Abstract
Low levels of pharmaceutical compounds have been detected in aquatic environments worldwide, but their human and ecological health risks associated with low dose environmental exposure is largely unknown due to the large number of these compounds and a lack of information. Therefore prioritization and ranking methods are needed for screening target compounds for research and risk assessment. Previous efforts to rank pharmaceutical compounds have often focused on occurrence data and have paid less attention to removal mechanisms such as human metabolism. This study proposes a simple prioritization approach based on number of prescriptions and toxicity information, accounting for metabolism and wastewater treatment removal, and can be applied to unmeasured compounds. The approach was performed on the 200 most-prescribed drugs in the U.S. in 2009. Our results showed that under-studied compounds such as levothyroxine and montelukast sodium received the highest scores, suggesting the importance of removal mechanisms in influencing the ranking, and the need for future environmental research to include other less-studied but potentially harmful pharmaceutical compounds.
Keywords: pharmaceuticals, prioritization, screening, ranking, risk assessment, prescription drugs, water, environment
1 Introduction
Low levels of pharmaceutically active compounds (PhACs) have been measured in surface waters, groundwater, drinking water sources, and wastewater effluent throughout the world (Vulliet et al., 2011; Dougherty et al., 2010; Fick et al., 2009; Zhao et al., 2009; Choi et al., 2008; Roberts and Thomas, 2006; Karthikeyan and Meyer, 2006; Bendz et al., 2005; Carballa et al., 2004; Yang and Carlson, 2004; Huggett et al., 2003; Calamari et al., 2003; Sacher et al., 2003; Andreozzi et al., 2003; Kolpin et al., 2002; Hirsch et al., 1999; Belfroid et al., 1999; Ternes, 1998). These compounds include antibiotics, analgesics, antidepressants, beta-blockers, hormones and hormone-mimics. Because PhACs are designed to target specific metabolic and biological pathways, there is concern that, even though levels found in the environment are much lower than the therapeutically effective doses (Webb et al., 2003), some compounds may disrupt key processes in sensitive non-target organisms, including certain human populations such as children and pregnant women.
Human PhACs enter aquatic environments primarily through the discharge of treated and untreated wastewater to surface water bodies, or to aquifers from septic systems and groundwater recharge. Following consumption of these drugs, both metabolites and unmetabolized PhACs are generally excreted in urine and feces. Metabolism rates vary greatly across compounds, from <1% to >95% metabolized before excretion (Physicians’ Desk Reference, 2009). Discharged into sewage or septic systems, these PhACs then undergo additional transformations or physical removal (e.g., sorption to solids and settling) within wastewater treatment plants (WWTPs), but removal efficiency is highly variable across compounds and can be substantially less than 100% (Metcalfe et al., 2010; Batt et al., 2006; Joss et al., 2006, 2005; Jones et al., 2005; Carballa et al., 2004; Ternes et al., 1999; Ternes, 1998). Due to the relatively long half-lives and hydrophilic nature of many PhACs, they cannot be treated by WWTPs as effectively as other contaminants (Batt et al., 2006), resulting in their persistence and accumulation to measurable levels in aquatic ecosystems. However, little is actually known about the potential human and ecological health risks that PhACs or PhAC-mixtures pose at environmentally relevant concentrations.
This lack of knowledge is primarily due to the large number of PhACs used in the U.S. and throughout the world. According to the Orange Book (Approved Drug Products with Therapeutic Equivalence Evaluations) published by the U.S. Food and Drug Administration, over 10,000 prescription drugs and about 300 over-the-counter drugs are currently in use and produced. For a majority of these compounds, no data are available on their fate and transport in the environment, their toxicity to organisms and humans at environmentally relevant doses, or no measurement has been made yet to determine their environmental concentrations. This is partly because chemical analysis methods for detecting and determining most of these compounds in the environment are still under development, and even for those that can be measured, the currently available analytical methods and toxicity tests are expensive and time-consuming. Therefore, a prioritizing approach is crucial for researchers to select the most important and relevant target compounds.
Given the large number of PhACs, many environmental studies base their selection of compounds on ease of measurement or detection (Schwab et al., 2005), brand name recognition (Foster et al., 2010; Nieto et al., 2010; Redshaw et al., 2008), or certain categories of concern (Schultz et al., 2010). Overall, current studies largely focus on common antibiotics such as trimethoprim, ciprofloxacin, sulfamethoxazole and erythromycin. This is not too surprising as antibiotics are the largest class of pharmaceuticals among all prescription, over-the-counter, and veterinary pharmaceuticals. A large proportion of the studies that chose to look at pharmaceuticals other than antibiotics focused on compounds with high consumption or prescription rates (e.g. atenolol, furosemide, codeine, acetaminophen, carbamazepine, verapamil, metformin, amitriptyline). However, a systematic approach that accounts for environmental loadings, fate and transport, as well as toxicity of PhACs will be most helpful in screening of common PhACs for potentially harmful compounds to the health of humans and aquatic organisms.
An increasing number of studies have proposed comprehensive approaches to rank or prioritize the risk of PhAC compounds in the environment using different criteria and models (Fick et al., 2010; Kumar and Xagoraraki, 2010; Cooper et al., 2008; Sanderson et al., 2004). Most of these studies began with PhACs’ occurrences in the environment and ranked their potential impacts based on toxicological information either from toxicity tests or estimated with quantitative structure-activity relationship (QSAR) models. Therefore, the compounds of which environmental measurements were not yet conducted or available could not be included in the ranking. Only very few studies started their analyses from the usage of PhACs (Cooper et al., 2008), and even fewer had considered influence on their ranking of PhACs imposed by various removal mechanisms, such as human metabolism (Perazzolo et al., 2010) and WWTP treatment (Lienert et al., 2007). We hypothesize that the ranks for certain compounds estimated by these methods may actually change substantially if attenuation terms were taken into consideration.
The main goals of this research are (1) to assess the feasibility of developing an approach that uses readily available data to prioritize PhAC compounds based on potential risk to ecological and human health, and (2) to use the developed approach to suggest a list of priority compounds. In this study, we developed a quantitative risk-based ranking approach, combining number of prescriptions, metabolism, WWTP removal, and multiple toxic endpoints, in order to identify compounds that pose potentially high risk to human and ecosystems. This approach can be used to provide guidance to both researchers and regulators in a simple and meaningful way on the risk of PhACs to human and ecological health, including previously unmeasured PhACs.
2 Methods
We started with a list of the top 200 prescription drugs (http://www.rxlist.com) in 2009 including both generic and brand name pharmaceuticals, and developed a ranking system for the active ingredients in these 200 drugs to calculate a Toxic Load (TL), a risk-based metric we developed incorporating annual mass production, human metabolism, WWTP removal, and the toxicity of each compound. In general, the toxic load for a compound can be described by the ratio of its mass loading into the environment and toxicity threshold of a certain endpoint:
| (1) |
Thus a compound with a large mass loading to the environment that also is toxic a low thresholds will have a hihg toxic loading to the environment. Details of the TL calculation wwre given below..
2.1 Estimating Mass Loadings
The mass loading of each PhAC to aquatic ecosystems was calculated as:
| (2) |
where MLi = mass loading of compound i to aquatic ecosystems after being consumed, excreted, and passing through a WWTP; Pi is the mass of compound i prescribed per year (kg/yr) calculated from published prescription data; ui = the fraction of a compound i that is actually utilized (ingested, inhaled, etc.) by the consumer as opposed to being disposed of (ui was assumed to be equal to 1 in our study due to the lack of data); ei = the fraction of compound i that is excreted unmetabolized after ingestion or inhalation; di = the fraction of compound i that is discharged from a typical WWTP without being chemically transformed or removed by sorption and settling.
Production (Pi), or consumer use of prescription pharmaceuticals, in kilograms per year, was calculated in two ways: (1) based on published sales data, as done in other published studies (Zuccato et al., 2006; Calamari et al., 2003), or (2) the number of prescriptions filled. The two methods were compared and the results were linearly related (R2=0.82, p<0.001). More data were available for the drugs on the list for the “# of prescriptions filled" method, thus data from this method was used for all subsequent calculations.
The number of prescriptions filled (#Rx) along with estimates of prescription type and dose, were used to convert #Rx to total production (kg/yr) in the U.S.. A maximum daily maintenance dose for an average adult was obtained for each drug on the 2009 list from Rxlist.com and, if available, the average time period for taking the drug (per prescription filled) was specified. If this information was not available or the drug was indicated for long-term use (months or longer), it was assumed that each prescription supported the patient for 30 days, with the exception of antibiotics, anti-inflammatory agents and opiate agonists. In these latter categories, an average time period of 14 days was used. The total production of each prescription drug from 2009 was then calculated as follows:
| (3) |
The percentage of excretion as unchanged (unmetabolized) parent compound (ei) was found for each drug in either the Rxlist website, PDR (Physicians’ Desk Reference, 2009) or AHFS Drug Information (American Society of Hospital Pharmacists, 2007). Zero metabolism was applied to topically and opthalmically used drugs (assuming no absorption into the human body). As a result of no experimental data for removal rates through wastewater treatment for many of the PhACs, the STPWIN module in the EPI Suite software (U.S. EPA, 2011) was used to estimate removal rate percentages (di), based on an EPA draft method using Kows. This program predicts the removal of chemicals in a typical activated sludge-based sewage treatment plant. Three processes that may contribute to removal are considered: biodegradation, sorption to sludge, and air stripping. The program assumes a standard system design and set of default operating conditions.
2.2 Toxicity Dose (TD)
No single toxicity estimate was used for the final analysis because of the multiple target organisms considered, multiple potential toxicity endpoints, and the limited consistency in data availability across compounds for each endpoint and organism. Thus, twelve different toxic endpoints were considered: (1) Adult Minimum Initial Dose, (2) Human LOAEL (Lowest-Observed-Adverse-Effect-Level), (3) Rat LD50 (lethal dose for 50% of test population), (4) Rat LOAEL, (5) Mouse LD50, (6) Mouse LOAEL, (7) Algae 96-hr EC50 (concentration at which 50% of test population exhibit toxic effect), (8) Algae Chronic Value (ChV, concentration showing no significant toxic effect during a 30-day exposure period), (9) Daphnid 48-hr LC50 (lethal concentration for 50% of test population), (10) Daphnic Chronic Value, (11) Fish 96-hr LC50, and (12) Fish Chronic Value. Ideally, human NOAEL (No-Observed-Adverse-Effect-Level) data, perhaps the most relevant endpoint for human health, should also be considered in this analysis. However, these data were not available for most of the compounds.
LD50s from acute rat and mouse studies were accessible for the majority of the pharmaceuticals on the 2009 list. Most of the rat and mouse LD50s, as well as some of the human, rat and mouse LOAELs (reported as TDLo, or Lowest Published Toxic Dose; reproductive and endocrine disrupting effects were included for hormones or hormone-mimics) were obtained from the RTECS database (http://ccinfoweb.ccohs.ca/rtecs/search.html). Adult minimum therapeutic initial dose, considered as a potentially useful indicator for the threshold impact on the general healthy population, were obtained from either Rxlist.com or PDR (Physicians’ Desk Reference, 2009) for all the drugs except those which took effect through non-oral pathways (i.e. topical, ophthalmic, or inhalant drugs). Experimental data for ecological effects were not available for most of the drugs on our list; therefore toxicity information was obtained from the ECOSAR module of the EPI Suite (U.S. EPA, 2011), which estimates 96-hr EC50 and ChVs for algae, 48-hr LC50s and ChVs for daphnid and 96-hr LC50 and ChVs for fish. These estimations were based on structure-activity analysis of these compounds.
2.3 Estimating Toxic Loads
As mentioned above, in order to quantify and compare the potential health impacts of pharmaceuticals, we calculated a compound’s “toxic load" as:
| (4) |
where MLi is the mass loading of compound i (kg/yr), TDij is the toxic dose of compound i for toxicity endpoint j (smaller TD indicates a more toxic compound), and TLi,j is the toxic load of compound i considering toxicity endpoint j (mg/day for human endpoints, mg/kg for mouse/rat endpoints, mg/L for aquatic endpoints). TL was used as the metric for ranking PhACs and was log-transformed in order to obtain a normal distribution of the data.
2.4 Calculating Priority Scores
Because TLs for different endpoints have different units, standard normal deviates were calculated for each log-transformed TL value, in order to standardize and compare ranks between endpoints. This is named as a priority score (PS) of compound i for endpoint j:
| (5) |
where logTLij is the log transformed toxic loading of drug i for endpoint j; is the mean of log transformed toxic loads of all drugs for endpoint j; Std(logTLj) is the standard deviation of log transformed toxic loads of all drugs for endpoint j.
We also calculated overall priority scores and combined priority scores for human, mammal and aquatic endpoints by averaging the PSs across all endpoints or for endpoints within the same category. Overall and categorical priority scores for different drug classes were calculated similarly; but instead of using individual toxic loads, these were calculated by summing toxic loads of all drugs in the same class, assuming that drugs in the same therapeutic class have similar modes of action. In this case, i in equation (5) refers to class i instead of an individual compound.
3 Results
3.1 Annual prescribed mass (P) and mass loading (ML)
The mass prescribed per year (P; kg/yr) was estimated for all top 200 U.S. prescription pharmaceutical compounds in 2009. P ranged over 8 orders of magnitude, from less than 1 kg/yr to over 107 kg/yr (Figure 1(a); P estimates for all compounds are provided in Table S1).
Figure 1.
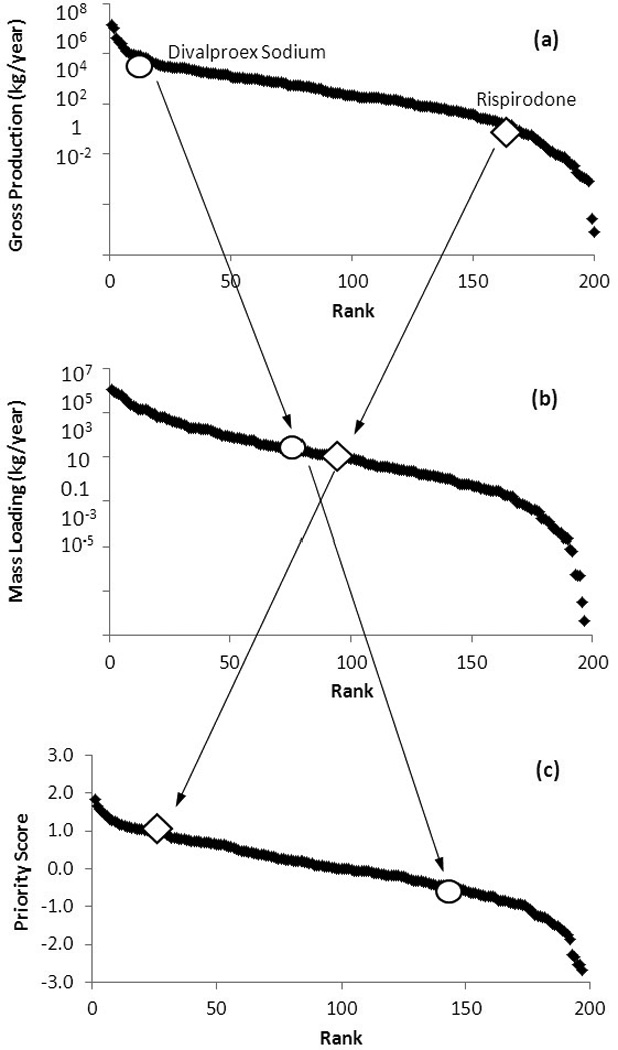
Relationship and difference between (a) production, (b) mass loading and (c) priority scores. Changes in the ranks of divalproex sodium (circle) and risperidone (diamond) are highlighted.
Metabolism and WWTP-removal substantially attenuate the input (mass loading) of most PhACs into the environment. For half of the compounds investigated, less than 10% of P will be delivered to aquatic environments (Table S1) assuming 100% of prescribed pharmaceuticals are consumed by the patient and that all human excreta undergoes secondary wastewater treatment.
The unaltered fraction, ei×di, varies over several orders of magnitude (10−5 to over 0.9), but some general trends are observable across drug classes (Figure 2). Contraceptives, antiulcers, and non-steroid anti-inflammatory drugs (NSAIDs) are removed to the greatest degree (ei×di=10−4 to 10−3). There is intermediate removal of antihypertensives, anticonvulsants and antidepressants (ei×di=10−3 to 10−1), and relatively low removal of bronchodilators, antiinfectives, antidiabetics and antibiotics (ei×di=10−1 to >0.9).
Figure 2.
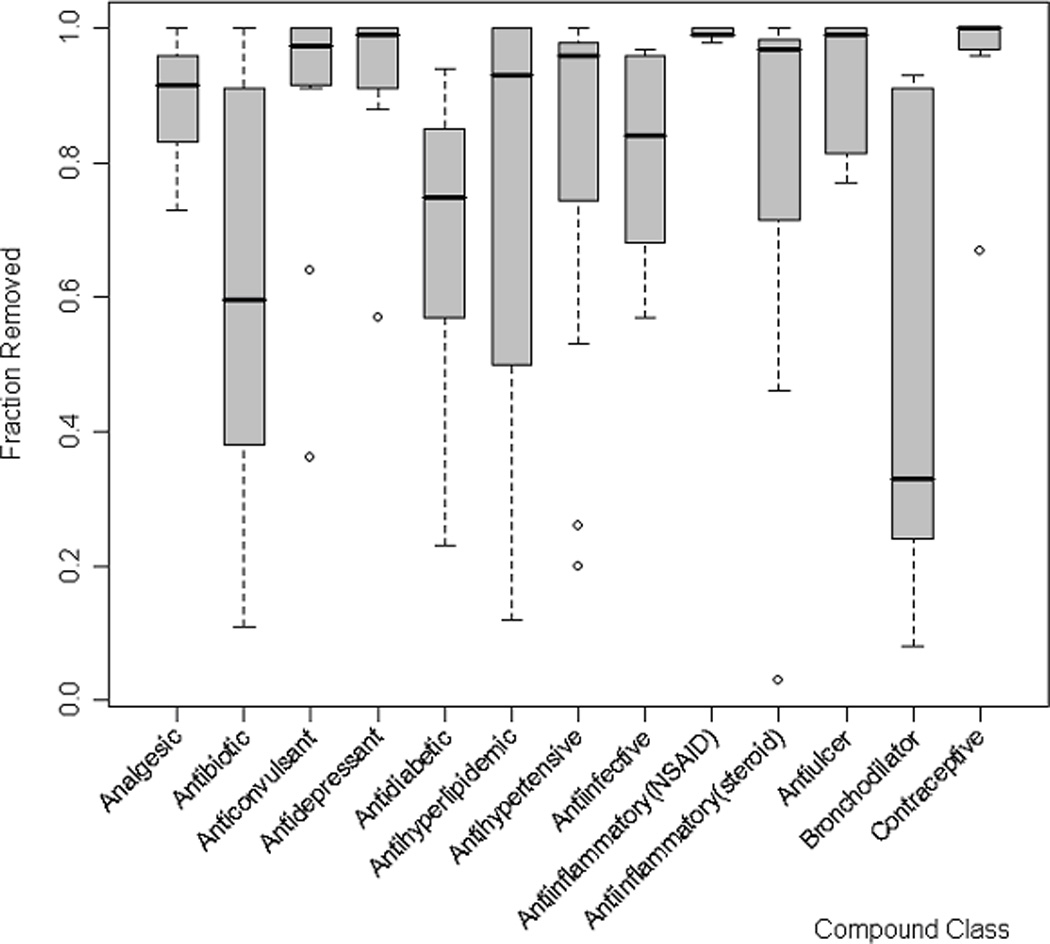
Removed fraction (δremoval) varies among compound classes. Only classes containing 5 or more compounds are shown. δremoval = fraction metabolized * fraction removed through WWTP treatment.
Despite the substantial removal of many compounds, 46 PhACs are loaded to the aquatic environment at rates greater than 104 kg/yr, with a maximum ML of 106 kg/yr (Metformin HCl). Of the top 20 compounds in mass loadings, only 12 also appeared on the list of top 20 produced compounds and 7 on the list of top 20 prescribed drugs (Table 1). This indicates that including metabolism and WWTP removal substantially shifted the relative ranking of compounds. For example, divalproex sodium was ranked at #10 in P, but its rank in ML moved to #77; in the meanwhile, risperidone had a rank of #162 in P, but was ranked at #89 in ML (Figure 1(a) and 1(b). This showed how much the ranks could be affected when additional information was incorporated into the ranking scheme.
Table 1.
Top 20 prescribed, produced and environmentally loaded PhACs.
| Rank | Top 20 Prescribed | Top 20 Produced | Top 20 Loaded | |||
|---|---|---|---|---|---|---|
| Compound | # of Prescriptions |
Compound | Production (kg/yr) |
Compound | Mass Loading (kg/yr) |
|
| 1 | Acetaminophen | 197,921 | Potassium Chloride | 1.46 × 107 | Metformin HCl | 1.10 × 106 |
| 2 | Hydrocodone Bitartrate | 125,813 | Acetaminophen | 1.11 × 107 | Polyethylene Glycol | 8.73 × 105 |
| 3 | Hydrochlorothiazide | 114,809 | Metformin HCl | 4.41 × 106 | Amoxicillin Trihydrate | 8.24 × 105 |
| 4 | Lisinopril | 97,442 | Ranitidine HCl | 2.79 × 106 | Cephalexin | 7.48 × 105 |
| 5 | Levothyroxine Sodium | 92,389 | Gabapentin | 2.52 × 106 | Ranitidine HCl | 6.52 × 105 |
| 6 | Simvastatin | 82,584 | Amoxicillin Trihydrate | 1.74 × 106 | Trimethoprim | 4.39 × 105 |
| 7 | Amoxicillin | 71,934 | Ibuprofen | 1.25 × 106 | Furosemide | 4.02 × 105 |
| 8 | Metoprolol Succinate | 68,821 | Cephalexin | 1.20 × 106 | Levothyroxine Sodium | 3.03 × 105 |
| 9 | Amlodypine Besylate | 63,734 | Methocarbamol | 9.40 × 105 | Fluticasone Propionate | 2.36 × 105 |
| 10 | Metformin HCl | 58,761 | Divalproex Sodium | 9.18 × 105 | Gabapentin | 2.00 × 105 |
| 11 | Ethinyl Estradiol | 53,324 | Polyethylene Glycol | 8.90 × 105 | Atenolol | 1.83 × 105 |
| 12 | Azithromycin | 49,902 | Levothyroxine Sodium | 8.32 × 105 | Hydrochlorothiazide | 1.53 × 105 |
| 13 | Albuterol Sulfate | 49,587 | Metoprolol Succinate | 8.26 × 105 | Ciprofloxacin HCl | 1.46 × 105 |
| 14 | Oxycodone HCl | 46,685 | Trimethoprim | 6.88 × 105 | Acetaminophen | 1.38 × 105 |
| 15 | Alprazolam | 44,467 | Furosemide | 6,62 × 105 | Clopidogrel Bisulfate | 1.38 × 105 |
| 16 | Atorvastatin Calcium | 44,150 | Sulfamethoxazole | 6.14 × 105 | Levetiracetam | 1.26 × 105 |
| 17 | Fluticasone Propionate | 42,683 | Ciprofloxacin HCl | 4.57 × 105 | Levofloxacin | 1.00 × 105 |
| 18 | Atenolol | 41,139 | Omeprazole | 4.29 × 105 | Sulfamethoxazole | 9.57 × 104 |
| 19 | Omeprazole | 39,700 | Guaifenesin | 3.83 × 105 | Fexofenedine HCl | 8.71 × 104 |
| 20 | Zolpidem Tartrate | 36,980 | Carisoprodol | 3.54 × 105 | Valacyclovir HCl | 6.58 × 104 |
3.2 Toxic Load (TL) and Priority Score (PS) Ranking
TLs are presented for 197 out of the 200 compounds across the 12 toxicity endpoints (Table S1). For the other three drugs (omega-3 acid ethyl esters, insulin and influenza hemagglutinin), removal and toxicological data were not available. Thirteen PhACs (ranitidine HCl, atenolol, clopidogrel bisulfate, furosemide, tramadol HCl, levothyroxine sodium, simvastatin, hydrochlorothiazide, trimethoprim, acetaminophen, bupropion HCl, lamotrigine, and levetiracetam) appear in the top 20 in at least 6 of the 12 endpoint categories, suggesting potential toxicity across a broad range of endpoints. Of particular note is ranitidine HCl, which ranked in the top 20 in all 12 TL categories.
The compounds that rank highly in multiple TL categories and those that tend to be toxic for both human and aquatic species should receive more attention. To combine the TLs into a single ranking metric, priority scores were generated for all 197 compounds. The approach used took into account missing TD data, which led to an inability to calculate TLs in some categories for some compounds. The top 20 overall compounds are presented in Table 2, assuming all TL categories can be weighted equally. Table 2 also presents the top 20 compounds in PS for adult humans, mouse/rat or aquatic species. Overall, 11 of the top 20 compounds are also in the top 20 list for mass loading. However, only 7 of these top 20 compounds were in the top 20 list for production (Table 1). This indicates further shifts in relative ranks due to the introduction of toxicity information into the ranking scheme. For example, ranks of divalproex sodium and risperidone further in-/decreased to #141 and #19 in PS, respectively, from #77 and #89 in ML (Figure 1 (b) and (c).
Table 2.
Top 10 individual priority scores over all toxicity endpoints and over human, mammal, and aquatic toxicity endpoints.
| All Endpoints (N=12) | Human Endpoints (N=2) | Mouse/Rat Endpoints (N=4) | Aquatic Endpoints (N=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Compound | n | Priority Score |
Compound | n | Priority Score |
Compound | n | Priority Score |
Compound | n | Priority Score |
| 1 | Levothyroxine Sodium | 12 | 1.82 | Levothyroxine Sodium | 2 | 3.13 | Fluticasone Propionate | 2 | 2.07 | Montelukast Sodium | 6 | 2.03 |
| 2 | Ranitidine HCl | 12 | 1.65 | Fluticasone Propionate | 2 | 2.46 | Ranitidine HCl | 4 | 1.95 | Clopidogrel Bisulfate | 6 | 1.77 |
| 3 | Clopidogrel Bisulfate | 9 | 1.57 | Hydrochlorothiazide | 2 | 1.57 | Furosemide | 4 | 1.78 | Levothyroxine Sodium | 6 | 1.65 |
| 4 | Fluticasone Propionate | 10 | 1.51 | Furosemide | 2 | 1.44 | Levetiracetam | 4 | 1.70 | Simvastatin | 6 | 1.61 |
| 5 | Furosemide | 12 | 1.44 | Ranitidine HCl | 2 | 1.36 | Metformin HCl | 4 | 1.65 | Ranitidine HCl | 6 | 1.55 |
| 6 | Montelukast Sodium | 9 | 1.40 | Lisinopril | 1 | 1.25 | Clopidogrel Bisulfate | 1 | 1.59 | Telmisartan | 6 | 1.51 |
| 7 | Trimethoprim | 10 | 1.30 | Rosuvastatin Calcium | 1 | 1.22 | Atenolol | 4 | 1.52 | Bupropion HCl | 6 | 1.43 |
| 8 | Atenolol | 12 | 1.27 | Ciprofloxacin HCl | 2 | 1.21 | Hydrochlorothiazide | 3 | 1.50 | Trimethoprim | 6 | 1.42 |
| 9 | Tramadol HCl | 12 | 1.26 | Trimethoprim | 1 | 1.19 | Levothyroxine Sodium | 4 | 1.43 | Nitrofuratoin | 6 | 1.37 |
| 10 | Simvastatin | 11 | 1.23 | Estrogen, conjugated | 1 | 1.17 | Triamterene | 2 | 1.42 | Tramadol HCl | 6 | 1.36 |
| 11 | Hydrochlorothiazide | 11 | 1.16 | Clonidine | 2 | 1.17 | Tramadol HCl | 4 | 1.32 | Hydroxyzine Pamoate | 6 | 1.35 |
| 12 | Acetaminophen | 12 | 1.15 | Topiramate | 2 | 1.13 | Fexofenadine HCl | 1 | 1.32 | Acetaminophen | 6 | 1.29 |
| 13 | Metformin HCl | 12 | 1.14 | Fexofenadine HCl | 2 | 1.13 | Acetaminophen | 4 | 1.30 | Amoxicillin Trihydrate | 6 | 1.28 |
| 14 | Bupropion HCl | 12 | 1.12 | Alendronate Sodium | 1 | 1.09 | Gabapentin | 4 | 1.27 | Hydroxychloroquine Sulfate | 6 | 1.28 |
| 15 | Olmesartan Medoxomil | 7 | 1.10 | Risperidone | 2 | 1.06 | Risperidone | 4 | 1.24 | Pioglitazone HCl | 6 | 1.27 |
| 16 | Sulfamethoxazole | 9 | 1.09 | Lamotrigine | 2 | 1.06 | Lamotrigine | 4 | 1.24 | Sulfamethoxazole | 6 | 1.23 |
| 17 | Pioglitazone HCl | 8 | 1.07 | Atenolol | 2 | 1.04 | Polyethylene Glycol | 1 | 1.20 | Irbesartan | 6 | 1.22 |
| 18 | Levetiracetam | 12 | 1.06 | Tadalafil | 1 | 1.02 | Citalopram HBr | 4 | 1.20 | Furosemide | 6 | 1.21 |
| 19 | Risperidone | 12 | 1.06 | Oxycodone HCl | 2 | 0.99 | Hydrocodone Bitartrate | 3 | 1.15 | Atenolol | 6 | 1.18 |
| 20 | Citalopram HBr | 12 | 1.04 | Simvastatin | 2 | 0.98 | Penicillin V Potassium | 2 | 1.12 | Trazodone HCl | 6 | 1.18 |
Average priority scores for compound classes showed a larger span than single compound priority scores (Table 3). Thyroid hormone ranked high in both the human and the overall priority scores, although only one drug belonged to this class (levothyroxine sodium). Analgesic and antihypertensive drugs showed up in the top 10 of all four categories, followed by antidiabetics, antibiotics, antiulcers, bronchodilators, antidepressants and diuretics, which showed up in the top 10 of overall and two of the combined endpoint categories. The antiasthmatic class, which also contained only one compound, montelukast sodium, ranked first in the overall priority scores, resulting from its high rankings in aquatic endpoints.
Table 3.
Top 10 drug classes in priority scores over all toxicity endpoints and over human, mammal, and aquatic toxicity endpoints.
| Rank | All Endpoints | Human Endpoints | Mouse/Rat Endpoints | Aquatic Endpoints | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | n | Priority Score |
Class | n | Priority Score |
Class | n | Priority Score |
Class | n | Priority Score |
|
| 1 | Antiasthmatic | 1 | 2.27 | Thyroid Hormone | 1 | 7.19 | Antiulcer | 7 | 2.45 | Antiasthmatic | 1 | 3.51 |
| 2 | Antibiotic | 15 | 1.68 | Bronchodilator | 5 | 0.58 | Antidiabetic | 5 | 2.04 | Antibiotic | 15 | 2.71 |
| 3 | Thyroid Hormone | 1 | 1.40 | Diuretic | 4 | −0.02 | Bronchodilator | 5 | 1.63 | Antidiabetic | 5 | 0.92 |
| 4 | Antidiabetic | 5 | 1.11 | Antihypertensive | 23 | −0.14 | Analgesic | 12 | 1.42 | Thyroid Hormone | 1 | 0.52 |
| 5 | Antiulcer | 7 | 0.97 | Antiulcer | 7 | −0.14 | Antibiotic | 15 | 1.06 | Antihypertensive | 23 | 0.43 |
| 6 | Bronchodilator | 5 | 0.54 | Analgesic | 12 | −0.14 | Diuretic | 4 | 0.83 | Antiulcer | 7 | 0.35 |
| 7 | Analgesic | 12 | 0.46 | Antipsychotic | 4 | −0.15 | Antidepressant | 11 | 0.59 | Antidepressant | 11 | 0.17 |
| 8 | Antidepressant | 11 | 0.26 | Menopause Therapy | 1 | −0.15 | Antihypertensive | 23 | 0.16 | Anticoagulant | 2 | 0.14 |
| 9 | Antihypertensive | 23 | 0.25 | Erectile Dysfunction Treatment | 1 | −0.15 | Anticonvulsant | 12 | 0.14 | Analgesic | 12 | 0.01 |
| 10 | Diuretic | 4 | 0.19 | Antiparkinsonian | 1 | −0.15 | Antiinfective | 5 | −0.07 | Antihyperlipidemic | 10 | −0.01 |
4 Discussion
This study was based on the 2009 top 200 prescription drug list in the U.S. (Rxlist). A preliminary study was conducted from 2004 to 2006, examining a similar list of top prescribed drugs in 2003 (data not shown). A comparison between the two lists revealed large discrepancies, that is, more than 30% of the drugs on the 2009 list did not appear on the 2003 list. Furthermore, it seems that the amounts and types of anti-depresssants, anti-hypertensives and birth control drugs used have been increasing over the years, as well as many relatively new drugs that have only been on the market for less than two decades, such as montelukast and carvedilol. For example, of the top 10 ranked drugs across all endpoints (Table 2), 8 were “new" drugs that did not show up on the 2003 list (levothyroxine sodium, fluticasone propionate, montelukast sodium, clopidegrel bisulfate, simvastatin, telmisartan, rosuvastatin calcium, levitiracetam), of which 4 (montelukast sodium, clopidegrel bisulfate, telmisartan, rosuvastatin calcium) were only prescribed in branded forms. This may further contribute to the lack of information on these compounds, and to our lack of understanding of the environmental impact of changing PhACs as a whole over time.
Basing the prioritization on prescription rather than occurrence data allowed us to examine these “new" drugs, for which measurements in the natural water systems rarely exist In addition, occurrence or measurements data are usually site-specific, which can be an advantage for site-specific studies, but may become inappropriate in prioritization methods which tend to study PhACs on a broader scale, as these data are often associated with large spatial and temporal variation and uncertainties.
Concerns have long been raised upon the interactions among different pharmaceuticals in the aquatic environment. An early USGS study (Kolpin et al., 2002) found that the detection of multiple drugs was common, with a median of 7 and up to 38 found in one sample, indicating that interaction between compounds may be important. However, the ability of individuals PhACs or PhAC-mixtures to induce toxic effects at these concentrations, and the most appropriate toxicity endpoints and indicator organisms, remain topics of on-going investigation and debate. A study that exposed human cell lines to a suite of PhACs at environmentally relevant concentrations found that the drug mixture inhibited the growth of human embryonic cells, activated stress response signaling protein kinases, produced morphological changes, and induced over expression of glutathione S transferase P1 gene, suggesting that a mixture of drugs at ng/L levels can inhibit cell proliferation by affecting their physiology and morphology (Pomati et al., 2006). On the other hand, Webb et al. (2003) argue that the potential daily exposure to approximately 60 PhACs in water supplies is at least three orders of magnitude lower than the daily therapeutic dose and therefore exposure to these PhACs through drinking water does not pose an unacceptable human health risk. Other studies have come to similar conclusions, finding no environmental PhAC human health risks (World Health Organization, 2012; Bruce et al., 2010; Schwab et al., 2005; Webb et al., 2003; Schulman et al., 2002; Christensen, 1998). However, few other studies have explored on such additive, synergistic or antagonistic effects (Escher et al., 2005).
As a preliminary consideration on the potential risk of drug mixtures, we calculated the priority scores of different therapeutic classes, assuming additive effect due to similar modes of action, which could be a source of potential uncertainties due to oversimplification. However, the results still provide some meaningful implications. Many of the drug classes that ranked high in priority scores (Table 3) have not drawn much attention in previous environmental studies, which tended to focus on the most commonly used and/or largest groups such as antibiotics (Figure 3). For example, the environmental impact of the potentially harmful antiasthmatic drug to aquatic organisms, montelukast sodium (Tables 2 & 3), has not been studied at all. It is also worth noting that summed TL of a certain category does not necessarily increase with increasing number of drugs, indicating that common consumption or detection of a number of different drugs in a specific usage category is not a strong enough criterion to justify the concentration of studies that have been previously performed on such drug groups.
Figure 3.
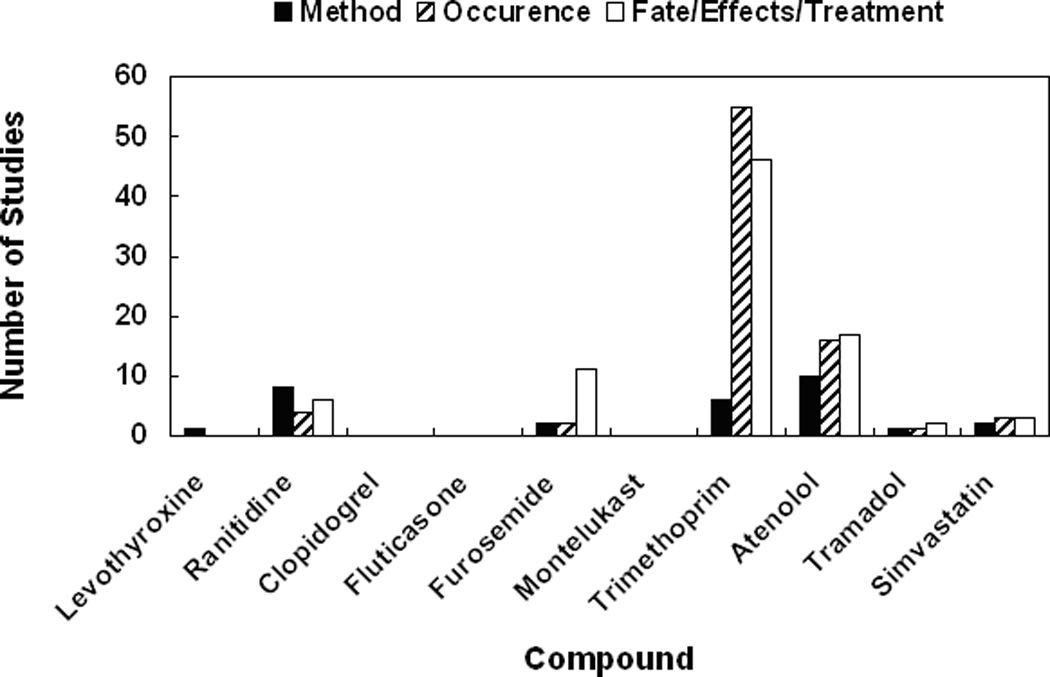
Number of studies found in a “Web of Science" search for the top 10 compounds in overall priority score using keyword “<compound name> AND environment" as of October, 2010. The search results were divided into three categories: studies on method for determining or detecting the compound, on environmental occurrences of the compound or on fate, effect or treatment of the compound.
For the sake of simplicity, this study focused on generic human prescription pharmaceuticals and did not include veterinary or over-the-counter drugs, as prescription or sales data were largely unavailable for these compounds. This may result in an underestimation of the health impact of PhACs as a whole. However, in conjunction with our main goals, the approach developed can be easily applied to other compounds such as patented drugs, chemotherapy drugs, and other drugs utilized or prescribed in hospital settings, as the data become easily accessible. In addition, this study only considered certain toxicity outcomes, but our approach can also be applied to other important toxicity endpoints, such as carcinogenicity and endocrine disruption.
Only the unmetabolized portions of the parent compounds are considered here. While metabolism makes many lipid soluble drugs more water soluble, it also can render them them inert. There are not enough data at this time to consider metabolites for all the drugs. Drugs with active metabolites should be examined in the future, as many drugs have active metabolites that are more potent than original or parent drug (Sherer, 2006).
This paper utilized only currently available and readily accessible data, in order to assess the feasibility of our general approach. This would inevitably introduce uncertainties that are commonly associated with data-intensive studies. One uncertainty lies in the assumption that all filled prescriptions were completely consumed by the patient, because detailed information on the disposal of un-consumed pharmaceuticals is not available. In fact, it is possible that the actual disposal rate of such un-consumed drugs is a large fraction of the total production that was calculated and used in this study. Previous studies have showed that consumers may throw away the unused pharmaceuticals immediately after treatment is finished (Slack et al., 2005). Thus, PhACs may enter the environment through direct disposal into the sewage system. If drugs that are disposed of, especially through the sewage system, make up a significant portion of the total yearly production amount, excluding such information may result in an underestimation of the health impact. However, it is most likely, as several studies have demonstrated, that only a small percentage of pharmaceuticals are actually released into environment through household disposal (Bound et al., 2006; Bound and Voulvoulis, 2005).
The uncertainty from relying on model estimations, as substitutes for the mostly non-existing measured data, such as the WWTP removal rates and ecotoxicity, is largely unknown. For example, the STP model used for estimating WWTP removals assumes a standard wastewater treatment plant setting and is not appropriate for predicting removal rates at a specific plant. While WWTP removal rates have been measured for a few pharmaceuticals in several studies (Castiglioni et al., 2006; Clara et al., 2005; Joss et al., 2005), these rates are likely to vary significantly among different WWTPs or different treatment procedures, thus for the purpose of developing a generalizable screening method they were not used here. However, for those PhAC compounds of which measured WWTP removal rates were available, our model outputs are generally within the range of measured data (Gros et al., 2010; Onesios et al., 2009; Bound and Voulvoulis, 2005), and for the few compounds that have different model and measured removal rates, the model tends to predict smaller removal rates, rendering more conservative results. In addition, around 20% of the households in the U.S. utilize septic systems (U.S. Census Bureau, 2008), although PhAC removal rates in septic systems are less studied (Conn et al., 2010). Furthermore, PhACs can enter the environment through WWTP sludge (Jelic et al., 2011), thus using WWTP removal rates alone may lead to an underestimation of their risk. Finally, the ecological risks posed by PhACs are difficult to assess because of the multiple organisms - from microbes to vertebrates and multiple possible endpoints (reproductive success, growth rate) that comprise a healthy ecosystem. Of the thousands of PhACs used in the U.S. and throughout the world, aquatic toxicity studies have been conducted for relatively few compounds and organisms.
Significant uncertainties may also rise from using the toxicity data that are currently available. With regards to the adult human toxicity dose data, because therapeutic dose varies with such factors as age and health status, it is possible that the health impact was overestimated. The reduced accountability of using “lowest reported" toxic doses as LOAELs rather than developing LOAELs from studies may result in an underestimation of the health impacts. Incomplete toxicity dose information results in incomplete TLs, therefore each category of TLs does not contain the same number of compounds, presenting a major difficulty. Moreover, it is known that there are significant interspecies and intraspecies uncertainties in regards to toxicity doses. Very few studies to date have explored extrapolating toxicity of pharmaceuticals amongst species, especially between organisms and human (Schreiber et al., 2011; Berninger and Brooks, 2010). Even metabolism and adult doses, which are derived from human pharmacological studies, show a significant variation among individuals. All of our calculations were based on the most conservative single-point estimates (e.g. maximum daily dose, lowest metabolism rate), aimed at making protective predictions but probably resulted in an overestimation of the effect on potential health impacts.
In general, although there might be large uncertainties associated with many different aspects and parameters of this approach, it is unlikely that these uncertainties would affect the final ranks significantly, due to the remarkably large range of production, attenuation and toxicity terms used in ranking (Figure 1). For instance, using model estimates for WWTP removal rates may result in one of the largest uncertainties discussed above, and the measured rates generally vary within a factor of 10 among different WWTPs and conditions (Rosario-Ortiz et al., 2010), which is outweighed by differences in the final priority scores among compounds spanning over several orders of magnitude. Therefore, our prioritization method can still be considered a robust approach under the influence of various uncertainties.
5 Conclusions
In this paper, a simple, quantitative risk-based ranking approach was developed and evaluated for prioritizing environmental PhACs. This approach combines estimates of production, attenuation and toxicity thresholds for multiple endpoints, and draws on publicly available data to quantitatively rank PhACs based on their potential ecological and human health impacts. While there were a lot of uncertainties associated with this approach, we hypothesized that differences in our ranking metrics amongst these compounds would be large enough to overcome the given uncertainties, and this simple and environmentally relevant approach may be used as a valuable tool for screening of target PhACs in future environmental research and regulations.
Supplementary Material
Acknowledgments
We would like to thank Martha Chang and Angela M. Renfroe for their work on initial data collection and analysis, and thank Dr. Laurel Schaider for her help on reviewing the manuscript. This study was made possible by Harvard University Center for the Environment (HUCE) and Kresge Center Grant ES00002 from the National Institute of Environmental Health Sciences (NIEHS), NIH. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- American Society of Hospital Pharmacists. AHFS drug information. Bethesda, Md: Published by authority of the Board of Directors of the American Society of Hospital Pharmacists; 2007. [Google Scholar]
- Andreozzi R, Raffaele M, Nicklas P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere. 2003;50:1319–1330. doi: 10.1016/s0045-6535(02)00769-5. [DOI] [PubMed] [Google Scholar]
- Batt AL, Bruce IB, Aga DS. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environmental Pollution. 2006;142:295–302. doi: 10.1016/j.envpol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Belfroid A, Van der Horst A, Vethaak A, Schafer A, Rijs G, Wegener J, Cofino W. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Science of the Total Environment. 1999;225:101–108. doi: 10.1016/s0048-9697(98)00336-2. [DOI] [PubMed] [Google Scholar]
- Bendz D, Paxeus N, Ginn T, Loge F. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Hoje River in Sweden. Journal of Hazardous Materials. 2005;122:195–204. doi: 10.1016/j.jhazmat.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Berninger JP, Brooks BW. Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicology Letters. 2010;193:69–78. doi: 10.1016/j.toxlet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Bound J, Kitsou K, Voulvoulis N. Household disposal of pharmaceuticals and perception of risk to the environment. Environmental Toxicology and Pharmacology. 2006;21:301–307. doi: 10.1016/j.etap.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bound J, Voulvoulis N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environmental Health Perspectives. 2005;113:1705–1711. doi: 10.1289/ehp.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce GM, Pleus RC, Snyder SA. Toxicological Relevance of Pharmaceuticals in Drinking Water. Environmental Science & Technology. 2010;44:5619–5626. doi: 10.1021/es1004895. [DOI] [PubMed] [Google Scholar]
- Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R. Strategic survey of therapeutic drugs in the rivers Po and Lambro in northern Italy. Environmental Science & Technology. 2003;37:1241–1248. [Google Scholar]
- Carballa M, Omil F, Lema J, Llompart M, Garcia-Jares C, Rodriguez I, Gomez M, Ternes T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Research. 2004;38:2918–2926. doi: 10.1016/j.watres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Castiglioni S, Zuccato E, Crisci E, Chiabrando C, Fanelli R, Bagnati R. Identification and measurement of illicit drugs and their metabolites in urban wastewater by liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2006;78:8421–8429. doi: 10.1021/ac061095b. [DOI] [PubMed] [Google Scholar]
- Choi K, Kim Y, Park J, Park CK, Kim M, Kim HS, Kim P. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Science of the Total Environment. 2008;405:120–128. doi: 10.1016/j.scitotenv.2008.06.038. [DOI] [PubMed] [Google Scholar]
- Christensen F. Pharmaceuticals in the environment - A human risk? Regulatory Toxicology and Pharmacology. 1998;28:212–221. doi: 10.1006/rtph.1998.1253. [DOI] [PubMed] [Google Scholar]
- Clara M, Strenn B, Gans O, Martinez E, Kreuzinger N, Kroiss H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Research. 2005;39:4797–4807. doi: 10.1016/j.watres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Conn KE, Lowe KS, Drewes JE, Hoppe-Jones C, Tucholke MB. Occurrence of Pharmaceuticals and Consumer Product Chemicals in Raw Wastewater and Septic Tank Effluent from Single-Family Homes. Environmental Engineering Science. 2010;27:347–356. [Google Scholar]
- Cooper ER, Siewicki TC, Phillips K. Preliminary risk assessment database and risk ranking of pharmaceuticals in the environment. Science of the Total Environment. 2008;398:26–33. doi: 10.1016/j.scitotenv.2008.02.061. [DOI] [PubMed] [Google Scholar]
- Dougherty JA, Swarzenski PW, Dinicola RS, Reinhard M. Occurrence of Herbicides and Pharmaceutical and Personal Care Products in Surface Water and Groundwater around Liberty Bay, Puget Sound, Washington. Journal of Environmental Quality. 2010;39:1173–1180. doi: 10.2134/jeq2009.0189. [DOI] [PubMed] [Google Scholar]
- Escher B, Bramaz N, Eggen R, Richter M. In vitro assessment of modes of toxic action of pharmaceuticals in aquatic life. Environmental Science & Technology. 2005;39:3090–3100. doi: 10.1021/es048590e. [DOI] [PubMed] [Google Scholar]
- Fick J, Lindberg RH, Tysklind M, Larsson DGJ. Predicted critical environmental concentrations for 500 pharmaceuticals. Regulatory Toxicology and Pharmacology. 2010;58:516–523. doi: 10.1016/j.yrtph.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Fick J, Soederstrom H, Lindberg RH, Phan C, Tysklind M, Larsson DGJ. Contamination of Surface, Ground, and Drinking Water from Pharmaceutical Production. Environmental Toxicology and Chemistry. 2009;28:2522–2527. doi: 10.1897/09-073.1. [DOI] [PubMed] [Google Scholar]
- Foster HR, Burton GA, Basu N, Werner EE. Chronic Exposure to Fluoxetine (Prozac) Causes Developmental Delays in Rana Pipiens Larvae. Environmental Toxicology and Chemistry. 2010;29:2845–2850. doi: 10.1002/etc.345. [DOI] [PubMed] [Google Scholar]
- Gros M, Petrović M, Ginebreda A, Barceló D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environment International. 2010;36:15–26. doi: 10.1016/j.envint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Hirsch R, Ternes T, Haberer K, Kratz K. Occurrence of antibiotics in the aquatic environment. Science of the Total Environment. 1999;225:109–118. doi: 10.1016/s0048-9697(98)00337-4. [DOI] [PubMed] [Google Scholar]
- Huggett D, Khan I, Foran C, Schlenk D. Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent. Environmental Pollution. 2003;121:199–205. doi: 10.1016/s0269-7491(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Jelic A, Gros M, Ginebreda A, Cespedes-Sanchez R, Ventura F, Petrovic M, Barcelo D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Research. 2011;45:1165–1176. doi: 10.1016/j.watres.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Jones O, Voulvoulis N, Lester J. Human pharmaceuticals in wastewater treatment processes. Critical Reviews in Environmental Science and Technology. 2005;35:401–427. [Google Scholar]
- Joss A, Keller E, Alder A, Gobel A, McArdell C, Ternes T, Siegrist H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Research. 2005;39:3139–3152. doi: 10.1016/j.watres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Joss A, Zabczynski S, Gobel A, Hoffmann B, Loffler D, McArdell C, Ternes T, Thomsen A, Siegrist H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Research. 2006;40:1686–1696. doi: 10.1016/j.watres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K, Meyer M. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Science of the Total Environment. 2006;361:196–207. doi: 10.1016/j.scitotenv.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Kolpin D, Furlong E, Meyer M, Thurman E, Zaugg S, Barber L, Buxton H. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environmental Science & Technology. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kumar A, Xagoraraki I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: A proposed ranking system. Science of the Total Environment. 2010;408:5972–5989. doi: 10.1016/j.scitotenv.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Lienert J, Gudel K, Escher BI. Screening method for ecotoxicological hazard assessment of 42 pharmaceuticals considering human metabolism and excretory routes. Environmental Science & Technology. 2007;41:4471–4478. doi: 10.1021/es0627693. [DOI] [PubMed] [Google Scholar]
- Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andrews DM. Antidepressants and their Metabolites in Municipal Wastewater, and Downstream Exposure in an Urban Watershed. Environmental Toxicology and Chemistry. 2010;29:79–89. doi: 10.1002/etc.27. [DOI] [PubMed] [Google Scholar]
- Nieto A, Peschka M, Borrull F, Pocurull E, Maria Marce R, Knepper TP. Phosphodiesterase type V inhibitors: Occurrence and fate in wastewater and sewage sludge. Water Research. 2010;44:1607–1615. doi: 10.1016/j.watres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Onesios KM, Yu JT, Bouwer EJ. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: a review. Biodegradation. 2009;20:441–466. doi: 10.1007/s10532-008-9237-8. [DOI] [PubMed] [Google Scholar]
- Perazzolo C, Morasch B, Kohn T, Magnet A, Thonney D, Chevre N. Occurrence and Fate of Micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part I: Priority List for Environmental Risk Assessment of Pharmaceuticals. Environmental Toxicology and Chemistry. 2010;29:1649–1657. doi: 10.1002/etc.221. [DOI] [PubMed] [Google Scholar]
- Physicians’ Desk Reference. Physicians’ desk reference. 63th edition. Oradell, NJ: Medical Economics Co.; 2009. [Google Scholar]
- Pomati F, Castiglioni S, Zuccato E, Fanelli R, Vigetti D, Rossetti C, Calamari D. Effects of a complex mixture of therapeutic drugs at environmental levels on human embryonic cells. Environmental Science & Technology. 2006;40:2442–2447. doi: 10.1021/es051715a. [DOI] [PubMed] [Google Scholar]
- Redshaw CH, Cooke MP, Talbot HM, McGrath S, Rowland SJ. Low biodegradability of fluoxetine HCl, diazepam and their human metabolites in sewage sludge-amended soil. Journal of Soils and Sediments. 2008;8:217–230. [Google Scholar]
- Roberts P, Thomas K. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Science of the Total Environment. 2006;356:143–153. doi: 10.1016/j.scitotenv.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Rosario-Ortiz FL, Wert EC, Snyder SA. Evaluation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater. Water Research. 2010;44:1440–1448. doi: 10.1016/j.watres.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Sacher F, Gabriel S, Metzinger M, Wenz M. Pharmaceuticals in ground and surface waters. Chimia. 2003;57:29–31. [Google Scholar]
- Sanderson H, Johnson D, Reitsma T, Brain R, Wilson C, Solomon K. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regulatory Toxicology and Pharmacology. 2004;39:158–183. doi: 10.1016/j.yrtph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Gündel U, Franz S, Küster A, Rechenberg B, Altenburger R. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regulatory Toxicology and Pharmacology. 2011;61:261–275. doi: 10.1016/j.yrtph.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Schulman L, Sargent E, Naumann B, Faria E, Dolan D, Wargo J. A human health risk assessment of pharmaceuticals in the aquatic environment. Human and Ecological Risk Assessment. 2002;8:657–680. [Google Scholar]
- Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM. Antidepressant Pharmaceuticals in Two US Effluent-Impacted Streams: Occurrence and Fate in Water and Sediment, and Selective Uptake in Fish Neural Tissue. Environmental Science & Technology. 2010;44:1918–1925. doi: 10.1021/es9022706. [DOI] [PubMed] [Google Scholar]
- Schwab B, Hayes E, Fiori J, Mastrocco F, Roden N, Cragin D, Meyerhoff R, D’Aco V, Anderson P. Human pharmaceuticals in US surface waters: A human health risk assessment. Regulatory Toxicology and Pharmacology. 2005;42:296–312. doi: 10.1016/j.yrtph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Sherer J. Pharmaceuticals in the environment. American Journal of Health-System Pharmacy. 2006;63:174–178. doi: 10.2146/ajhp050123. [DOI] [PubMed] [Google Scholar]
- Slack R, Zerva P, Gronow J, Voulvoulis N. Assessing quantities and disposal routes for household hazardous products in the United Kingdom. Environmental Science & Technology. 2005;39:1912–1919. doi: 10.1021/es0404062. [DOI] [PubMed] [Google Scholar]
- Ternes T. Occurrence of drugs in German sewage treatment plants and rivers. Water Research. 1998;32:3245–3260. [Google Scholar]
- Ternes T, Stumpf M, Mueller J, Haberer K, Wilken R, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants - I. Investigations in Germany, Canada and Brazil. Science of the Total Environment. 1999;225:81–90. doi: 10.1016/s0048-9697(98)00334-9. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Current Housing Reports. Washington D.C.: 2008. American housing survey for the united states: 2007; table 1a-4 selected equipment and plumbingC all housing units; pp. H150–H107. [Google Scholar]
- U.S. Environmental Protection Agency. Exposure Assessment Tools and Models: Estimation Program Interface (EPI) Suite. 2011 http://www.epa.gov/oppt/exposure/pubs/episuite.htm.
- Vulliet E, Cren-Olive C, Grenier-Loustalot MF. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environmental Chemistry Letters. 2011;9:103–114. [Google Scholar]
- Webb S, Ternes T, Gibert M, Olejniczak K. Indirect human exposure to pharmaceuticals via drinking water. Toxicology Letters. 2003;142:157–167. doi: 10.1016/s0378-4274(03)00071-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Pharmaceuticals in Drinking-Water. WHO Library Cataloguing-in-Publication Data. 2012 http://www.who.int/water_sanitation_health/publications/2011/pharmaceuticals/en/index.html.
- Yang S, Carlson K. Routine monitoring of antibiotics in water and wastewater with a radioimmunoassay technique. Water Research. 2004;38:3155–3166. doi: 10.1016/j.watres.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Ying GG, Wang L, Yang JF, Yang XB, Yang LH, Li X. Determination of phenolic endocrine disrupting chemicals and acidic pharmaceuticals in surface water of the Pearl Rivers in South China by gas chromatography-negative chemical ionization-mass spectrometry. Science of the Total Environment. 2009;407:962–974. doi: 10.1016/j.scitotenv.2008.09.048. [DOI] [PubMed] [Google Scholar]
- Zuccato E, Castiglioni S, Fanelli R, Reitano G, Bagnati R, Chiabrando C, Pomati F, Rossetti C, Calamari D. Pharmaceuticals in the environment in Italy: Causes, occurrence, effects and control. Environmental Science and Pollution Research. 2006;13:15–21. doi: 10.1065/espr2006.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


