Abstract
Background and Purpose
Stimulation of the A1 adenosine receptor and angiotensin II receptor type‐1 (AT1 receptor) causes vasoconstriction through activation of cytochrome P450 4A (CYP4A) and ERK1/2. Thus, we hypothesized that acute angiotensin II activation alters the vasomotor response induced by the non‐selective adenosine receptor agonist, NECA, in mouse mesenteric arteries (MAs).
Experimental Approach
We used a Danish Myo Technology wire myograph to measure muscle tension in isolated MAs from wild type (WT), A1 receptor and A2B receptor knockout (KO) mice. Western blots were performed to determine the expression of AT1 receptors and CYP4A.
Key Results
Acute exposure (15 min) to angiotensin II attenuated the NECA‐dependent vasodilatation and enhanced vasoconstriction. This vasoconstrictor effect of angiotensin II in NECA‐treated MAs was abolished in A1 receptor KO mice and in WT mice treated with the A1 receptor antagonist DPCPX, CYP4A inhibitor HET0016 and ERK1/2 inhibitor PD98059. In MAs from A2B receptor KO mice, the vasoconstrictor effect of angiotensin II on the NECA‐induced response was shown to be dependent on A1 receptors. Furthermore, in A2B receptor KO mice, the expression of AT1 receptors and CYP4A was increased and the angiotensin II‐induced vasoconstriction enhanced. In addition, inhibition of KATP channels with glibenclamide significantly reduced NECA‐induced vasodilatation in WT mice.
Conclusions and Implications
Acute angiotensin II stimulation enhanced A1 receptor‐dependent vasoconstriction and inhibited A2B receptor‐dependent vasodilatation, leading to a net vasoconstriction and altered vasomotor response to NECA in MAs. This interaction may be important in the regulation of BP.
Abbreviations
- CYP
cytochrome P450
- KO
knock out
- MAs
mesenteric arteries
- NECA
5′‐(N‐ethylcarboxamido) adenosine
- PE
phenylephrine
- WT
wild type
- 20‐HETE
20‐hydroxyeicosatetraeonic acid
- DPCPX
1,3‐Dipropyl‐8‐cyclopentylxanthine
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes c |
| A 1 receptor | CYP4A |
| A 2B receptor | ERK1 |
| AT 1 receptor | ERK2 |
| Ion channels b | PKCα |
| K ATP channels |
| LIGANDS | |
|---|---|
| 20‐HETE | L‐NAME |
| Adenosine | Losartan |
| Angiotensin II | NECA |
| Arachidonic acid | PD98059 |
| DPCPX | Phenylephrine (PE) |
| Glibenclamide |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,2013b,2013c).
Introduction
Adenosine is a metabolite of adenine nucleotide degradation generated in both extracellular and intracellular compartments in response to various physiological and pathological stimuli. Once released, adenosine regulates vascular tone by activating four subtypes of adenosine receptors. These adenosine receptors are GPCRs, namely, A1, A2A, A2B and A3. A1 and A3 receptor activation causes vasoconstriction, while stimulation of A2A and A2B receptors produces vasodilatation (Ohisalo, 1987; Mubagwa et al., 1996; Olanrewaju and Mustafa, 2000; Olanrewaju et al., 2000; Mubagwa and Flameng, 2001; Jacobson and Gao, 2006; Hein et al., 2013). In mouse aorta, A1 receptor‐dependent smooth muscle contraction involves cytochrome P450 epoxygenase type 4A (CYP4A), PKCα and MAPKs, mainly p44/42 MAPK (ERK1/2) (Ansari et al., 2009; Sanjani et al., 2011; Kunduri et al., 2013a; Kunduri et al., 2013b). Moreover, A1 receptor‐mediated vasoconstriction occurs through the activation of PKCα, leading to ERK1/2 phosphorylation in coronary artery smooth muscle cells (Tawfik et al., 2005; Ansari et al., 2009). In contrast, A2A receptor‐dependent vasodilatation involves the activation of KATP channels (Ponnoth et al., 2012b; Sharifi‐Sanjani et al., 2013; Zhou et al., 2013). The mechanism involved in the vasodilatation mediated through A2B receptors is less well established and may possibly involve the activation of KATP channels (Berwick et al., 2010).
Interestingly, stimulation of angiotensin II receptor type 1 (AT1 receptor) activates PLC, generates 20‐hydroxyeicosatetraeonic acid (20‐HETE) metabolite from the degradation of arachidonic acid by CYP4A, causing ERK1/2‐dependent vasoconstriction and the development of hypertension (McGiff and Laniado‐Schwartzman, 1988; Harder et al., 1994; Haller et al., 1996; McGiff et al., 1996; Muthalif et al., 1998; 2000; Zaman et al., 2002). Notably, studies have revealed that inhibiting A1 receptors attenuates hypertension in spontaneously hypertensive rats and Dahl salt‐sensitive rats (Uehara et al., 1995; Kost et al., 2000). Moreover, in chronic angiotensin II‐ and l‐NAME‐induced hypertension in mice, genetic deletion of A1 receptors attenuates arteriolar vasoconstriction and hypertension (Gao et al., 2011; Lee et al., 2012). Recently, increased adenosine production and activation of A2B receptors in kidneys of mice were implicated in chronic angiotensin II‐dependent hypertension (Zhang et al., 2013). Previously, we demonstrated that endothelium‐dependent contractile responses to adenosine analogues in spontaneously hypertensive rat (SHR) aorta are mediated by A1 receptors and vasodilatation is mediated by A2 receptors (Fahim and Mustafa, 2001). Moreover, in SHR aorta, endothelium‐dependent adenosine receptor‐mediated vasodilatation was attenuated (Fahim et al., 2001). Thus, a complex relationship exists between adenosine and angiotensin II signalling with important implications for chronic angiotensin II‐dependent hypertension.
Previously, we have reported that all four adensine receptors are present in mesenteric arteries (MAs) of mice and that the A1 receptor mediates vasoconstriction, while the A2B receptor mediates vasodilatation (Teng et al., 2013). We hypothesized that acute exposure to angiotensin II alters adenosine receptor‐dependent regulation of vascular tone in systemic arteries. In this study, we examined the effects of acute angiotensin II stimulation on the non‐selective adenosine agonist, 5′‐(N‐ethylcarboxamido) adenosine (NECA)‐induced concentration‐response curves in isolated MAs using a Danish Myo Technology (DMT) wire myograph and identified a critical role for the A1 and A2B receptors in the response of MAs to NECA after acute angiotensin II‐pretreatment.
Methods
Animals
All animal protocols were approved by the IACUC of West Virginia University Health Sciences Center. Male C57BL/6J wild type (WT), A1 receptor knock out (KO) and A2B receptor KO mice used for the experiments were raised as described previously (Teng et al., 2013). Animals aged 14–18 weeks and body wt 25 ± 3 g were used for isolation of MAs. Experiments were conducted in accordance with national legislation and with the Declaration of Helsinki regarding the use of experimental animals.
Muscle tension measurement
Muscle tension measurements were performed using a DMT myograph (Danish Myo Technology A/S, Aarhus, Denmark) as described previously (Teng et al., 2013). Briefly, after anaesthesia with pentobarbital sodium (100 mg·kg‐1 i.p.), the mesentery with intestines from mice was excised and placed in cold oxygenated (5% CO2 and 95% O2) modified Krebs–Henseleit physiological salt solution (PSS, in mM: NaCl 120, NaHCO3 25, KCl 4.7, KH2PO4 1.2, CaCl2 1.8, MgSO4 1.2, glucose 15 and EDTA 0.05, pH 7.4). First‐order MAs were carefully dissected from the mesentery under a microscope in ice‐cold PSS. The MA rings (3–5 mm long, 75–150 µm diameter) were mounted on the DMT myograph in PSS continuously oxygenated at 37°C. The MAs were stretched to a preload of 300 mg and equilibrated for 90 min. During the equilibration period, MAs were tested for viability using KCl (50 mM). The presence of an intact endothelium was tested by assessing the response to ACh (1 μM) of MAs precontracted with phenylephrine (PE; 1 μM). NECA‐induced responses (0.5, 5, 50 and 500 nM and 5 μM) were performed in MAs precontracted with PE (1 μM). The MAs were incubated with various pharmacological agents, and NECA‐induced responses were obtained and normalized to % contraction obtained with PE (1 μM). The stable contraction to PE was attained in 10 min, and muscle tension measurements at this time were used to quantify data. In all of our muscle tension measurement experiments, the endothelia were intact. ACh‐induced relaxations were −86.35 ± 3.211, −81.48 ± 5.868 and −91.34 ± 4.421 (% contractions to PE) in MAs obtained from WT, A2B and A1 receptor KO mice, respectively. Similar levels of PE‐induced stable contractions were observed whenever serial NECA‐induced responses were performed.
The role of A1 receptors was investigated by incubating MAs with the A1 receptor antagonist DPCPX (1 μM) (Li et al., 1998; Tawfik et al., 2005; Ansari et al., 2009; Pradhan et al., 2014). Furthermore, A1 receptor KO mice were used to determine the role of the A1 receptor. To study the effect of CYP4A, MAs were pre‐incubated with HET0016 (10 μM) (Ponnoth et al., 2012a; Kunduri et al., 2013a), an inhibitor of CYP4A and 20‐HETE production. In addition, the role of ERK1/2 was determined by using the inhibitor PD98059 (1 μM) (Ponnoth et al., 2012a; El‐Awady et al., 2013; Kunduri et al., 2013b). In addition, the role of KATP channels in NECA‐induced vasodilatation was investigated by pre‐incubation of the MAs with glibenclamide (1 μM), a KATP channel blocker (Fujii et al., 1999; Ponnoth et al., 2012b).
Western blotting
Western blot analyses were performed as described previously (Ponnoth et al., 2012a; Kunduri et al., 2013a). Briefly, MAs isolated from WT and A2B receptor KO mice were homogenized with 125 μL of RIPA lysis and extraction buffer (Peirce #89901 mixed with 1 mM of Na3VO4, 1 mg·mL‐1 of leupeptin and 1 mM NaF), vortexed and centrifuged for 10 min at 10 000 g at 4°C. Supernatants were stored at −80°C. Protein was measured by Lowry's method using Bio‐Rad Laboratories protein assay concentrate (Hercules, CA, USA). The samples (2–5 µg of total protein) were loaded on slab gels (10% acrylamide; 1.5 mm thick), separated by SDS–PAGE, and transferred to nitrocellulose membranes (Hybond‐ECL). Membranes were blocked with 5% dried skimmed milk for 45 min and incubated overnight with primary antibodies. The 1:1000 dilutions were used for CYP4A and AT1 receptor antibodies. All membranes were stripped and probed for β‐actin. Secondary antibodies were HRP conjugated. Membranes were developed using enhanced chemiluminescence (Amersham BioSciences, Amersham, UK) and X‐ray film. Densitometry analysis was made using Multi Guage V3.0 (Fuzifilm Medical Systems, CT, USA) software. The data obtained were normalized to actin and presented as the normalized ratio (% change) of target protein to WT protein expression.
Statistical analysis
Data are presented as mean ± SEM % contraction of MAs observed with 1 μM PE. The data from the concentration response curves were analysed between groups at the same concentrations. The comparison between two groups was made using Student's t‐test. Experiments comprising three or more groups were compared using one‐way ANOVA followed by Tukey's test to compare the significance between various groups. P < 0.05 was considered statistically significant. For all data, two or more MA rings from one animal were averaged together and presented as one ‘n’; MAs from a minimum of six animals were tested and analysed in each group of experiments.
Materials
NECA, phenylephrine (PE), ACh, angiotensin II, DPCPX, PD98059 and all other chemicals and buffers were purchased from Sigma‐Aldrich (St. Louis, MO, USA). HET0016 and glibenclamide were purchased from Cayman Chemical (Ann Arbor, MI, USA). Antibodies AT1 receptor (product # sc‐1173), CYP4A (sc‐271983) and actin (sc‐47778) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
Results
Acute angiotensin II stimulation causes NECA‐induced vasoconstriction in MAs
With an aim to investigate whether angiotensin II stimulation alters adenosine‐induced vascular responses, we first assessed angiotensin II‐induced vasoconstriction. We observed that angiotensin II concentration‐dependently (1, 10 and 100 nM) produced vasoconstriction (Figure 1A and B). As shown in Figure 1A, angiotensin II‐induced vasoconstriction rapidly waned. Next, we determined the effect of acute angiotensin II stimulation on NECA‐induced vascular responses. We used the non‐selective adenosine agonist NECA to determine the effect on all adenosine receptors. First, we measured NECA‐induced responses without angiotensin II pretreatment (Control, Figure 2A) and then exposed the MAs to angiotensin II (10 nM) and performed NECA‐induced responses in the same tissues (Figure 2B). Angiotensin II pretreatment inhibited the vasodilatation produced by high concentrations of NECA and facilitated a vasoconstrictor response to low doses of NECA that was not observed in control tissues that were not pretreated with angiotensin II (Figure 2C). To make sure that the altered responses to NECA were indeed due to angiotensin II stimulation, we performed serial NECA concentration–response experiments. Reproducible serial NECA‐induced responses were achieved in MAs (Figure 2D). Losartan (1 μM, AT1 receptor blocker) completely blocked the angiotensin II‐induced vasoconstriction in MAs (0.49 ± 0.34% to PE). Furthermore, the vasoconstrictor response to NECA after angiotensin II stimulation was also blocked by losartan pretreatment (Figure 2E), suggesting that acute angiotensin II stimulation of AT1 receptors altered the NECA‐induced responses to produce vasoconstriction.
Figure 1.
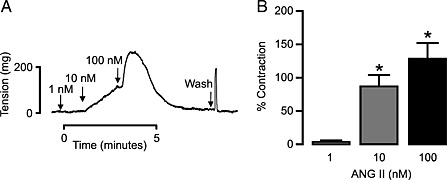
Angiotensin (ANG) II induces concentration‐dependent vasoconstrictions in MAs. (A) Representative trace showing angiotensin II concentration‐dependent (1, 10 and 100 nM) vasoconstriction. (B) Bar graph summarizes ANG II‐induced concentration‐dependent muscle tension generated in MAs from WT mice (n = 6). *P < 0.05 compared with vasoconstriction observed at 1 nM angiotensin II.
Figure 2.
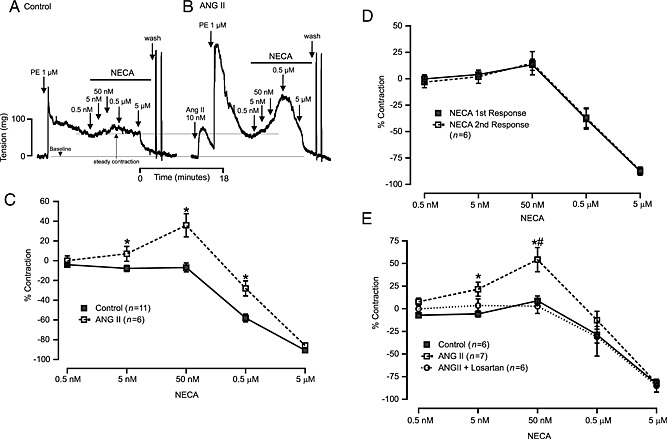
Acute angiotensin (ANG) II stimulation alters NECA‐induced responses in MAs from WT mice. (A) Control tracing. (B) NECA‐induced responses after ANG II (10 nM) stimulation (bottom line represents baseline, and upper line shows stable contraction to PE). (C) Summarizes quantification of NECA‐induced responses in control and ANG II‐stimulated MAs. (D) Graph shows that responses of MAs to a series of increasing concentrations of NECA were comparable. (E) Effect of losartan (1 μM, 10 min) on NECA‐induced altered responses after ANG II stimulation. The graph shows that losartan pre‐treatment completely blocked altered responses to NECA after ANG II stimulation. Asterisk (*) indicates p < 0.05 compared with control, and number sign (#) denotes p < 0.05 compared with losartan‐treated response.
To determine the role of the endothelium, we examined the NECA‐induced responses in endothelium‐denuded MAs. In these vessels, the ACh‐induced vascular relaxation was −6.53 ± 2.0 (% contraction to PE). In MAs not pretreated with angiotensin II, NECA‐induced relaxation was partially attenuated by removal of the endothelium (Figure 3A). As shown in Figure 3B, partial attenuation of the NECA‐induced vasodilatation was observed in angiotensin II treated MAs in endothelium‐denuded MAs. In addition, NECA‐induced vasoconstriction was absent in endothelium‐denuded MA (Figure 3B) compared with MAs with intact endothelium (Figure 2C). These data suggest that NECA‐induced vasodilatation is partially endothelium‐dependent and that the effects of angiotensin II on NECA‐induced vasoconstrictions are largely endothelium‐dependent.
Figure 3.
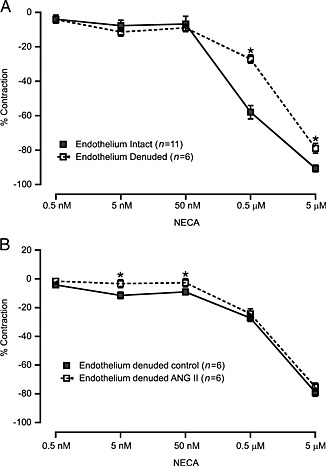
Endothelium plays an important role in vasodilatation to NECA. After endothelium denudation, we performed NECA responses in MAs. (A) Graph shows that NECA‐induced vascular responses were partially dependent on endothelium. (B) Denuded MAs were exposed to angiotensin (ANG) II (10 nM), and serial NECA‐induced responses were performed. The graph shows that the contraction to NECA was absent, and a significant inhibition of relaxation was observed after ANG II stimulation. * P < 0.05 compared with control.
A1 receptors, CYP4A and ERK1/2 mediate the altered NECA‐induced response following angiotensin II stimulation in MAs
After establishing that the vascular responses to NECA were changed following angiotensin II stimulation, we next wanted to determine the signalling mechanism of the angiotensin II‐induced vasoconstriction. Thus, we performed muscle tension measurements to angiotensin II (10 nM) in MAs incubated (15 min) with various pharmacological inhibitors, namely, the A1 receptor antagonist DPCPX (1 μM), CYP4A inhibitor HET0016 (10 μM) and ERK1/2 inhibitor PD98059 (1 μM).
We found that DPCPX (Figure 4A) and HET0016 (Figure 4B) significantly reduced vasoconstrictor responses to angiotensin II. Pretreatment of MAs with the A1 receptor antagonist DPCPX (1 μM) or CYP4A inhibitor HET0016 (10 μM) for 15 min completely abolished the vasoconstriction observed to NECA after angiotensin II stimulation (Figure 4C). We also observed that pretreatment with the ERK1/2 inhibitor PD98059 (1 μM) significantly inhibited angiotensin II‐induced vasoconstriction (Figure 5A) and NECA‐induced contractions in angiotensin II‐treated MAs (Figure 5B). Taken together, these data show that the enhanced vasoconstriction to NECA after angiotensin II treatment occurs through activation of the A1 receptor and involves signalling through CYP4A and ERK1/2.
Figure 4.
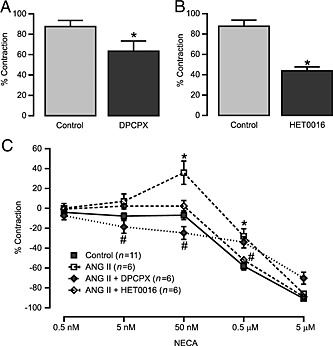
Acute angiotensin (ANG) II stimulation alters the A1 receptor‐ and CYP4A‐dependent NECA‐induced responses in MAs from WT mice. Pre‐incubation of MAs with the A1 receptor antagonist DPCPX 1 μM (Control n = 10, DPCPX n= 6) (A) and CYP4A inhibitor HET0016 10 μM (Control n = 7, HET0016 n = 7) (B) significantly inhibited ANG II‐induced (10 nM) vasoconstrictions. (C) ANG II‐induced altered responses to NECA were blocked by DPCPX and HET0016. * P < 0.05 compared with control, and # P < 0.05 compared with ANG II‐stimulated response.
Figure 5.
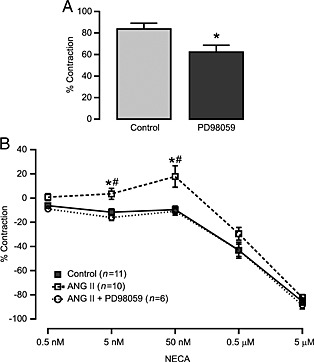
ERK1/2 inhibition reduces angiotensin (ANG) II‐induced vasoconstriction and altered responses to NECA in MAs from WT mice. (A) PD98059 (1 μM), an ERK1/2 inhibitor, significantly reduced ANG II (10 nM)‐induced vasoconstrictions (control n = 10, PD98059 n= 6; *P < 0.05 compared with control). (B) Altered response to NECA after ANG II stimulation was also blocked by PD98059. *P < 0.05 compared with control NECA response, and #P < 0.05 compared with response after PD98059 treatment.
A1A and A2B receptors regulate angiotensin II‐ and NECA‐induced vasoconstriction in MAs
To determine the role of the A2B receptor and substantiate the role of the A1 receptor in angiotensin II‐induced vasoconstriction and the altered responses to NECA, we performed muscle tension measurements in MAs obtained from A2B receptor KO mice. We observed that angiotensin II‐induced vasoconstriction in MAs from A2B KO mice was significantly higher than that from WT mice (Figure 6A). Furthermore, DPCPX (1 μM) and HET0016 (10 μM) (Figure 6B and C, respectively) significantly reduced angiotensin II‐induced vasoconstriction. Western blot analysis of MAs revealed that the expression of AT1 receptors and CYP4A was higher in A2B receptor KO mice compared with WT mice (Figure 7A and B). In contrast to observations in WT mice, NECA alone (without angiotensin II pretreatment) induced a vasoconstrictor response in A2B receptor KO mice, suggesting un‐opposed A1 receptor activation. Similar to WT mice, acute angiotensin II stimulation potentiated NECA‐induced vasoconstriction (Figure 8). Pre‐incubation of A2B receptor KO MAs with DPCPX (1 μM, 15 min) completely abolished NECA‐induced vasoconstriction (Figure 8). The CYP4A inhibitor HET0016 (10 μM) partially but not significantly attenuated the enhanced vasoconstriction to NECA following angiotensin II stimulation (Figure 8). These data suggest that A2B receptor expression limits NECA‐induced contraction by (1) reducing AT1 receptor and CYP4A expression and by (2) providing an opposing vasodilator signalling pathway in vascular smooth muscle.
Figure 6.
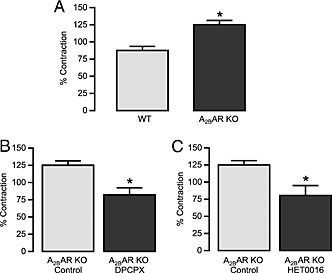
Angiotensin (ANG) II‐induced vasoconstrictions in MAs obtained from A2B receptor (A2BAR) KO mice. (A) Significantly higher vasoconstrictions to ANG II were observed in MAs from A2B receptor KO mice (WT n = 10, A2BAR KO n = 6); (B) DPCPX (1 μM) significantly reduced ANG II‐induced vasoconstrictions (control n = 6, DPCPX n = 6), and (C) HET0016 (10 μM) significantly attenuated ANG II‐induced vasoconstrictions (control n = 6, HET0016 n = 8). * P < 0.05 compared with respective controls.
Figure 7.
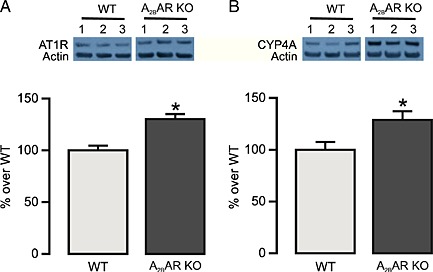
Western blot analysis of AT1 receptors (AT1R) and CYP4A in MAs from WT and A2B receptor (A2BAR) KO mice. Top panel represents the expression of AT1 receptors (A) and CYP4A (B) in MAs from three mice. Bottom panel summarizes the quantification of data obtained from six mice in each group. * P < 0.05 compared with WT.
Figure 8.
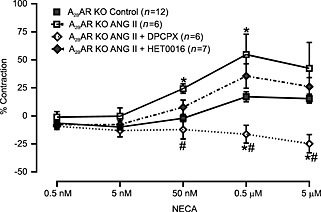
NECA‐induced responses were altered by angiotensin (ANG) II stimulation in MAs from A2B receptor KO mice. ANG II stimulation altered NECA‐induced responses in MAs. These altered responses were completely abolished when MAs were pretreated (15 min) with DPCPX (1 μM) and partly reduced by HET0016 (10 μM). *P < 0.05 compared with control NECA response. # P < 0.05 compared with ANG II‐stimulated responses.
Angiotensin II stimulation inhibits A2B receptor‐dependent vasodilatation in MAs
To determine whether angiotensin II stimulation inhibits NECA‐induced vasodilatation, we examined MAs obtained from A1 receptor KO mice. As shown in Figure 9A, acute angiotensin II stimulation significantly reduced NECA‐induced vasodilatation in MAs from A1 receptor KO mice. Notably, the PE‐induced vasoconstriction observed in control and angiotensin II‐stimulated MAs were 181.5 ± 26.39 and 212.3 ± 28.72 (mg tension). One potential mechanism for these effects of angiotensin II is through the inhibition of KATP channels. To test this hypothesis, we examined NECA‐induced responses in MAs pre‐incubated with the KATP channel blocker glibenclamide (1 μM). As shown in Figure 9B, glibenclamide significantly attenuated NECA‐induced vasodilatation in MAs from WT mice.
Figure 9.
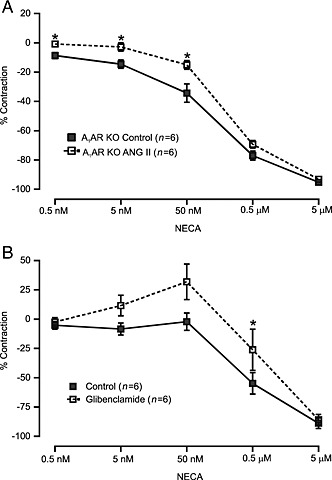
Acute angiotensin (ANG) II stimulation inhibits NECA‐induced vasodilatation in MAs. (A) We found that after ANG II stimulation, NECA‐induced vasodilator responses were partly but significantly inhibited in MAs obtained from A1 receptor KO mice. (B) In MAs from WT mice, the KATP channel blocker, glibenclamide (1 μM), significantly reduced NECA‐induced vasodilatation. *P < 0.05 compared with control.
Discussion
In the present study, we found that acute angiotensin II stimulation significantly altered adenosine receptor‐dependent vascular tone in MAs. Angiotensin II pretreatment of MAs resulted in the response to NECA being vasoconstriction. This vasoconstrictor response was A1 receptor‐dependent and mediated by CYP4A and ERK1/2. Angiotensin II stimulation also inhibited the A2B receptor‐dependent vasodilatation. These data suggest an intricate relationship between adenosine receptors and angiotensin II‐induced vascular tone regulation in MAs.
Angiotensin II is a potent vasoconstrictor; we observed concentration‐dependent vasoconstriction in mouse isolated MAs (Figure 1). Angiotensin II receptors are rapidly desensitized, and the muscle tension generated diminishes in whole animals, isolated vessels and in cultured cells (Abdellatif et al., 1991; Sasamura et al., 1994; Paul et al., 2006). Similarly, angiotensin II‐induced vasoconstriction rapidly diminished in MAs (Figure 1A). Evidently, angiotensin II activates CYP4A‐dependent metabolism of arachidonic acid into 20‐HETE, which constricts blood vessels. In addition, CYP4A contributes to the development of angiotensin II‐dependent hypertension (McGiff and Laniado‐Schwartzman, 1988; Harder et al., 1994; Haller et al., 1996; McGiff et al., 1996; Muthalif et al., 1998; 2000; Zaman et al., 2002). PLC, 20‐HETE and ERK1/2 pathways are involved in the regulation of vascular tone by adenosine through the activation of A1 receptors (Rogel et al., 2005; Jacobson and Gao, 2006; Ponnoth et al., 2012a; Kunduri et al., 2013a; Kunduri et al., 2013b). Thus, we believe that acute angiotensin II stimulation may alter adenosine receptor‐dependent signalling, establishing an important role for adenosine in the regulation of vascular tone in MAs. We found that stimulation of MAs with angiotensin II uncovered a NECA‐induced vasoconstriction and inhibited NECA‐induced vasodilatation (Figure 2A, B and C). After angiotensin II stimulation, at low concentrations, NECA produced significant vasoconstriction that was not observed in NECA‐treated MAs that were not treated with angiotensin II. We found that altered responses to NECA after angiotensin II stimulation were due to the stimulation of AT1 receptors (Figure 2E). Importantly, responses to a series of increasing concentrations of NECA were identical (Figure 2D), suggesting that there is no desensitization of the NECA response (Figure 2D).
Adenosine receptors are present on endothelium as well as smooth muscle cells of arteries (Ohisalo, 1987; Mubagwa et al., 1996; Mubagwa and Flameng, 2001; Jacobson and Gao, 2006; Hein et al., 2013). Thus, we determined the role of the endothelium in NECA‐induced vascular responses and subsequent effects of acute angiotensin II stimulation. We found that NECA‐induced vasodilatation was partially endothelium‐ dependent (Figure 3A). Furthermore, in the absence of endothelium, angiotensin II stimulation inhibited NECA‐induced vasodilatation (Figure 3B). In addition, no vasoconstriction to NECA was noted in endothelium‐denuded vessels after angiotensin II stimulation (Figures 2C and 3B). Previously, in mouse aorta, we demonstrated that the endothelium mediates A3 receptor‐dependent vasoconstriction through the generation of TXB2 by COX‐1 (Ansari et al., 2007). Similarly, no vasoconstriction to adenosine receptor stimulation was observed in endothelium‐denuded mouse aorta (Nayeem et al., 2008; Ponnoth et al., 2012b). Our data suggest that the endothelium plays a role in A1 receptor‐dependent vasoconstriction in MAs, which involves the activation of CYP4A possibly through the generation of 20‐HETE (Figures 3B and 4C). Overall, acute angiotensin II stimulation of AT1 receptors alters the adenosine receptor‐dependent regulation of vascular tone in MAs.
In the quest to identify the mechanism for the altered responses to NECA induced by angiotensin II, we first investigated the mechanism of angiotensin II‐induced vasoconstriction in MAs. We found that angiotensin II‐induced vasoconstriction in MAs involves the A1 receptor (Figure 4A) and CYP4A (Figure 4B). Angiotensin II‐induced vasoconstriction has been shown to be mediated through CYP4A activation, leading to 20‐HETE production, resulting in MAPK activation (McGiff and Laniado‐Schwartzman, 1988; Harder et al., 1994; Haller et al., 1996; McGiff et al., 1996; Muthalif et al., 1998; 2000; Zaman et al., 2002). Our data are in agreement with these findings. However, inhibition of angiotensin II‐induced vasoconstriction by an A1 receptor antagonist was intriguing and needs further investigation. Possibly, inhibition of A1 receptors by DPCPX, in part, blocks further downstream activation of CYP4A, PLC and ERK1/2, which mediates angiotensin II‐induced vasoconstriction. Another possibility is that DPCPX may directly inhibit angiotensin II receptors. Notably, our data revealed that enhanced vasoconstriction to NECA after angiotensin II pretreatment was A1 receptor‐dependent (Figure 4C). Furthermore, CYP4A plays an important role in the enhanced vasoconstriction to NECA observed in MAs after angiotensin II pretreatment (Figure 4C). In addition, our data revealed that angiotensin II‐induced vasoconstriction and altered responses to NECA in MAs were ERK1/2 dependent (Figures 5A and B). Evidently, the constrictor effect of A1 receptors was enhanced in the presence of angiotensin II, as mentioned previously (Lai et al., 2006). Moreover, in chronic angiotensin II and l‐NAME hypertension mouse models, genetic deletion of A1 receptors has been shown to attenuate arteriolar and BP responses (Gao et al., 2011; Lee et al., 2012). Previously, we demonstrated that CYP4A and ERK1/2 are involved in the A1 receptor‐dependent contractile response in mouse aorta (Ponnoth et al., 2012a; Kunduri et al., 2013b; Kunduri et al., 2013a). In agreement, our data revealed that enhanced vasoconstriction to NECA in MAs following angiotensin II stimulation was completely dependent on the A1 receptor and mediated through CYP4A and ERK1/2 (Figures 4 and 5).
We further investigated the role of A1ARs in altered NECA‐induced responses using isolated MAs from A2BAR KO mice. Previously, we demonstrated that in MAs, the A1 receptor regulates vasoconstriction, while the A2B receptor regulates vasodilatation (Teng et al., 2013). In addition, the expression of A1 receptors was higher in MAs obtained from A2B receptor KO mice (Teng et al., 2013). We believe that the absence of A2B receptors may potentiate the contractile responses to NECA or angiotensin II. As expected, we found that angiotensin II‐induced vasoconstriction was higher in MAs obtained from A2B receptor KO mice (Figure 6A), possibly due to enhanced signalling through AT1 receptors and CYP4A, as protein expression of these proteins was greater in MAs from A2B receptor KO mice (Figure 7A and B). Angiotensin II‐induced vasoconstriction was reduced by A1 receptor and CYP4A inhibitors (Figure 6B and C). Thus, our data suggest an intricate regulation of angiotensin II‐induced contraction through A2B receptors. As the A2B receptor mediates vasodilatation in MAs (Teng et al., 2013), we found no vasodilatation to NECA in MAs obtained from A2B receptor KO mice (Figure 8). Furthermore, the vasoconstriction to NECA was potentiated after stimulation of MAs with angiotensin II (Figure 8). Importantly, the angiotensin II‐induced increased vasoconstrictor responses to NECA were abolished by the A1 receptor antagonist DPCPX and partially reduced by CYP4A inhibition (Figure 8). The elevated expression of CYP4A (Figure 7B) may account for the lack of inhibition of NECA‐induced vasoconstriction after angiotensin II stimulation by HET0016. Notably, our data suggest the possibility that an absence of A2B receptors could also potentiate the vasoconstrictor response to angiotensin II stimulation in MAs.
Recently, enhanced A2B receptor‐dependent production of endothelin‐1 in the kidneys was implicated in angiotensin II‐induced hypertension in mice, while no change in endothelin receptors was reported (Zhang et al., 2013). In addition, angiotensin II led to enhanced production of adenosine in the kidneys whereas the blood levels of adenosine were unchanged (Zhang et al., 2013). However, no vascular reactivity data were recorded in this study. In the scenario of unchanged levels of adenosine in the blood, altered responses to angiotensin II‐induced stimulation of adenosine receptors may contribute to the regulation of BP in angiotensin II‐induced hypertension. An absence of A2B receptors enhances angiotensin II‐induced vasoconstriction in MAs (Figure 6A), whereas it suppresses the production of endothelin‐1, a potent vasoconstrictor, from kidneys in angiotensin II‐induced hypertension in mice (Zhang et al., 2013). This complicated relationship between adenosine receptors and angiotensin II signalling warrants further detailed investigations, as it is involved in the regulation of BP in chronic hypertension models.
After establishing the role of the A1 receptor in vasoconstriction, we further determined the role of A2B receptors in the vasodilator response to NECA in MAs pretreated with angiotensin II. To address this, we performed muscle tension studies in MAs obtained from A1 receptor KO mice. According to our previous findings, A2B receptor expression was higher in MAs obtained from A1 receptor KO mice. In addition, the vasodilator response to NECA was increased in MAs from A1 receptor KO mice (Teng et al., 2013). Similarly, an increased vasodilator response to NECA was observed in the present study (data not shown). Furthermore, acute angiotensin II stimulation significantly inhibited this NECA‐induced vasodilatation in MAs obtained from A1 receptor KO mice (Figure 9A). MAs lack functional A2A receptors, which have previously been shown to mediate vasodilatation (Teng et al., 2013). Previously, we demonstrated that A2A receptor‐dependent vasodilatation of aorta occurred through the activation of KATP channels (Ponnoth et al., 2012b). In addition, KATP channels are involved in A2A receptor‐dependent vasodilatation in coronary reactive hyperaemia (Sharifi‐Sanjani et al., 2013; Zhou et al., 2013). Interestingly, angiotensin II inhibits KATP channels of rat mesenteric arterial smooth muscle cells (Hayabuchi et al., 2001) and aortic smooth muscle cells (Sampson et al., 2007). Also, A2B receptor‐dependent activation of KATP channels has been implicated in the regulation of coronary flow (Berwick et al., 2010). Similarly, we found that inhibition of KATP channels produces vasoconstriction to NECA in MA from WT mice (Figure 9B). Probably, inhibition of KATP channels by angiotensin II may contribute to the inhibition of NECA‐induced dilation, enhancing the vasoconstrictor response after angiotensin II stimulation in MA.
In conclusion, our data reveal that acute angiotensin II stimulation potentiates endothelium‐ and A1 receptor‐dependent vasoconstriction mediated by CYP4A and ERK1/2 in MAs. Angiotensin II also inhibited the A2B receptor‐dependent vasodilatation, thus augmenting the vasoconstrictor response to NECA. In addition, the expression of AT1 receptor and CYP4A was increased and the contraction to angiotensin II enhanced in MAs from A2B receptor KO mice. Thus, a complex interaction exists between adenosine receptors and angiotensin II signalling pathways, which contribute to an altered vascular tone in MAs, and this interaction may be important in the regulation of BP. Findings from the current study suggest that adenosine may regulate the angiotensin II‐mediated altered vascular tone and potentially contribute to BP regulation in angiotensin II‐dependent hypertension. Further studies are needed to investigate the role of A1 and A2B receptors in the regulation of vascular tone contributing to high BP in murine models of chronic angiotensin II‐induced hypertension so as to substantiate the involvement of adenosine receptors in angiotensin II‐dependent hypertension. In addition, determining the various factors involved and delineating their mechanisms will provide novel therapeutic targets for the control of BP in angiotensin II‐induced hypertension.
Author contributions
V. R. Y., M. A. N. and S. J. M. conceived and designed the research; S. L. T. provided the KO mice; V. R. Y. performed the experiments and analysed the data; V. R. Y. drafted the manuscript; V. R. Y., M. A. N., S. J. M. and S. L. T. revised and edited the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This study was supported by NIH grants HL114559, HL027339, HL09444, HL071802 and U54GM104942.
Yadav, V. R. , Nayeem, M. A. , Tilley, S. L. , and Mustafa, S. J. (2015) Angiotensin II stimulation alters vasomotor response to adenosine in mouse mesenteric artery: role for A1 and A2B adenosine receptors. British Journal of Pharmacology, 172: 4959–4969. doi: 10.1111/bph.13265.
References
- Abdellatif MM, Neubauer CF, Lederer WJ, Rogers TB (1991). Angiotensin‐induced desensitization of the phosphoinositide pathway in cardiac cells occurs at the level of the receptor. CircRes 69: 800–809. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The concise guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA et al. (2013b). The concise guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol 170: 1607–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013c). The concise guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari HR, Nadeem A, Tilley SL, Mustafa SJ (2007). Involvement of COX‐1 in A3 adenosine receptor‐mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol 293: H3448–H3455. [DOI] [PubMed] [Google Scholar]
- Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J et al. (2009). A(1) adenosine receptor‐mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 297: H1032–H1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD (2010). Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation 17: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Awady MS, Rajamani U, Teng B, Tilley SL, Mustafa SJ (2013). Evidence for the involvement of NADPH oxidase in adenosine receptors‐mediated control of coronary flow using A and A knockout mice. Physiol Rep 1: e00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M, Hussain T, Mustafa SJ (2001). Role of endothelium in adenosine receptor‐mediated vasorelaxation in hypertensive rats. Fundam Clin Pharmacol 15: 325–334. [DOI] [PubMed] [Google Scholar]
- Fahim M, Mustafa SJ (2001). Evidence for the presence of A(1) adenosine receptors in the aorta of spontaneously hypertensive rats. Br J Pharmacol 134: 1760–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Onaka U, Goto K, Abe I, Fujishima M (1999). Impaired isoproterenol‐induced hyperpolarization in isolated mesenteric arteries of aged rats. Hypertension 34: 222–228. [DOI] [PubMed] [Google Scholar]
- Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sallstrom J et al. (2011). Adenosine A(1)‐receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol 301: R1669–R1681. [DOI] [PubMed] [Google Scholar]
- Haller H, Lindschau C, Erdmann B, Quass P, Luft FC (1996). Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res 79: 765–772. [DOI] [PubMed] [Google Scholar]
- Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB et al. (1994). Formation and action of a P‐450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol 266: H2098–H2107. [DOI] [PubMed] [Google Scholar]
- Hayabuchi Y, Davies NW, Standen NB (2001). Angiotensin II inhibits rat arterial KATP channels by inhibiting steady‐state protein kinase A activity and activating protein kinase Ce. J Physiol 530: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TW, Xu W, Ren Y, Kuo L (2013). Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A(2)A receptor activation. Cardiovasc Res 99: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG (2006). Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost CK Jr, Herzer WA, Rominski BR, Mi Z, Jackson EK (2000). Diuretic response to adenosine A(1) receptor blockade in normotensive and spontaneously hypertensive rats: role of pertussis toxin‐sensitive G‐proteins. J Pharmacol Exp Ther 292: 752–760. [PubMed] [Google Scholar]
- Kunduri S, Dick G, Nayeem M, Mustafa S (2013a). Adenosine A receptor signaling inhibits BK channels through a PKCalpha‐dependent mechanism in mouse aortic smooth muscle. Physiol Rep 1 e00037: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA (2013b). Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C‐alpha, and ERK1/2. J Cardiovasc Pharmacol 62: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N et al. (2006). Contribution of adenosine receptors in the control of arteriolar tone and adenosine‐angiotensin II interaction. Kidney Int 70: 690–698. [DOI] [PubMed] [Google Scholar]
- Lee DL, Bell TD, Bhupatkar J, Solis G, Welch WJ (2012). Adenosine A1‐receptor knockout mice have a decreased blood pressure response to low‐dose ANG II infusion. Am J Physiol Regul Integr Comp Physiol 303: R683–R688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS et al. (1998). Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res 80: 357–364. [DOI] [PubMed] [Google Scholar]
- McGiff JC, Laniado‐Schwartzman M (1988). Arachidonic acid metabolites and blood pressure control. Clin Physiol Biochem 6: 179–187. [PubMed] [Google Scholar]
- McGiff JC, Steinberg M, Quilley J (1996). Missing links: cytochrome P450 arachidonate products a new class of lipid mediators. Trends Cardiovasc Med 6: 4–10. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Flameng W (2001). Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res 52: 25–39. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Mullane K, Flameng W (1996). Role of adenosine in the heart and circulation. Cardiovasc Res 32: 797–813. [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR et al. (1998). 20‐Hydroxyeicosatetraenoic acid mediates calcium/calmodulin‐dependent protein kinase II‐induced mitogen‐activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A 95: 12701–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE et al. (2000). Angiotensin II‐induced hypertension: contribution of Ras GTPase/Mitogen‐activated protein kinase and cytochrome P450 metabolites. Hypertension 36: 604–609. [DOI] [PubMed] [Google Scholar]
- Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS et al. (2008). Role of CYP epoxygenases in A2A AR‐mediated relaxation using A2A AR‐null and wild‐type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohisalo JJ (1987). Regulatory functions of adenosine. Med Biol 65: 181–191. [PubMed] [Google Scholar]
- Olanrewaju HA, Mustafa SJ (2000). Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35: 171–177. [DOI] [PubMed] [Google Scholar]
- Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ (2000). Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 279: H650–H656. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan MA, Kreutz R (2006). Physiology of local renin‐angiotensin systems. Physiol Rev 86: 747–803. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucl Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C et al. (2012a). Role of omega‐hydroxylase in adenosine‐mediated aortic response through MAP kinase using A2A‐receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 302: R400–R408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Jamal Mustafa S (2012b). CYP‐epoxygenases contribute to A2A receptor‐mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol 303: R1003–R1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan I, Zeldin DC, Ledent C, Mustafa JS, Falck JR, Nayeem MA (2014). High salt diet exacerbates vascular contraction in the absence of adenosine A(2)A receptor. J Cardiovasc Pharmacol 63: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A, Bromberg Y, Sperling O, Zoref‐Shani E (2005). Phospholipase C is involved in the adenosine‐activated signal transduction pathway conferring protection against iodoacetic acid‐induced injury in primary rat neuronal cultures. Neurosci Lett 373: 218–221. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Davies LM, Barrett‐Jolley R, Standen NB, Dart C (2007). Angiotensin II‐activated protein kinase C targets caveolae to inhibit aortic ATP‐sensitive potassium channels. Cardiovasc Res 76: 61–70. [DOI] [PubMed] [Google Scholar]
- Sanjani MS, Teng B, Krahn T, Tilley S, Ledent C, Mustafa SJ (2011). Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double‐knockout mice. Am J Physiol Heart Circ Physiol 301: H2322–H2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura H, Dzau VJ, Pratt RE (1994). Desensitization of angiotensin receptor function. Kidney Int 46: 1499–1501. [DOI] [PubMed] [Google Scholar]
- Sharifi‐Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B et al. (2013). Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol 304: H1294–H1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ (2005). Role of A1 adenosine receptors in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 288: H1411–H1416. [DOI] [PubMed] [Google Scholar]
- Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ (2013). Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol 61: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Numabe A, Hirawa N, Kawabata Y, Nagoshi H, Kaneko H et al. (1995). A new adenosine subtype‐1 receptor antagonist, FK‐838, attenuates salt‐induced hypertension in Dahl salt‐sensitive rats. Am J Hypertens 8: 1189–1199. [DOI] [PubMed] [Google Scholar]
- Zaman MA, Oparil S, Calhoun DA (2002). Drugs targeting the renin‐angiotensin‐aldosterone system. Nat Rev Drug Discov 1: 621–636. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R et al. (2013). Elevated ecto‐5′‐nucleotidase‐mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112: 1466–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Teng B, Tilley S, Mustafa SJ (2013). A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A‐mediated H2O2 production and KATP opening in isolated mouse hearts. Am J Physiol Heart Circ Physiol 305: H1668–H1679. [DOI] [PMC free article] [PubMed] [Google Scholar]


