Abstract
Rapid alkalinization factor (RALF) is a peptide signal that plays a role in plant cell expansion. We have recently proposed that AtRALF1 negatively regulates root cell elongation and lateral root formation by opposing the effects of brassinosteroid (BR). We reported 6 AtRALF1-inducible cell wall-related genes and 2 P450 monooxygenase -encoding genes involved in the BR biosynthetic pathway. The AtRALF1-inducible genes implicated in cell wall remodeling were not downregulated by brassinolide (BL) treatment alone; their induction was only compromised following simultaneous treatment with AtRALF1 and BL. We further examined the cell wall-remodeling gene EXPANSIN A5 (AtEXPA5), which is upregulated by BL and has been shown to positively affect root cell elongation. Herein, we report that AtEXPA5 expression is downregulated by AtRALF1 in a dose-dependent manner in the roots and hypocotyls of Arabidopsis plants. AtEXPA5 is also downregulated in plants that overexpress AtRALF1, and it is upregulated in plants in which the AtRALF1 gene is partially silenced. The AtRALF1 peptide is also able to repress AtEXPA5 induction following a pre-treatment with BL. A schematic diagram showing the gene regulatory network connecting the recently reported genes with the regulation of cell expansion by AtEXPA5 is presented.
Keywords: Arabidopsis thaliana, brassinolide, cell elongation, expansin, peptide hormone
Cell-cell communication is essential in several growth and developmental processes in plants. Peptide signals regulate a variety of developmental processes and have been demonstrated to be involved in cellular signaling.1,2 Rapid alkalinization factor (RALF) genes are present throughout the plant kingdom and can exhibit either ubiquitous or tissue-specific expression patterns.3-6 RALF proteins have an N-terminal signal sequence and a conserved C-terminal region that covers the active peptide.3,4 The inhibitory activities of RALF peptides on root growth, hypocotyl elongation and pollen tube growth are all related to their roles as negative regulators of cell expansion.3,5,7,8
The primary cell wall is composed of polysaccharides, structural proteins, and enzymes.9 Among cell wall proteins, expansins induce cell wall loosening by disrupting the hydrogen bonds between cellulose microfibrils and cross-linking glycans, resulting in cell expansion.10 The concerted activities of plant hormones, such as auxin, brassinosteroid (BR) and ethylene, control expansin gene expression, and therefore, their functions in cell expansion.10
The AtRALF1 gene is mainly expressed in roots, and the protein it encodes inhibits cell elongation when applied exogenously.5,7 Recent studies have shown that FERONIA, which is a ubiquitously expressed receptor kinase in Arabidopsis plants, is an AtRALF1 receptor and that root growth inhibition is mediated by the inhibition of the plasma membrane H+-ATPase2.11 Our recent study highlighted a role for AtRALF1 in cell expansion through interference with the BR signaling pathway. We have reported 6 AtRALF1-inducible genes, including 4 involved in cell wall rearrangement and 2 in BR biosynthesis.12 Both genes involved in BR biosynthesis, CONSTITUTIVE PHOTOMORPHISM AND DWARFISM (CPD) and DWARF4 (DWF4), were downregulated by brassinolide (BL) treatment. However, the 4 AtRALF1-inducible genes related to cell wall remodeling, the proline-rich proteins AtPRP1 and AtPRP3, the hydroxyproline-rich glycoprotein AtHRPG2 and the xyloglucan endotransglucosylase TCH4, were not repressed by BL itself but were induced at lower levels following simultaneous treatment with AtRALF1 and BL. We selected an expansin gene, AtEXPA5, and evaluated its response following exposure to AtRALF1. AtEXPA5 was induced by BL treatment, played a regulatory role in the elongation of both roots and hypocotyls, and was downregulated by ethylene and upregulated in edt1 roots, which is an enhanced drought-tolerant mutant with a well-developed root system.13-16
Roots of Arabidopsis plants treated with increasing doses of the recombinant AtRALF1 peptide (HisAtRALF1) showed proportional decreases in AtEXPA5 gene expression (Fig. 1A). The downregulation of AtEXPA5 expression was noted at concentrations as low as 0.5 μM, clearly opposing the effects of BL (Fig. 1A).13,14 AtEXPA5 expression levels in the hypocotyls of Arabidopsis plants treated with AtRALF1 were also downregulated in these tissues at an even lower AtRALF1 concentration of 0.1 μM (Fig. 1B). Plants treated with BL for 6 h showed expected increases in AtEXPA5 expression; however, when they were pre-treated with BL for 5.5 h and then exposed to AtRALF1 for 30 min, the mRNA levels of AtEXPA5 were reduced to approximately 50% of the control levels (Fig. 1C).
Figure 1.
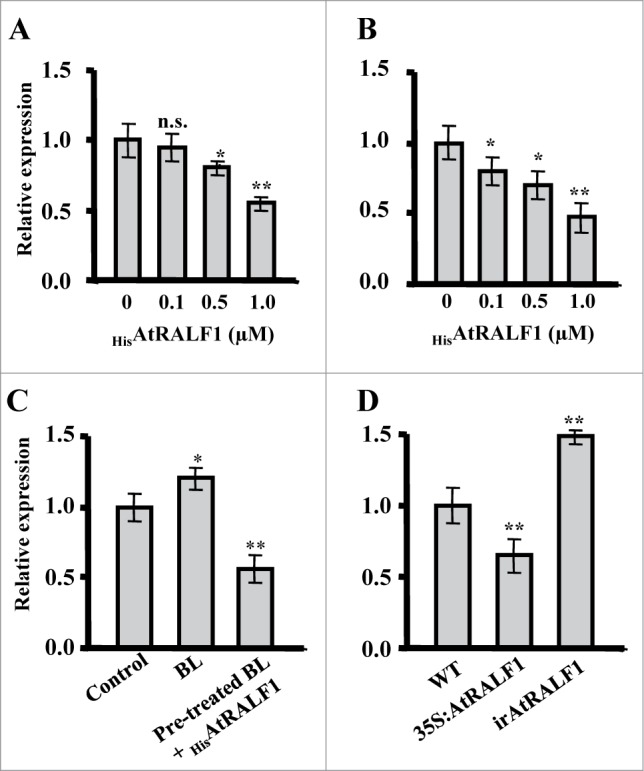
Quantitative RT-PCR gene expression analyses of EXPANSIN A5 (AtEXPA5, At3 g29030). (A) HisAtRALF1-treated 10-d-old wild-type plants. Total RNA was extracted from roots after 30 min of treatment with 0, 0.1, 0.5 and 1 μM of the peptide. (B) Dark-grown 4-d-old wild-type plants treated with HisAtRALF1. Total RNA was extracted from hypocotyls after 30 min of treatment with 0, 0.1, 0.5 and 1 μM of the peptide. (C) Arabidopsis plants (10-d-old) treated with 100 nM of brassinolide (BL) for 6 h or pre-treated with 100 nM of BL for 5.5 h and treated with 1 μM of HisAtRALF1 for 30 min. Control plants were treated with H2O. Total RNA was extracted from the roots after each treatment. (D) AtRALF1-overexpressing (35S:AtRALF1), AtRALF1-silencing (irAtRALF1) and wild-type (WT) plants. Total RNA was extracted from roots of 10-d-old plants. Error bars indicate SD (* = p value < 0.05,** = p value < 0.01, t-test). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, At1 g13440) expression was used as a control. All experiments were repeated at least 3 times (independent biological replicates).
To confirm the effects observed in the exogenously treated plants, we modulated the endogenous levels of AtRALF1 in plants expressing the AtRALF1 gene under the control of a strong 35S promoter (35S:AtRALF1) and plants with a partially silenced AtRALF1 gene (irAtRALF1). AtEXPA5 was downregulated in the 35S:AtRALF1 plants to approximately 60% of the AtEXPA5 mRNA levels found in the control plants (Fig. 1D). That is in agreement with our previous report that 35S:AtRALF1 plants show a compromised response when treated with exogenous BL.12 AtEXPA5 also showed low levels of expression in the detiolated2 (det2) mutant, which is defective in BR biosynthesis, and in the brassinosteroid-insensitive1 (bri1), which is insensitive to BR.14 In irAtRALF1, AtEXPA5 was upregulated by a factor of 1.5 (Fig. 1D). BL increased the level of AtEXPA5 when applied exogenously,14 suggesting an antagonistic effect between AtRALF1 and BR in the regulation of genes involved in cell expansion. Moreover, AtEXPA5 controlled hypocotyl elongation through the transcription factor BZR1, which is involved in BR signaling.14 Curiously, ethylene also reduced AtEXPA5 expression levels, regulating hypocotyl elongation.15 Ethylene and BR interact in plant growth and development, and BR promotes ethylene biosynthesis.17 AtRALF1 and ethylene may act together by sharing components of the pathway or function in parallel, achieving the same effects.
A molecular model illustrating the interactions between AtRALF1 and BR in the regulation of cell expansion-related genes shows 5 cell wall rearrangement genes and 2 involved in the BR biosynthetic pathway (Fig. 2). According to the proposed model, AtRALF1 induces the cell wall remodeling genes AtPRP1, AtPRP3, and AtHRGP2. These genes, among others, are then responsible for cell wall hardening and the blocking of further elongation. The TCH4 gene undergoes more complex regulation because it is controlled by both AtRALF1 and BL. Enzymes such as TCH4 play roles in the entire elongation process and not only in the final hardening stage, which may characterize one of the hypotheses explaining such complex regulation. Finally, the AtEXPA5 gene may play a role in elongation only, and therefore, it is repressed by AtRALF1 to initiate cell wall stiffening. Other genes, such as the BL-downregulated genes CPD and DWF4, are induced by AtRALF1 and may work in concert with BL during the elongation process.12,18,19
Figure 2.
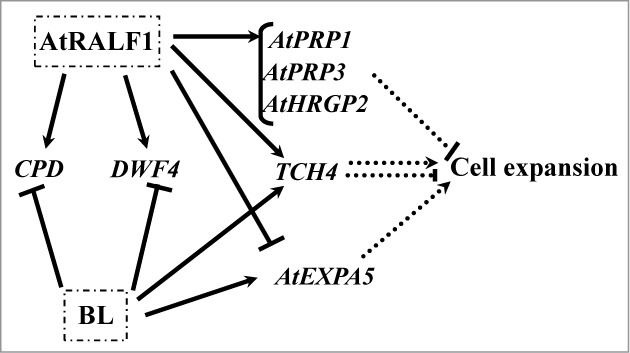
Schematic diagram of the AtRALF1 and BL gene regulatory network for cell expansion. Arrowed solid lines represent induction, and solid lines with bars at the end represent repression. Dashed lines represent the final effects of the actions of the genes in the cell expansion process. Dashed lines ending with arrowheads represent activation, and dashed lines ending with bars represent inhibition.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Antonio Francisco de Campos Amaral for technical assistance.
Funding
This research was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil. T.B. was supported by fellowships from FAPESP. M.C.S.F. and D.S.M. are research fellows of CNPq.
References
- 1. Matsubayashi Y, Sakagami Y. Peptide hormones in plants. Annu Rev Plant Biol 2006; 57:649-74; PMID:16669777; http://dx.doi.org/ 10.1146/annurev.arplant.56.032604.144204 [DOI] [PubMed] [Google Scholar]
- 2. Moura DS, Silva-Filho MCS. Plant peptide hormones, from defense to pollen self incompatibility, cell fate and development: small peptides as signaling molecules in plants. In: Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues (1st Edition), Teixeira da Silva JA. (ed), Global Science Books, London, UK, 2006, pp 1-7 [Google Scholar]
- 3. Pearce G, Moura DS, Stratmann J, Ryan CA. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci USA 2001; 98: 12843-7; PMID:11675511; http://dx.doi.org/ 10.1073/pnas.201416998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guerreiro JR, Pearce G, Silva-Filho MC, Moura DS. RALF peptides. Handbook of Biologically Active Peptides; Kastin AJ (ed.), 2013, pp. 46-49. [Google Scholar]
- 5. Morato do Canto A, Ceciliato PHO, Ribeiro B, Ortiz-Morea FA, Franco Garcia AA, Silva-Filho MC, Moura DS. Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiol Biochem 2014; 75:45-54; PMID:24368323; http://dx.doi.org/ 10.1016/j.plaphy.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 6. Murphy E, De Smet I. Understading the RALF family: a tale of many species. Trends Plant Sci 2014; 19:pii:S1360-85; PMID:24999241; http://dx.doi.org/ 10.1016/j.tplants.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Mingossi FB, Matos JL, Rizzato AP, Medeiros AH, Falco MC, Silva-Filho MC, Moura DS. SacRALF1, a peptide signal from the grass sugarcane (Saccharum spp.), is potentially involved in the regulation of tissue expansion. Plant Mol Biol 2010; 73:271-81; PMID:20148351; http://dx.doi.org/ 10.1007/s11103-010-9613-8 [DOI] [PubMed] [Google Scholar]
- 8. Covey PA, Subbaiah CC, Parsons RL, Pearce G, Lay FT, Anderson MA, Ryan CA, Bedinger PA. A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol 2010; 153:703-15; PMID:20388667; http://dx.doi.org/ 10.1104/pp.110.155457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 1993; 3:1-30; PMID:8401598; http://dx.doi.org/ 10.1111/j.1365-313X.1993.tb00007.x [DOI] [PubMed] [Google Scholar]
- 10. Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D. The growing world of expansins. Plant Cell Physiol 2002; 43:1436-44; PMID:12514240; http://dx.doi.org/ 10.1093/pcp/pcf180 [DOI] [PubMed] [Google Scholar]
- 11. Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014; 343:408-11; PMID:24458638; http://dx.doi.org/ 10.1126/science.1244454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergonci T, Ribeiro B, Ceciliato PHO, Guerrero-Abad JC, Silva-Filho MC, Moura DS. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot 2014; 65:2219-30; PMID:24620000; http://dx.doi.org/ 10.1093/jxb/eru099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Müssig C, Fischer S, Altmann T. Brassinosteroid-regulated gene expression. Plant Physiol 2002; 129:1241-51; PMID:12114578; http://dx.doi.org/ 10.1104/pp.011003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park CH, Kim T, Son S, Hwang J, Lee SC, Chang SC, Kim S, Kim SW, Kim S. Brassinosteroids control AtEXPA5 gene expression in Arabidopsis thaliana. Phytochemistry 2010; 71:380-87; PMID:20035956; http://dx.doi.org/ 10.1016/j.phytochem.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 15. Son S, Chang SC, Park CH, Kim S. Ethylene negatively regulates EXPA5 expression in Arabidopsis thaliana. Physiol Plant 2012; 144:254-62; PMID:22145846; http://dx.doi.org/ 10.1111/j.1399-3054.2011.01552.x [DOI] [PubMed] [Google Scholar]
- 16. Xu P, Cai XT, Wang Y, Xing L, Chen Q, Xiang CB. HDG11, upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. J Exp Bot 2014; 65:4285-95; PMID:24821957; http://dx.doi: 10.1093/jxb/eru202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arteca JM, Arteca RN. Brassinosteroid-induced exaggerated growth in hydroponically grown Arabidopsis plants. Physiol Plant 2001; 112: 104-12; PMID:11319021; http://dx.doi.org/ 10.1034/j.1399-3054.2001.1120114.x [DOI] [PubMed] [Google Scholar]
- 18. Mathur J, Molnár G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, Schell J, Koncz C, Szekeres M. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 1998; 14:593-602; PMID:9675902; http://dx.doi.org/ 10.1046/j.1365-313X.1998.00158.x [DOI] [PubMed] [Google Scholar]
- 19. Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 2002; 130:1319-34; PMID:12427998; http://dx.doi.org/ 10.1104/pp.011254 [DOI] [PMC free article] [PubMed] [Google Scholar]


