Abstract
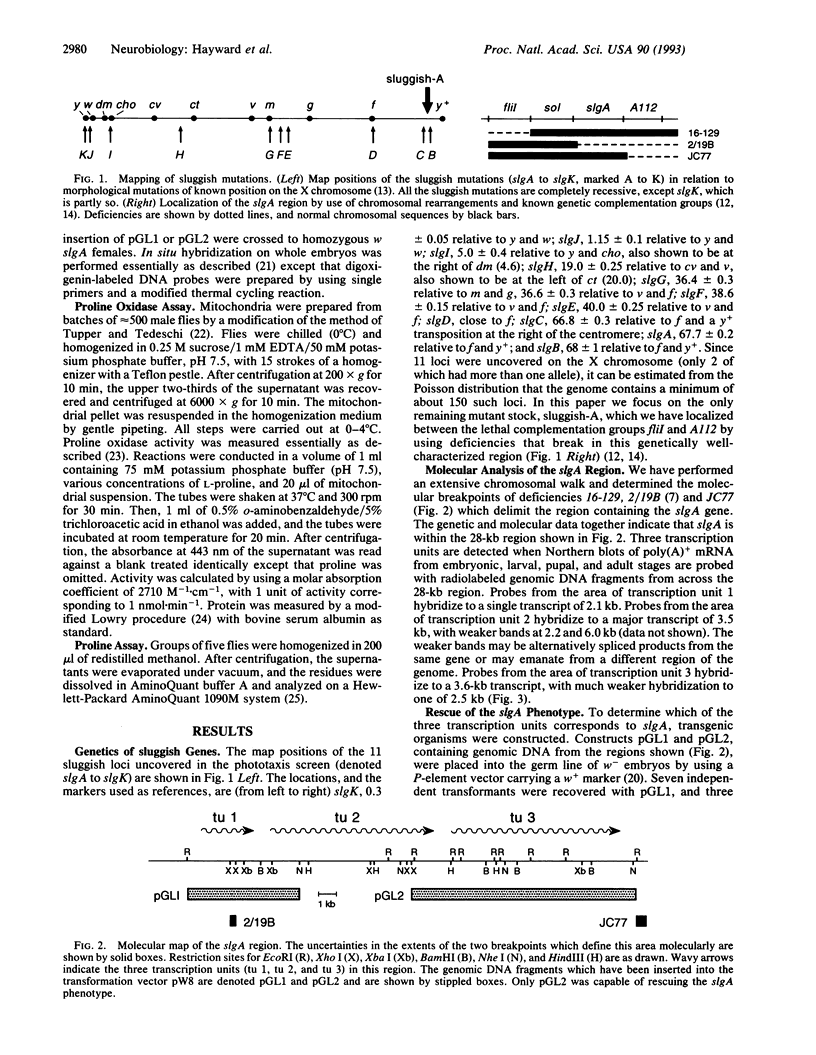
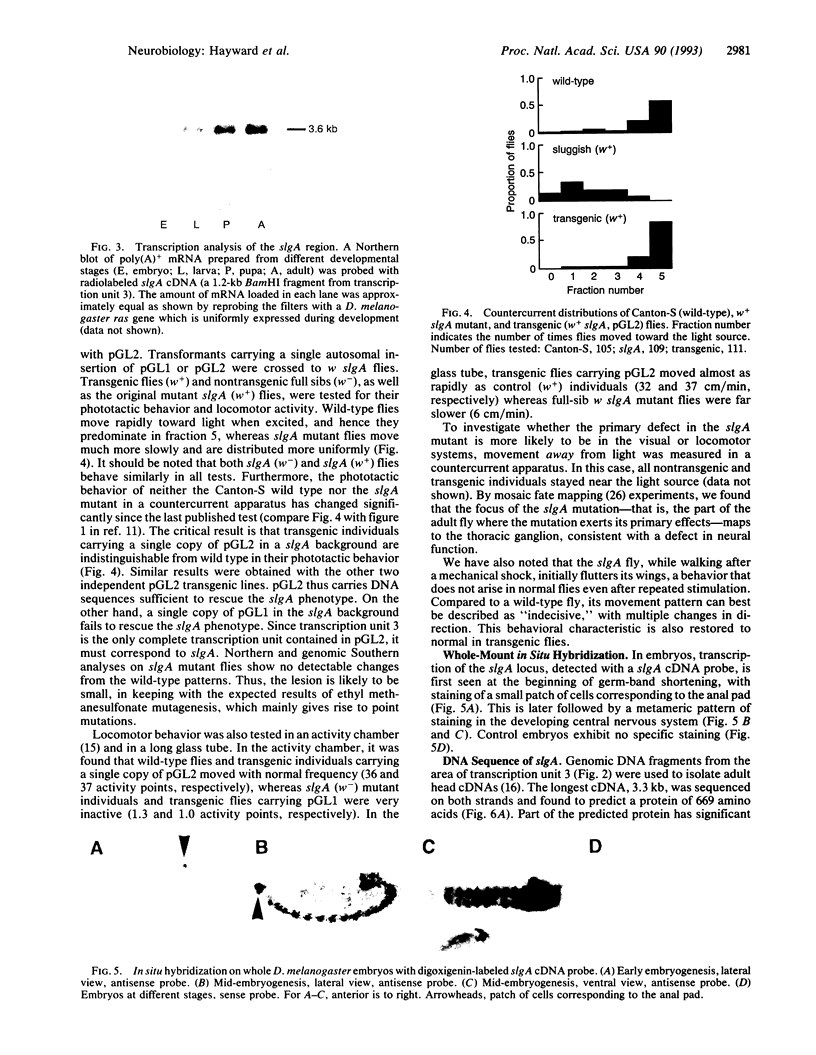
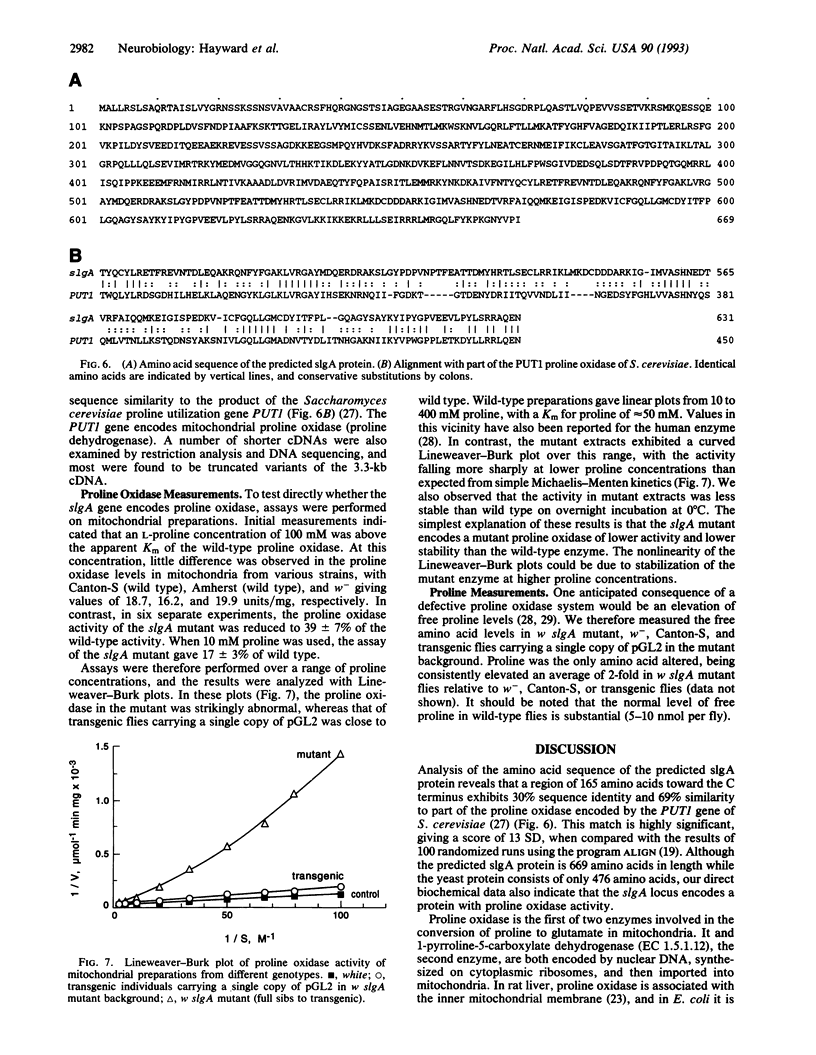
Certain gene mutations in Drosophila melanogaster cause sluggish motor activity. We have localized the transcription unit of the sluggish-A gene to a 14.7-kb region at the base of the X chromosome and have cloned corresponding cDNAs. The predicted protein product has significant sequence similarity to Saccharomyces cerevisiae proline oxidase (EC 1.5.99.8), a mitochondrial enzyme which catalyzes the first step in the conversion of proline to glutamate. In the mutant fly, mitochondrial proline oxidase activity is reduced and has kinetic properties different from those of the wild type, providing further evidence that the gene encodes proline oxidase. Indeed, the free proline level in mutant flies is elevated. When the mutant is rescued by transformation, the proline oxidase and free proline levels, as well as the motor and phototactic behavior, are restored to normal. During embryonic development the sluggish-A transcript is predominantly expressed in the nervous system. Significantly, it has previously been reported that a mouse mutant, PRO/Re, which has reduced proline oxidase activity and elevated free proline levels, also exhibits sluggish behavior.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson J. L., Baker L. G., Stephenson J. T., Wood J. M. Proline dehydrogenase from Escherichia coli K12. Properties of the membrane-associated enzyme. Eur J Biochem. 1983 Jul 15;134(1):77–82. doi: 10.1111/j.1432-1033.1983.tb07533.x. [DOI] [PubMed] [Google Scholar]
- Attwell D., Bouvier M. Cloners quick on the uptake. Curr Biol. 1992 Oct;2(10):541–543. doi: 10.1016/0960-9822(92)90024-5. [DOI] [PubMed] [Google Scholar]
- Banerjee U., Renfranz P. J., Pollock J. A., Benzer S. Molecular characterization and expression of sevenless, a gene involved in neuronal pattern formation in the Drosophila eye. Cell. 1987 Apr 24;49(2):281–291. doi: 10.1016/0092-8674(87)90569-1. [DOI] [PubMed] [Google Scholar]
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. From the gene to behavior. JAMA. 1971 Nov 15;218(7):1015–1022. [PubMed] [Google Scholar]
- Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988 Aug 26;54(5):723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Delaney S. J., Hayward D. C., Barleben F., Fischbach K. F., Miklos G. L. Molecular cloning and analysis of small optic lobes, a structural brain gene of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7214–7218. doi: 10.1073/pnas.88.16.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Caron M. G., Blakely R. D. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992 May;8(5):915–926. doi: 10.1016/0896-6273(92)90206-s. [DOI] [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Hagedorn C. H., Phang J. M. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986 Jul;248(1):166–174. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Benzer S. Mapping of behaviour in Drosophila mosaics. Nature. 1972 Dec 29;240(5383):527–535. doi: 10.1038/240527a0. [DOI] [PubMed] [Google Scholar]
- Jones K. R., Rubin G. M. Molecular analysis of no-on-transient A, a gene required for normal vision in Drosophila. Neuron. 1990 May;4(5):711–723. doi: 10.1016/0896-6273(90)90197-n. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Krakower C. A., Manaligod J. R., Justice P., Wong P. W. Biochemical, morphological and hybrid studies in hyperprolinemic mice. Biomedicine. 1975 May;22(3):209–216. [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow T. A., Merriam J. Phototactic and geotactic behavior of countercurrent defective mutants of Drosophila melanogaster. Behav Genet. 1977 Nov;7(6):447–455. doi: 10.1007/BF01066780. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., Kelly L. E., Coombe P. E., Leeds C., Lefevre G. Localization of the genes shaking-B, small optic lobes, sluggish-A, stoned and stress-sensitive-C to a well-defined region on the X-chromosome of Drosophila melanogaster. J Neurogenet. 1987 Jan;4(1):1–19. doi: 10.3109/01677068709102329. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Benzer S. Monoclonal antibody cross-reactions between Drosophila and human brain. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7641–7645. doi: 10.1073/pnas.80.24.7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler J. V., Wang A., Hakim A. Toxicity of L-proline toward rat hippocampal neurons. Brain Res. 1988 Jul 19;456(1):168–172. doi: 10.1016/0006-8993(88)90358-7. [DOI] [PubMed] [Google Scholar]
- Oyanagi K., Tsuchiyama A., Itakura Y., Tamura Y., Nakao T., Fujita S., Shiono H. Clinical, biochemical and enzymatic studies in type I hyperprolinemia associated with chromosomal abnormality. Tohoku J Exp Med. 1987 Apr;151(4):465–475. doi: 10.1620/tjem.151.465. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Smouse D., Miklos G. L. Developmental genetics of loci at the base of the X chromosome of Drosophila melanogaster. Genetics. 1989 Feb;121(2):313–331. doi: 10.1093/genetics/121.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Pflugfelder G. O., Schwarz H., Roth H., Poeck B., Sigl A., Kerscher S., Jonschker B., Pak W. L., Heisenberg M. Genetic and molecular characterization of the optomotor-blind gene locus in Drosophila melanogaster. Genetics. 1990 Sep;126(1):91–104. doi: 10.1093/genetics/126.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden C. S., Pirrotta V., Jan L. Y. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987 Oct 23;51(2):165–173. doi: 10.1016/0092-8674(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Tedeschi H. Microelectrode studies on the membrane properties of isolated mitochondria. Proc Natl Acad Sci U S A. 1969 Jun;63(2):370–377. doi: 10.1073/pnas.63.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic T. S., Hyde D. R., O'Tousa J. E. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics. 1991 Apr;127(4):761–768. doi: 10.1093/genetics/127.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Brandriss M. C. Proline utilization in Saccharomyces cerevisiae: sequence, regulation, and mitochondrial localization of the PUT1 gene product. Mol Cell Biol. 1987 Dec;7(12):4431–4440. doi: 10.1128/mcb.7.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Wuarin J. P., Dudek F. E. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990 Nov 30;250(4985):1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]





