associated with the expression of the transcription factors master-switch genes that trigger the Th1 and Th17 lymphocyte differentiation. Considered together, these data let us propose that variability in the Th1 and Th17 immuno-inflammatory response induced by the different serotypes of A. actinomycetemcomitans is associated, at least to a certain degree, with the CC chemokines and receptors that they express; however, functional studies are necessary to confirm our hypothesis.
ACKNOWLEDGMENTS
The authors declare no competing financial interests. This study was supported by grants FONDECYT 11100298 and 1140904.
REFERENCES
- 1.Araujo-Pires AC, Francisconi CF, Biguetti CC, Cavalla F, Aranha AM, Letra A, et al. Simultaneous analysis of T helper subsets (Th1, Th2, Th9, Th17, Th22, Tfh, Tr1 and Tregs) markers expression in periapical lesions reveals multiple cytokine clusters accountable for lesions activity and inactivity status. J Appl Oral Sci. 2014;22(4):336–346. doi: 10.1590/1678-775720140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Díaz-Zúñiga J, Melgar-Rodríguez S, Alvarez C, Monasterio G, Benítez A, Ciuchi P, et al. T-lymphocyte phenotype and function triggered by Aggregatibacter actinomycetemcomitans is serotype-dependent. 10.1111/jre.12270.J Periodontal Res. 2015 doi: 10.1111/jre.12270. [DOI] [PubMed] [Google Scholar]
- 3.Díaz-Zúñiga J, Yáñez JP, Alvarez C, Melgar-Rodríguez S, Hernández M, Sanz M, et al. Serotype-dependent response of human dendritic cells stimulated with Aggregatibacter actinomycetemcomitans. J Clin Periodontol. 2014;41(3):242–251. doi: 10.1111/jcpe.12205. [DOI] [PubMed] [Google Scholar]
- 4.Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A. Comparison of CCL28, interleukin-8, interleukin-1beta and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J Periodontal Res. 2013;48(1):44–51. doi: 10.1111/j.1600-0765.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujita H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci. 2013;72(1):3–8. doi: 10.1016/j.jdermsci.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 7.Garlet GP, Avila-Campos MJ, Milanezi CM, Ferreira BR, Silva JS. Actinobacillus actinomycetemcomitans-induced periodontal disease in mice: patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 2005;7(4):738–747. doi: 10.1016/j.micinf.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Garlet TP, Fukada SY, Saconato IF, Avila-Campos MJ, Silva TA, Garlet GP, et al. CCR2 deficiency results in increased osteolysis in experimental periapical lesions in mice. J Endod. 2010;36(2):244–250. doi: 10.1016/j.joen.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38(2):210–217. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- 10.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol. 2001;69(3):331–339. [PubMed] [Google Scholar]
- 13.Kabashima H, Yoneda M, Nagata K, Hirofuji T, Maeda K. The presence of chemokine (MCP-1, MIP-1alpha, MIP-1beta, IP-10, RANTES)-positive cells and chemokine receptor (CCR5, CXCR3)-positive cells in inflamed human gingival tissues. Cytokine. 2002;20(2):70–77. doi: 10.1006/cyto.2002.1985. [DOI] [PubMed] [Google Scholar]
- 14.Kochi Y, Suzuki A, Yamamoto K. Genetic basis of rheumatoid arthritis: a current review. Biochem Biophys Res Commun. 2014;452(2):254–262. doi: 10.1016/j.bbrc.2014.07.085. [DOI] [PubMed] [Google Scholar]
- 15.Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, et al. CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol. 2009;183(4):2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- 16.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391(6665):344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88(7):633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 18.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64(7):995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 19.Perry MB, MacLean LM, Brisson JR, Wilson ME. Structures of the antigenic O-polysaccharides of lipopolysaccharides produced by Actinobacillus actinomycetemcomitans serotypes a, c, d and e. Eur J Biochem. 1996;242(3):682–688. doi: 10.1111/j.1432-1033.1996.0682r.x. [DOI] [PubMed] [Google Scholar]
- 20.Perry MB, MacLean LL, Gmür R, Wilson ME. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect Immun. 1996;64(4):1215–1219. doi: 10.1128/iai.64.4.1215-1219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Yang B, Yin Y, Leng X, Jiang Y, Zhang L, et al. Aberrant CD200/CD200R1 expression and its potential role in Th17 cell differentiation, chemotaxis and osteoclastogenesis in rheumatoid arthritis. Rheumatology. 2015;54(4):712–721. doi: 10.1093/rheumatology/keu362. [DOI] [PubMed] [Google Scholar]
- 23.Repeke CE, Ferreira SB, Jr, Vieira AE, Silveira EM, Avila-Campos MJ, Silva JS, et al. Dose-response met-RANTES treatment of experimental periodontitis: a narrow edge between the disease severity attenuation and infection control. PLoS One. 2011;6(7): doi: 10.1371/journal.pone.0022526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder HE, Graf-de Beer M. Stereologic analysis of chronic lymphoid cell infiltrates in human gingiva. Arch Oral Biol. 1976;21(9):527–537. doi: 10.1016/0003-9969(76)90018-2. [DOI] [PubMed] [Google Scholar]
- 26.Shadidi KR, Aarvak T, Henriksen JE, Natvig JB, Thompson KM. The chemokines CCL5, CCL2 and CXCL12 play significant roles in the migration of Th1 cells into rheumatoid synovial tissue. Scand J Immunol. 2003;57(2):192–198. doi: 10.1046/j.1365-3083.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Sugano N, Nishihara R, Suzuki K, Tanaka H, Ito K. Differential effects of five Aggregatibacter actinomycetemcomitans strains on gingival epithelial cells. Oral Microbiol Immunol. 2008;23(6):455–458. doi: 10.1111/j.1399-302X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 28.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86(4):306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- 29.Subasinghe NL, Lanter J, Markotan T, Opas E, McKenney S, Crysler C, et al. A novel series of N-(azetidin-3-yl)-2-(heteroarylamino)acetamide CCR2 antagonists. Bioorg Med Chem Lett. 2013;23(4):1063–1069. doi: 10.1016/j.bmcl.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Nishihara T, Ishihara Y, Amano K, Shibuya N, Moro I, et al. Murine macrophage interleukin-1 release by capsularlike serotype-specific polysaccharide antigens of Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59(1):18–23. doi: 10.1128/iai.59.1.18-23.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia M, Hu S, Fu Y, Jin W, Yi Q, Matsui Y, et al. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J Allergy Clin Immunol. 2014;134(3):634–644. doi: 10.1016/j.jaci.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi N, Kawasaki M, Yamashita Y, Nakashima K, Koga T. Role of the capsular polysaccharide-like serotype-specific antigen in resistance of Actinobacillus actinomycetemcomitans to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1995;63(12):4589–4594. doi: 10.1128/iai.63.12.4589-4594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]