Abstract
Several studies have described phenotypic changes in the offspring of mice exposed to a variety of environmental factors, including diet, toxins, and stress; however, the molecular pathways involved in these changes remain unclear. Using a high fat diet (HFD)-induced obesity mouse model, we examined liver gene expression in male offspring and analyzed chromatin of paternal spermatozoa. We found that the hepatic mRNA level of 7 genes (out of 20 evaluated) was significantly altered in HFD male offspring compared to control mice, suggesting that phenotypic changes in the offspring depend on parental diet. We examined 7 imprinted loci in spermatozoa DNA from HFD-treated and control fathers by bisulfite sequencing, but did not detect changes in DNA methylation associated with HFD. Using chromatin immunoprecipitation followed by high-throughput sequencing, we found differential histone H3-occupancy at genes involved in the regulation of embryogenesis and differential H3K4me1-enrichment at transcription regulatory genes in HFD fathers vs. control mice. These results suggest that dietary exposure can modulate histone composition at regulatory genes implicated in developmental processes.
Keywords: DNA methylation, epigenetics, epigenetic inheritance, high fat diet, histone H, obesity
Introduction
The field of epigenetics studies key functional elements that regulate gene expression.1,2 Epigenetics depends on chromatin, a complex comprising DNA, RNA, and proteins that package DNA. The smallest subunit of chromatin is the nucleosome, composed of 8 subunits containing histone H3, H4, H2A, and H2B. Histones can be posttranslationally modified by methylation, acetylation, phosphorylation, and other modifications; DNA can be covalently modified at cytosines, in a process known as CpG methylation. These modifications promote chromatin compaction and control the access of transcription factors to DNA. In addition to local chromatin changes, long-range chromatin interactions regulate gene expression, for example, by bringing enhancer and promoter elements together.
During development, chromatin states may be reset several times.3-5 Murine germ cells migrate to fetal gonads at mid gestation and undergo genome-wide DNA demethylation. Thus, genomic imprints are eradicated to allow the establishment of novel male- or female-specific imprinting patterns.6 A second reprogramming of chromatin states occurs upon fusion of the male and female genome soon after fertilization. In this way, epigenetic information that was acquired during parental development and adult life is thought to be lost, enabling the unique development of the offspring. However, several recent studies have suggested that some epigenetic changes induced by the environment in the parent may be inherited through the germline to the progeny.7 One of the original reports observing epigenetic changes in germ cells demonstrated DNA methylation changes in sperm after ancestral exposure to vinclozolin, an agricultural toxin.8 Many types of environmental challenges imposed on the parent, such as hunger, specific diets, toxins, or trauma have been found to influence the development of the offspring.3,9-12 Whereas some studies examined the phenotype of immediate offspring, others observed changes in several subsequent generations. The transmission of phenotypic changes to grandchildren (F3 generation) and following generations is also termed ‘transgenerational inheritance’ and assumes that the inducing agent has not been in direct contact with the offspring, whereas an effect on the immediately succeeding generation may be termed ‘intergenerational inheritance’.13,14 While environmental exposure of the mother can affect the efficiency of the pregnancy itself, treatment of the male parent is thought to mediate epigenetic information through sperm.
During spermatogenesis, nucleosomal histones are replaced by basic protamines leading to a tenfold higher compaction of spermatozoa DNA. This tightly packaged chromatin is critical for sperm motility. However, a small amount of histones (1–10%) is thought to remain in contact with DNA and to shuttle to the next generation of offspring.15-20 Recent studies have also detected specific histone tail modifications at retained histones and the presence of small RNA in sperm.3,10,16,19-21 After fertilization of the oocyte, protamines are stripped from the paternal genome and nucleosomes are reassembled.22 It remains to be shown whether H3 retention in sperm can transfer transgenerational epigenetic information.
Obesity is associated with human diseases including diabetes, cardiovascular diseases, cancer, and arthritis.23 The development of obesity depends on genetic and environmental factors. In particular, long-term intake of a high fat diet (HFD) can contribute to the development of obesity and diabetes in humans and rodents. Feeding C57BL/6J male mice with high fat diets can serve as a disease model since male mice develop obesity, hyperglycemia, and hyperlipidemia.24,25
A number of studies have reported that a parental diet containing high fat or imposing calorie restriction modulates the phenotype of the offspring.26-28 Under-nourishment in utero leads to methylome changes in the sperm of the male progeny and metabolic changes that are inherited to the subsequent generation.29 A high fat paternal diet in rodents changes the insulin and glucose levels in offspring serum and induces an abnormal response after glucose or insulin injections.26 The change in glucose tolerance in rodents is associated with deregulation of pancreatic islet gene expression, including upregulation of IL13 receptor α2 (IL13Ra2) mRNA level and reduced CpG methylation at the IL13Ra2 promoter regions.30 In addition, offspring fertility decreased after exposure of the father to a high fat diet.31
Obesity is associated with pathophysiologic conditions and may have health impacts even on subsequent generations. We hypothesized that the fat content of a diet may have an impact on chromatin composition during gamete development and this may have the potential to influence the reprogramming process that takes place during the transition from spermatogonia to spermatids.
Here we examined C57BL/6J male mice treated with high- vs. low-fat diets by looking at H3 retention and genomic imprints in the sperm, and examined liver mRNA expression of several fat synthesis-related genes in the offspring.
Results
Liver gene expression in male offspring of HFD- vs. LFD-treated fathers
Male mice (C57BL/6J) were fed for 10 weeks with a high fat diet (HFD), containing 60% of the caloric value derived from fat, or with a normal control low fat diet (LFD), with 10% of calories derived from fat (Fig. 1). The HFD treatment lead to male C57BL/6J mice obesity, with elevated blood glucose and impaired glucose tolerance, but without development of overt diabetes.24,25 HFD- and LFD-treated male mice were bred with female C57BL/6J mice. We examined liver gene expression in the offspring of HFD- and LFD-treated fathers. mRNA was obtained from male offspring livers at 8, 16, and 24 weeks of age and analyzed by quantitative real time-PCR. We examined 20 genes that play roles in fatty acid metabolism and oxidative stress response, since these pathways have been shown to be deregulated under high fat diet.32,33
Figure 1.
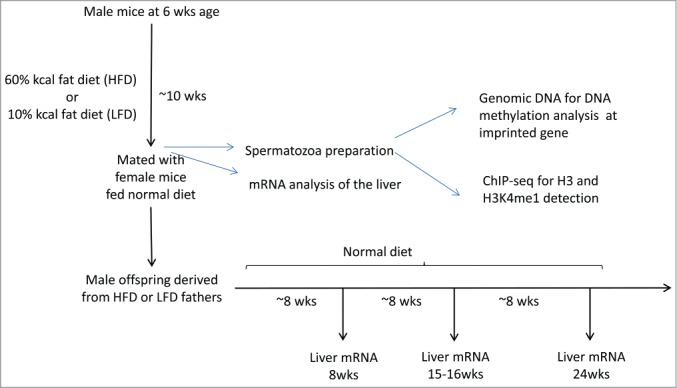
Experimental design for analyzing the epigenetic effect of high fat diet on male mice sperm cells and on offspring liver gene expression. Sperm cells were prepared from male mice fed a HFD for 10 weeks after weaning. Liver tissues were prepared from males and offspring. Chromatin was prepared and precipitated with antibodies against H3 and H3K4me1, followed by high-throughput sequencing (ChIP-Seq).
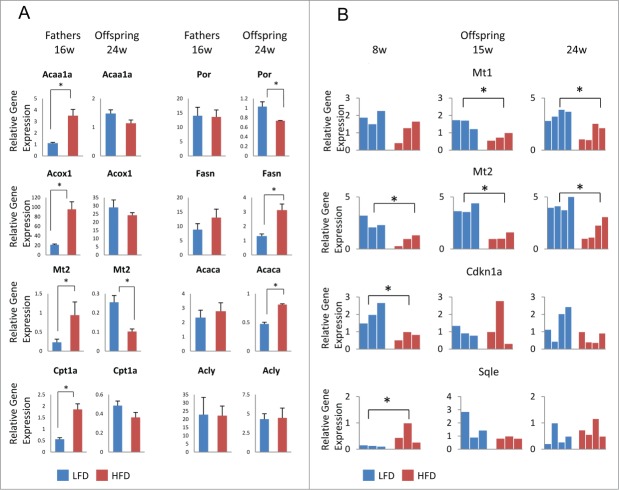
Five genes were differentially expressed between male offspring at 24 weeks of age from HFD vs. LFD-treated fathers, including metallothionein-1 and −2 (Mt1, Mt2), fatty acid synthase (Fasn), P450 cytochrome oxidoreductase (Por), and acetyl-CoA carboxylaseα (Acaca) (Fig. 2). Acaca, Fasn, and Por affect lipid metabolism in the liver. Mt1 and Mt2 play a role in the oxidative stress response and their deletion in mice resulted in increased drug sensitivity of the liver and development of obesity.34,35 Differential Mt1 and Mt2 gene expression was also detected at earlier time points (Fig. 2B). Two other genes, squalene epoxidase (Sqle), which is altered in obesity,33,36 and the cell cycle regulator cyclin-dependent kinase inhibitor 1a (Cdkn1a) displayed significant mRNA expression changes in HFD vs. LFD offspring at 8 weeks of age (Fig. 2B). We do not know why some genes showed significant expression changes in 24 week-old offspring in HFD compared to control. It is possible that increasing testosterone levels exacerbated the differential gene expression between HFD and LFD progeny, since, for example, testosterone normally represses lipogenic enzymes, such as Acaca and FasN.66 Alternatively, prolonged exposure to oxidative stress (e.g., in the case of Mt1) may aggravate the differences in gene expression between HFD and LFD offspring over time.34,35
Figure 2.
Aberrant gene expression in male offspring liver from high fat diet-treated fathers. (A) Real-time PCR analysis in liver from HFD- (red) and LFD-treated (blue) fathers (left panels) and 24-week old male offspring (right panels) for each of the following genes: Acaa1a, Por, Acox1, Fasn, Mt2, and Acaca. For fathers, each bar represents the mean (±SD) of an experiment using 3 animals performed in triplicate. For offspring, each bar represents the mean (±SD) of an experiment using 4 animals performed in triplicate. (B) Real-time PCR analysis in liver from male offspring at 8, 16, and 24 weeks of age from HFD- (red) and LFD-treated (blue) fathers for the genes Mt1, Mt2, Cdkn1a, and Sqle. Each bar represents the result of an experiment performed in triplicate from an individual animal. PCR data were normalized with respect to control Gapdh expression. *P-value < 0.05.
It should be noted that half of the analyzed genes (a total of 10) showed significant mRNA changes in the liver of HFD-treated fathers compared to LFD fathers (Fig. 2A, Fig. S1, and data not shown). However, genes were not necessarily affected in the same way in HFD-treated fathers and their offspring. For example, Mt2 expression was increased in HFD-treated father liver, but Mt2 mRNA levels were reduced in the HFD offspring liver (Fig. 2A). No significant differences were detected in liver gene expression between female offspring from HFD- and LFD-treated fathers (data not shown). We observed high gene expression variability between individual female mice, which may be caused by female hormones, since we did not synchronize for estrus cycles. Our data suggest that differences in dietary treatment of male mice can affect gene expression in male subjects of the successive generation.
Genomic imprints in HFD- and LFD-treated male sperm DNA
In order to address molecular mechanisms involved in the transference of diet-induced effects to the next generation, we examined the chromatin of diet-treated fathers. We first addressed the question of whether CG methylation was altered in sperm at differentially methylated regions (DMR) of genomic imprinted genes. Genomic imprinting is an epigenetic mechanism that ensures differential expression from the maternal or paternal chromosome at imprinted genes.6 Imprinted genes play roles in development and have impact on human diseases, including obesity and cancer.37 The differential gene expression is regulated by a short DNA sequence, the imprinting center region, which shows differential methylation.38 Deletion of the imprinting center causes disruption of the parent-specific gene expression pattern. These critical sequences acquire de novo CG methylation in the parental germ line, according to the sex of the parent.39 We hypothesized that differential diet exposure may directly perturb imprinting patterns during spermatogonia to spermatid formation.40 Such a perturbed methylation pattern could conceivably be transferred to offspring, since DMRs retain their DNA methylation pattern while the zygote undergoes global DNA demethylation, thus escaping zygotic reprogramming.39
Mature, motile spermatozoa were isolated from cauda epididymis (see Methods and Supplemental movie) of animals treated for 10 weeks with HFD or LFD (see Fig. 1 for experimental scheme). Five animals of each dietary treatment group were pooled, and genomic DNA was prepared. Using bisulfite sequencing, we examined CG methylation at 7 known Imprinting Control Regions (ICRs) that show complete CG methylation in sperm or are excluded from CG methylation in sperm.38 The ICR upstream of the H19 gene (which encodes a long non-coding RNA) controls the expression of H19 and insulin like growth factor 2 (Igf2) genes. The H19 ICR is highly methylated in sperm DNA and hypomethylated in oocytes, whereas embryonic stem (ES) cells, carrying a maternal (hypomethylated) and a paternal (methylated) copy, display intermediate methylation (Fig. S2). The H19 ICR displayed complete CG methylation in spermatozoa DNA derived from HFD- and LFD-treated fathers (Fig. S2). Six additional ICRs that are normally hypomethylated in male germ cells were examined, including the Zinc Finger protein gene Zac1, Small nuclear ribonucleoprotein polypeptide N (Snrpn), Long QT intronic transcript 1 (Lit1), Paternally expressed gene 1 (Peg1), Insulin like growth factor 2 receptor (Igf2r), and Paternally expressed gene 3 (Peg3). The methylation patterns were not significantly different between genomic DNA derived from HFD and LFD-fathers (Fig. S2). However, we cannot conclude that DNA methylation is not affected by diet since our ICR methylation analysis was very limited. Nevertheless, the male-specific CG methylation pattern at ICRs confirms the purity of our spermatozoa preparation.
H3 retention in spermatozoa
Although most of the histone proteins are replaced by protamines during spermatogenesis, several recent reports suggest that a portion of the haploid genome is retained in nucleosomes,16-20 with about 1–4% being retained in the murine genome. To address the effect of diet on sperm histone content, we performed chromatin immunoprecipitation (ChIP) using specific antibodies against histone H3. Chromatin was prepared from pooled HFD and LFD samples (5 animals each group) and their biological replicates. ChIP samples and their respective ‘input’ samples, serving as controls, were used for high-throughput sequencing analysis.
We first performed peak analysis to assess the highest H3-enrichment in the genome. About 820 peaks [false discovery rate (FDR) < 0.0001] were detected in the H3-ChIP from high fat diet sample 7 (HFD7) vs. about 550 peaks in the low fat diet sample 8 (LFD8). A second ChIP on independently-derived spermatozoa samples from a different set of diet-treated male mice revealed an even larger number of H3 peaks in high fat diet sample 4 (HFD4; 5603) compared to low fat diet sample 5 (LFD5; 926). About 42–43% of peaks showed an overlap with biological replicates. Despite variation in sample sets, the results suggest that HFD treatment is associated with increased H3-enrichment in sperm compared to LFD control samples. After peak evaluation, we identified the nearest neighboring genes and found that the majority of peaks in the HFD samples (53% HFD7 and 73% HFD4) were located near genes, in contrast to LFD samples (42% in LFD5 and 34% in LFD8). Furthermore, HFD samples had the highest share of peaks at promoter regions, whereas LFD samples showed the largest proportion in the coding region (Fig. 3A).
Figure 3.

H3 enrichment in HFD- and LFD-treated mice spermatozoa. (A) Distribution of H3 enrichment peaks among gene promoter regions, which include 5 kb upstream and 3 kb downstream the TSS, 5′UTR, 3′UTR, and coding sequence (CDS) in HFD7 vs. LFD8. (B) Heatmap of H3-ChIP signal intensities, normalized for input, around the TSS of protein-coding genes (23,350) in HFD7 and LFD8, at a 5 bp resolution. Profiles show the TSS plus 2 kb of upstream and 2 kb of downstream flanking region. Genes are ranked based on the number of CG sites. (C) Heatmap of H3-enrichment around the TSS of genes encoding microRNAs (1,516) in HFD7 and LFD8 samples. (D) Genome browser view presenting H3-enrichment, normalized to input, in HFD7 and LFD8 samples at genes (black) and CG islands (green bar).
Since H3 was enriched at promoter regions, we generated high-resolution H3 profiles based on normalized read density around transcriptional start sites (TSS) (23,350 genes). H3 retention was observed around TSS (Fig. 3B) and density plots revealed an enrichment profile with peaks at +1 nucleosome (peaking 100–160 bp downstream of the TSS), a slight dip in H3 density immediately upstream of the TSS, and another peak of H3 retention at −1 nucleosome. This enrichment profile was specific for TSSs of protein-coding genes and was not present at TSSs of miRNAs (Fig. 3C). H3 occupancy was most intense at CG-rich promoter regions (Fig. 3B,D), as was previously reported.16,19 Overall, we observed a higher normalized read density at protein-coding promoter regions in HFD (HFD7) compared to LFD (LFD8), consistent with the results of the peak analysis.
In order to identify genes with differential H3 retention we ranked all promoter regions based on differences in H3 occupancy comparing HFD7 to LFD8 (the H3 enrichment in HFD7 minus H3 occupancy in LFD8). Both high fat samples (HFD7 and HFD4) revealed a similar pattern of H3 retention at TSSs compared to LFD samples (LFD8 and LFD5) (Fig. 4A), indicating that a similar subset of genes was affected by the dietary treatment. Half of the genes (1,074) with greatest differences in H3 occupancy (HFD7 minus LFD8) displayed similar changes in H3 retention in biological replicates (HFD4 minus LFD5 > 10 normalized read density). Gene Ontology (GO) analysis for functional annotation revealed enrichment for genes involved in embryonic morphogenesis (FDR < 0.05), and about 150 genes play a role in transcription (P-value< 1.40E-05), including 125 DNA binding factors (Fig. 4B). The genes include the basic helix-loop-helix transcription factor Musculin (Msc), which inhibits myogenesis during normal development,41 Pou domain 3f3 (Pou3f3), critical for normal neocortex formation,42 and R1bcc1, a kinase interacting protein that upon deletion causes heart failure and liver degeneration in mice.43( Fig. 4C). Thus, HFD enhances H3 retention in spermatozoa at genes involved in the regulation of embryonic development.
Figure 4.
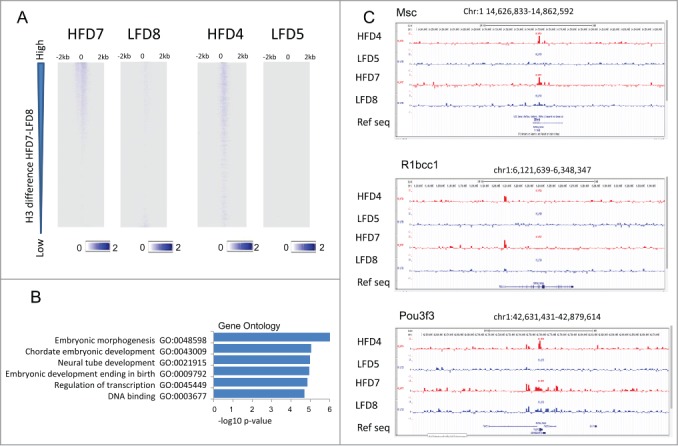
Differential H3 occupancy at regulatory genes in HFD- vs. LFD-treated mice. (A) Heatmap of H3-ChIP signal intensities, normalized to input, around TSS of protein-coding genes (23,350) and 2 kb of flanking sequences for samples HFD7 and LFD8 and their biological replicates, HFD4 and LFD5. Genes are ranked based on H3 occupancy difference at TSS (+500 bp to −500 bp) in HFD7 vs. LFD8, from high- to low-occupancy. (B) GO term analysis of genes highly enriched in H3 (1,074) in HFD samples (HFD7 and HFD4) vs. their control samples (LFD8 and LFD5). (C) Genome browser view presenting H3-enrichment, normalized to input, for sample sets HFD7 (red) and LFD8 (blue) and biological replicates HFD4 (red) and LFD5 (blue) at selected genes involved in transcription regulation.
H3K4me1 retention
Next, we considered the possibility that specific histone modifications may be retained in sperm and performed ChIP-Seq for the H3K4me1 modification on one set of dietary samples (HFD4 and LFD5). H3K4me1 is a histone modification present around TSSs and enhancer regions and frequently associated with active gene transcription.44 The H3K4me1 pattern in both HFD and LFD samples displayed enrichment around TSS in a pattern that was distinct from the H3 retention pattern (Fig. 5A). H3K4me1 was depleted around the TSS regions (−500 to +500 bp) and enrichment spread to 2 kb upstream or downstream the TSS. To identify genes with differential enrichment of H3K4me1 between the 2 dietary treatments, we ranked all protein-coding genes (23,350) based on the difference in H3K4me1 occupancy between HFD and LFD sample (Fig. 5B). The top 5% of genes with significant differential H3K4me1 enrichment (LFD > HFD) were mostly implicated in transcription regulation (FDR < 5.11E-13) and transcription factor activity (FDR < 7.94E-12) (Fig. 5B). Notably, the subset of transcription regulatory genes differentially enriched for H3K4me1 (158 genes) showed minimal overlap (only 9 genes) with transcription regulatory factors showing differential H3 retention (150 genes). Among the transcriptional regulator genes that were enriched in H3K4me1 LFD5 compared to HFD4 samples were the chromatin remodeling factor Lsh,45 the homeobox genes Hoxd11 and Hoxd13, the brain-specific angiogenesis inhibitor Bai3, the forkhead transcription factor Foxp2, and Foxa2, known to induce transdifferentiation of fibroblasts into hepatocytes.44,46 (Fig. 5C). This indicated that retention of H3K4me1 is influenced by dietary treatment and affected a discrete subset of genes involved in development regulatory processes.
Figure 5.
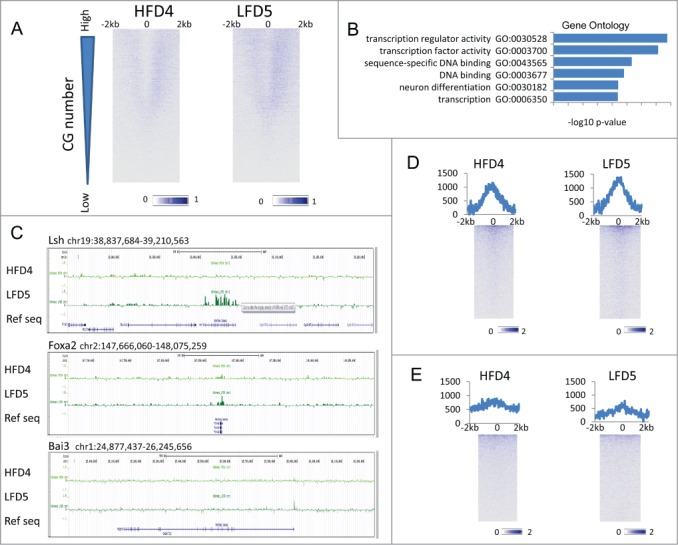
H3K4me1 enrichment at TSS and enhancer. (A) Heatmap of H3K4me1-ChIP signal intensities, normalized to input, around TSS of protein-coding genes (23,350) at a 5-bp resolution in HFD4 vs. LFD5 spermatozoa samples. Profiles show TSS and 2 kb of upstream and 2 kb of downstream flanking region. Genes were ranked based on the number of CG sites. (B) GO term analysis of the top 5% of genes highly enriched in H3K4me1 (1,169) in LFD5 vs. HFD4 samples. (C) Genome browser view representing H3K4me1 enrichment, normalized to input, for HFD4 (dark green) and LFD5 (light green) at selected genes encoding transcriptional regulators. (D) Heatmap of H3K4me1-ChIP signal intensities at enhancers (9,452) plus 2 kb of upstream and downstream flanking sequences, normalized to input, at a 25-bp resolution in HFD4 vs. LFD5 spermatozoa samples. Sequences were sorted based on highest enrichment in HFD4. Line graphs on top of heatmaps represents the sum of H3K4me1 enrichment profiles at each position. (E) Heatmap of H3K4me1-ChIP signal intensities at enhancers (8,701) in liver samples.
Finally, we profiled H3K4me1 modification at testis enhancers (n = 9,451) and liver enhancers (n = 8,701) in HFD and LFD samples.44 H3K4me1 enrichment was higher at testis enhancers (Fig. 5D) than at liver enhancers (Fig. 5E). Differentially enriched testis enhancers (LFD > HFD) were significantly enriched at genes involved in mesoderm formation and the formation of ectoderm derived tissues (FDR<0.05) (Fig. S3A). In contrast, differentially enriched liver enhancers were significantly enriched (FDR<0.05) at genes controlling lipid biosynthesis, fatty acid synthesis and the oxidation-reduction process (Fig. S3B).
Taken together, different dietary treatments were associated with specific histone retention in sperm at genes that are implicated in the regulation of development.
Discussion
In this study, we have examined H3 retention, H3K4me1 enrichment, and several imprints in the sperm of male mice fed with either a high- (HFD) or low-fat diet (LFD). We describe an altered H3 retention profile dependent on diet exposure affecting genes involved in cell fate decisions. In addition, we have examined the expression of genes in the liver of male offspring and found differential expression of several genes involved in the oxidative response pathway.
Multiple studies have reported an effect of parental dietary exposure on the phenotype of the next generation, including alterations in weight, fertility, and response to insulin. Some studies observed specific changes in gene expression induced by dietary exposure. For example, a low protein diet has been shown to alter gene expression in the liver of offspring.17 A diet poor in folate induced DNA methylation changes in the sperm and alterations of gene expression in the placenta.47 Using odor-induced fear conditioning in male mice, it was found that offspring also had enhanced sensitivity to the same odor, which was associated with hypomethylation at a specific olfactory receptor gene 48. Induction of a pre-diabetic state in fathers (feeding a high fat diet in combination with streptozotocin injection) altered pancreatic gene expression pattern in male offspring.26 In our study, we detected altered gene expression in the liver of HFD male offspring compared to LFD male offspring. Several of the differentially expressed genes play a role in lipid metabolism (Acaca, Fasn, Por, and Sqle) and are involved in the oxidative-reduction pathway (Mt1, Mt2, Por, and Sqle). Although we do not have any evidence that the de-regulation of those genes could affect the health of the offspring, these genes are known to play a role in the pathophysiology of the liver. Metallotheonein (Mt) isoforms are thought to modulate complex diseases, including cancer, kidney, heart malfunction, and diabetes.49 Mt1 and Mt2 are metal binding proteins, with a role in the prevention of oxidative stress. Mt1 and Mt2 are also expressed in the testis and are regulated in response to environmental stress.50 Mt1 and Mt2 knockout mice enhanced the HFD-induced obesity phenotype.34 P450 cytochrome oxidoreductase (Por) is a major player in cholesterol biosynthesis, drug metabolism, and the formation of reactive oxygen radicals; Por deficiency in humans causes a disease with perturbed steroidogenesis.51 Squalene epoxidase (Sqle) contains oxidoreductase activity and catalyzes an important oxygenation step in cholesterol biosynthesis. Sqle is a candidate gene for quantitative trait locus analysis of obesity.36 In addition to previously observed alterations in fertility, growth, and altered glucose metabolism, we report here an altered liver gene expression phenotype, suggesting that offspring may have an impaired oxidative/DNA damage response pathway influenced by parental diet.
In order to address the possibility that epigenetic information is modulated by diet high fat content and passed on through the sperm chromatin, we examined H3 composition of spermatozoa. Most of the histones are replaced with protamines during spermatogenesis, but about 1–10% of the mammalian genome retain nucleosomes composed of histones.13,14
Although histone retention in sperm has been confirmed by several studies, there is a discordance with respect to which genome compartment has the highest H3 retention. Whereas some studies found nucleosomes mainly at gene-poor regions,18 others report the greatest enrichment at gene-rich regions.15,16,20 Moreover, gene ontology analysis revealed that histone bound genes were enriched for developmental activity. For example, in human sperm, mononucleosomes are retained at promoter regions of genes encoding developmental transcription factors and signaling molecules.20 In mouse spermatozoa, nucleosomes are highly enriched at CG-rich regions, particularly at promoter regions and exons.16 It should be noted that even in studies reporting the highest nucleosome enrichment at gene-poor regions, a subset of nucleosomes are also bound to promoter regions with high-CG content.18 The antibody we used for ChIP-seq detects all H3 variants, including the prevalent variant in murine sperm H3.3.19 The H3 enrichment profile we observed is consistent with previous studies in several aspects: first, the highest enrichment of nucleosomes is found at gene-rich regions; second, the enrichment is high at promoter regions; third, the strongest enrichment is at CG island promoters and; finally, GO-term analysis reveals a significant enrichment for regulatory regions implicated in development.19,20 Notably, nucleosomes accumulate as a peak located immediately downstream of TSS.19 and, likewise we found a peak of H3 enrichment downstream of TSS (Supplemental Fig. 4). This feature is unique in sperm, since nucleosomes are depleted at TSS in somatic tissue.19 In short, our H3 retention profile in sperm is in agreement with those previous studies.
We report here differential H3 retention at promoter regions in HFD- compared to LFD-sperm. These regions are significantly enriched for genes involved in development and cell fate decisions, including Indian Hedgehog (Ihh), involved in mesoderm differentiation, Lim domain only 4 (Lmd4), involved in mammalian development, and Bone morphogenetic protein 4(BMP4), Eyes are absent 1 (Eya1) and Forkhead Box D3 (FoxD3), which is critical for reprogramming in liver progenitors and binds to the albumin promoter prior to gene expression during embryogenesis.52
Recent reports have detected specific histone modifications, such as H3K4me1 and H3K4me2, a mark of active genes, and H3K27me3, associated with repressed genes in sperm,16,20 indicating that histones that are exempt from protamine replacement may preserve their modifications. We examined H3K4me1, a mark at active promoters and poised or active enhancers.44 Our study revealed that H3K4me1 is highly enriched at promoters and enhancers in testis, with a modest enrichment at enhancers in liver. Dietary treatment modulates H3K4me1 retention at promoter regions, and the genes affected are implicated in the regulation of embryogenesis, although comprising a distinct set of genes than those enriched for H3. In particular, H3K4me1 enrichment at promoter regions of genes enconding transcription regulators was highly significant (FDR < 2.94E-16). Intriguingly, it included a number of genes encoding transcription factors that play a role in the hepatocyte network, including Foxa2 (Fig. 5C), a pioneering factor involved in the reprogramming of somatic cells to the liver lineage,46 and other key transcription factors critically involved in hepatic-specific gene expression, such as CCAAT/enhancer-binding protein α (CEBPa), Nuclear receptor subfamily 5, group A, member 2 (Nr5a2), LRH-1, and several forkhead proteins (Focp1, Foxp2, Foxf2, Foxg1, and Foxl2).52,53
Repressive histone modification H3K27me3 may contribute to gene suppression in the early embryo.19 Consistent with this view, H3K27me3 marks persist in the one-cell embryo, concurrently with a lack of H3K27me demethylase activity,54 suggesting a possible transmission of this epigenetic repressive mark from the sperm to the early embryo. Whether other histone modifications can be maintained through early reprogramming after fertilization of the oocyte (including H3K4me1) requires further investigation.
The molecular mechanisms for the selective retention of histones in spermatozoa remain unclear, but overrepresentation of genes involved in spermatogenesis has been observed.16 Whether transcription activity can influence the process of H3 retention at a given gene, and whether the diet can modulate the transcription state in germ cells is not known. However, out of 75 genes developmentally activated in male gametocytes and functionally important for germ cell development,55 we found differential H3K4me1-enrichment in HFD vs. LFD samples in the following genes: ATP dependent DNA helicase homolog (Hfm1), Left-right determination factor 2 (Lefty2), Mut S homolog 5 (Msh5), and SRY-box 2 (Sox2). We identified differential H3 retention in Foxl2, Follistatin (Fst), Gap junction protein β-3 (Gjb3) and Sidekick homolog 1 (Sdk1).
It should be noted that we did not find a consistent pattern of either H3 retention or H3K4me1 modification in spermatozoa at those genes that were differentially expressed in the offspring liver, although Por and Mt1 were included in the set of genes with differential H3K4me1 enrichment in LFD vs. HFD. An alternative hypothesis is that a molecular pathway is deregulated by the diet that in turn affects liver gene expression. In this context, it is noteworthy that differentially marked liver enhancers were significantly enriched for the GO term oxidation-reduction (FDR < 2.6E-08) (Fig. 3B). Another alternative explanation considers indirect regulation via master transcription factors, such as Foxa2 or forkhead proteins (see above). In addition, several metabolic parameters are perturbed in the offspring and higher reactive oxygen species have been detected in sperm from HFD-treated fathers.3
Finally, intergenerational inheritance may also be promoted by factors other than histone modification or DNA methylation. During maturation in the epididymis, most of the cytoplasm is extruded. Under the influence of RNases, glycosidases, and proteases, sperm achieve competence for motility.14 However, the elimination of nuclear and cytosolic factors is not absolute and some sperm components are delivered to the oocyte, including mRNA, miRNA, and transcription factors.14,56 Seminal fluid can also contribute to placental growth, which in turn can alter metabolic parameters in the offspring.57
The fate of paternal histones and histone modifications after fertilization remains unknown, but some retained histones stay associated with the paternal genome in the zygote.58,59 Whether those histones and their modifications could resist further reprogramming during cellular differentiation and directly influence gene expression in differentiated cells (such as liver cells) or could be propagated into subsequent generations remains a challenging topic of research.
Materials and Methods
Animal experiments
Diet-induced obese (DIO) C57BL/6J males and controls were obtained from The Jackson Laboratory (JAX) and were fed according to the protocol used by JAX. Male mice were fed a high-fat diet (HFD: D12492 with 60 kcal% fat from Research Diets, Inc.) or a normal control low fat diet (LFD: 12450B with 10 kcal% fat from Research Diets, Inc.) for 10 to 12 weeks starting at 6 weeks of age. HFD contains 25 g soybean oil and 245 g lard, whereas LFD is composed of 25 g soybean oil and 20 g lard. Thus, HFD contains more than 10-fold the amount of lard, which has a concentration of 0.72 mg/g cholesterol. DIO male mice show increased body weight, elevated blood glucose, and impaired glucose tolerance, without developing overt diabetes (http://jaxmice.jax.org/diomice/diomice-phenotypes.html). During mating, C57BL/6J females (normal in-house diet, 9% fat, Labdiet #5021) and HFD- or LFD-males were kept in a cage with a divider. Animals had access to food for 8 h during the day, then the divider was removed for 16 h during which no food was provided. Total RNA from livers was obtained by isolating the left lateral lobe of the liver in Trizol and grounding it with a pestle. Offspring were derived from the mating of 5 HFD fathers and 6 LFD fathers. HFD and LFD diet fathers were either euthanized for sperm collection or utilized for mating.
Spermatozoa chromatin preparation
Spermatozoa for ChIP-seq and bisulfite sequencing were isolated as previously described,60 using a swim-out method followed by the Percoll gradient procedure. In brief, the cauda epididymis was dissected and suspended in pre-warmed (37°C) PBS. Five animals for each experimental group (HFD7, LFD8) and biological replicas (HFD4, LFD5) were pooled. The epididymides were transferred in pre-warmed-K-RVFE sperm motility medium (Cook medical, A898571) and dissected with a needle to allow live mobile sperm cells to swim out. After incubation for 1, h cells were loaded onto a 2-step Percoll gradient [35% and 70% Percoll (GE Healthcare Biosciences)] and the gradient was centrifuged (650 g, 30 min). The spermatozoa fraction was collected from a layer between 35% and 70% Percoll solutions and as a pellet, and had a motility of over 95% (Supplemental Movie).
ChIP-seq
Spermatozoa chromatin was prepared as described previously,61 with slight modifications. Briefly, spermatozoa were washed in PBS and suspended in lysis buffer (0.1% SDS, 0.5% Triton X-100 in PBS) for 20 min on ice to eliminate white blood cell contamination. After spermatozoa was harvested by centrifugation, the cell pellet was washed in PBS and suspended in 0.05 mg/ml L-a-lysolecithin for 15 min on ice to eliminate acrosome of the sperm head. Cells were harvested by centrifugation and the cell pellet was suspended in 10 mM DTT for 30 min at 37°C to reduce disulfide bonds between protamines and solubilize chromatin. Cells were fixed with 1% formaldehyde and the reaction was stopped with 0.2 M glycine. After the cells were harvested, the cell pellet was suspended in sperm lysis buffer [20 mM HEPES-NaOH (pH 7.3), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% DOC and 0.1% SDS]. ChIP assay was performed as previously described.19,62 Briefly, chromatin was sonicated on ice to generate 150–1,000 bp DNA fragments. The following antibodies were used for immunoprecipitation: rabbit anti-H3 antibody (Abcam, ab1791, recognizing all H3 variants) and rabbit anti-H3K4me1 antibody (Abcam, ab8895). ChIP DNA libraries were made using the Illumina ChIP-seq library preparation kit and subjected to Solexa sequencing (Illumina) at the CCR-Sequencing Facility, National Cancer Institute. DNA libraries from input samples served as controls. Sequencing was performed on an Illumina Genome Analyzer IIx. Reads were aligned using mouse genome build mm9 as reference genome.
ChIP-Seq analysis
For visualization in the UCSC genome browser, read numbers were counted for each ChIP sample, including input samples, using the IGV tool (https://www.broadinstitute.org/igv/) with 1 kb windows. Read numbers were normalized based on total aligned reads, and input signals were subtracted from ChIP-seq sample signals. The total read numbers were as follows: HFD4 sample: 12,523,660 reads for H3K4me1; 10,093,476 reads for H3; and 8,150,084 reads for input; LFD5 sample: 10,264,167 reads for H3K4me1; 11,812,718 reads for H3; and 9,350,861 reads for input; HFD7 sample: 17,355,440 reads for H3; 18,883,447 reads for input; LFD8 sample: 19,866,498 reads for H3; 26,913,692 reads for input. Peak detection (genomic bins containing statistically significant ChIP-Seq enrichment) was performed for a 200 bp window using Partek Genomics Suite at an FDR < 0.0001. Each read was extended toward the interior of the sequenced fragment (about 150 bp) based on the strand of the alignment. For nearest neighboring gene analysis, a flanking region including 5,000 bp upstream and 3,000 bp downstream of the genes was chosen. For high-resolution profiles of H3- or H3K4me1-retention at promoter regions, the number of normalized ChIP-seq reads was tabulated in 5 bp windows around the TSS with 2 kb of upstream and 2 kb of downstream flanking sequences. Input values were subtracted from ChIP values. To identify genes with differential H3 occupancy, read density (normalized to input) in the immediate TSS region (−500 bp to plus +500 bp) was computed. The differences between HFD7 and LFD8 samples (H3 occupancy in HFD7 minus H3 occupancy in LFD8) were calculated and genes ranked from high to low, based on this difference (Fig. 4A). HFD7 and its biological replicate (HFD4) showed similar enrichment, compared to their controls (LFD8 and LFD5, respectively; Fig. 4A). The top 10% highest H3 occupancy difference between HFD7 and LFD8 (2,157 genes) were selected. Half of this subset (1,074) displayed a similar H3 occupancy difference in biological replica (HFD4 minus LFD5 > 10 reads). For functional annotation analysis of these promoter regions enriched for H3 we used David (http://david.abcc.ncifcrf.gov/). The P-value in David represents the EASE score, a modified Fisher exact P-value, as previously described.63 For Gene Ontology (GO) analysis of genomic regions enriched in H3K4me1, GREAT analysis was performed (GREAT version 3.0.0, http://bejerano.stanford.edu/great/public/html/). For P-value calculations a hypergeometric equation was applied, as previously outlined.64 The complete set of RefSeq genes was downloaded from the UCSC website (http://hgdownload.cse.ucsc.edu/goldenPath/mm9/database/refGene.txt.gz). Complete sets of miRNA sequences were downloaded from Ensembl BioMart GRCm38. Testis- and liver-specific enhancer have been downloaded from http://chromosome.sdsc.edu/mouse/download.html based on raw data deposited in GEO under the accession number GSE29184.
Quantitative RT-PCR analysis
Total RNA was extracted from liver samples using TRIzol reagent (Invitrogen) with DNase treatment. To synthesize first-strand cDNA from total RNA (1 μg), reverse transcription was performed using SuperScript III First-Strand Synthesis System (Invitrogen) with random hexamers. The quantitative real-time PCR was performed on MyiQ2 system (Bio-Rad) using iQ SYBR Green Supermix. Every experiment was performed at least 3 times using duplicates or triplicates. Primer sequences are listed in Table S1. For P-value computation Student's t-test was applied.
Bisulfite Sequencing
Genomic DNA was extracted from spermatozoa samples using DNeasy Blood & Tissue Kit (Qiagen; Purification of total DNA from animal sperm using the DNeasy Blood & Tissue Kit; Protocol 2). Five animals were pooled for each experimental group. Bisulfite conversion of genomic DNA was performed using Bisulfite Conversion Kit (Active Motif), as described previously 65. To monitor DNA methylation level of each ICR, the converted DNA was amplified under each PCR condition. PCR conditions and primer sequences are listed in Table S2. Sequencing data were visualized using the QUMA software (http://quma.cdb.riken.jp/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for members of the MCGP program for useful discussions on the manuscript. We thank Karen Saylor for excellent animal technical assistance. We thank Jyoti Shetty and Bao Tran for excellent sequencing work at the Sequencing Facility, FNLCR.
Frederick National Laboratory for Cancer Research is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academic Press; Washington, DC).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the National Library of Medicine, NIH.
References
- 1.Berger SL. The complex language of chromatin regulation during transcription. Nature 2007; 447:407-12; PMID:17522673; http://dx.doi.org/ 10.1038/nature05915 [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell 2007; 128:693-705; PMID:17320507; http://dx.doi.org/ 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci 2014; 16:297-305; PMID:25364281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science 2010; 330:622-7; PMID:21030646; http://dx.doi.org/ 10.1126/science.1190614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill PW, Amouroux R, Hajkova P. DNA demethylation, Tet proteins and 5-hydroxymethylcytosine in epigenetic reprogramming: an emerging complex story. Genomics 2014; 104:324-33; PMID:25173569; http://dx.doi.org/ 10.1016/j.ygeno.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 6.Das R, Hampton DD, Jirtle RL. Imprinting evolution and human health. Mamm Genome 2009; 20:563-72; PMID:19830403; http://dx.doi.org/ 10.1007/s00335-009-9229-y [DOI] [PubMed] [Google Scholar]
- 7.Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK. Nature, nurture and epigenetics. Mol Cell Endocrinol 2014; 398:42-52; PMID:25102229; http://dx.doi.org/ 10.1016/j.mce.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005; 308:1466-9; PMID:15933200; http://dx.doi.org/ 10.1126/science.1108190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl Res 2015; 165:12-7; PMID:24657180; http://dx.doi.org/ 10.1016/j.trsl.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martos SN, Tang WY, Wang Z. Elusive inheritance: Transgenerational effects and epigenetic inheritance in human environmental disease. Prog Biophys Mol Biol 2015; 118(1–2):44-54; PMID:25792089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol 2010; 88:938-44; PMID:20568270; http://dx.doi.org/ 10.1002/bdra.20685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nye MD, Fry RC, Hoyo C, Murphy SK. Investigating Epigenetic Effects of Prenatal Exposure to Toxic Metals in Newborns: Challenges and Benefits. Med Epigenet 2014; 2:53-9; PMID:24955086; http://dx.doi.org/ 10.1159/000362336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley M, Oakey RJ. Resetting for the next generation. Mol Cell 2012; 48:819-21; PMID:23273741; http://dx.doi.org/ 10.1016/j.molcel.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 14.Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet 2005; 6:633-42; PMID:16136654; http://dx.doi.org/ 10.1038/nrg1654 [DOI] [PubMed] [Google Scholar]
- 15.Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, Saida M, Steger K, Tedder P, Miller D. Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res 2009; 19:1338-49; PMID:19584098; http://dx.doi.org/ 10.1101/gr.094953.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, Beisel C, Schubeler D, Stadler MB, Peters AH. Repressive and active histone ethylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol 2010; 17:679-87; PMID:20473313; http://dx.doi.org/ 10.1038/nsmb.1821 [DOI] [PubMed] [Google Scholar]
- 17.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, et al.. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010; 143:1084-96; PMID:21183072; http://dx.doi.org/ 10.1016/j.cell.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carone BR, Hung JH, Hainer SJ, Chou MT, Carone DM, Weng Z, Fazzio TG, Rando OJ. High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm. Dev Cell 2014; 30:11-22; PMID:24998598; http://dx.doi.org/ 10.1016/j.devcel.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erkek S, Hisano M, Liang CY, Gill M, Murr R, Dieker J, Schubeler D, van der Vlag J, Stadler MB, Peters AH. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol 2013; 20:868-75; PMID:23770822; http://dx.doi.org/ 10.1038/nsmb.2599 [DOI] [PubMed] [Google Scholar]
- 20.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009; 460:473-8; PMID:19525931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casas E, Vavouri T. Sperm epigenomics: challenges and opportunities. Front Genet 2014; 5:330; PMID:25278962; http://dx.doi.org/ 10.3389/fgene.2014.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi SL, Henikoff S. Germline histone dynamics and epigenetics. Curr Opin Cell Biol 2007; 19:257-65; PMID:17467256; http://dx.doi.org/ 10.1016/j.ceb.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health 2001; 22:355-75; PMID:11274526; http://dx.doi.org/ 10.1146/annurev.publhealth.22.1.355 [DOI] [PubMed] [Google Scholar]
- 24.Alexander J, Chang GQ, Dourmashkin JT, Leibowitz SF. Distinct phenotypes of obesity-prone AKR/J, DBA2J and C57BL/6J mice compared to control strains. Int J Obes (Lond) 2006; 30:50-9; PMID:16231032; http://dx.doi.org/ 10.1038/sj.ijo.0803110 [DOI] [PubMed] [Google Scholar]
- 25.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes–related traits in mouse strains susceptible to diet-induced obesity. Diabetes 2003; 52:1958-66; PMID:12882911; http://dx.doi.org/ 10.2337/diabetes.52.8.1958 [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, Schatten H, Sun QY. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci USA 2014; 111:1873-8; PMID:24449870; http://dx.doi.org/ 10.1073/pnas.1321195111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008; 41:91-102; PMID:18515302; http://dx.doi.org/ 10.1677/JME-08-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod 2012; 27:1391-400; PMID:22357767; http://dx.doi.org/ 10.1093/humrep/des030 [DOI] [PubMed] [Google Scholar]
- 29.Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, Seisenberger S, Hore TA, Reik W, Erkek S, et al.. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 2014; 345:1255903; PMID:25011554; http://dx.doi.org/ 10.1126/science.1255903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010; 467:963-6; PMID:20962845; http://dx.doi.org/ 10.1038/nature09491 [DOI] [PubMed] [Google Scholar]
- 31.Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, Lane M. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep 2015; 3:e12336PMID:25804263; http://dx.doi.org/ 10.14814/phy2.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EJ, Kim E, Kwon EY, Jang HS, Hur CG, Choi MS. Network analysis of hepatic genes responded to high-fat diet in C57BL/6J mice: nutrigenomics data mining from recent research findings. J Med Food 2010; 13:743-56; PMID:20553184; http://dx.doi.org/ 10.1089/jmf.2009.1350 [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Sohn I, Ahn JI, Lee KH, Lee YS. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004; 340:99-109; PMID:15556298; http://dx.doi.org/ 10.1016/j.gene.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 34.Beattie JH, Wood AM, Newman AM, Bremner I, Choo KH, Michalska AE, Duncan JS, Trayhurn P. Obesity and hyperleptinemia in metallothionein (-I and -II) null mice. Proc Natl Acad Sci U S A 1998; 95:358-63; PMID:9419380; http://dx.doi.org/ 10.1073/pnas.95.1.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A 1994; 91:584-8; PMID:8290567; http://dx.doi.org/ 10.1073/pnas.91.2.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stylianou IM, Clinton M, Keightley PD, Pritchard C, Tymowska-Lalanne Z, Bunger L, Horvat S. Microarray gene expression analysis of the Fob3b obesity QTL identifies positional candidate gene Sqle and perturbed cholesterol and glycolysis pathways. Physiol Genomics 2005; 20:224-32; PMID:15598878; http://dx.doi.org/ 10.1152/physiolgenomics.00183.2004 [DOI] [PubMed] [Google Scholar]
- 37.Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet 2014; 15:517-30; PMID:24958438; http://dx.doi.org/ 10.1038/nrg3766 [DOI] [PubMed] [Google Scholar]
- 38.Lewis A, Reik W. How imprinting centres work. Cytogenet Genome Res 2006; 113:81-9; PMID:16575166; http://dx.doi.org/ 10.1159/000090818 [DOI] [PubMed] [Google Scholar]
- 39.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol 2014; 6:a018382; PMID:24492710; http://dx.doi.org/ 10.1101/cshperspect.a018382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal AC, Benjamin Neelon SE, Liu Y, Tuli AM, Fuemmeler BF, Hoyo C, Murtha AP, Huang Z, Schildkraut J, Overcash F, et al.. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genet Epigenet 2014; 6:37-44; PMID:25512713; http://dx.doi.org/ 10.4137/GEG.S18067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J, Webb R, Richardson JA, Olson EN. MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD. Proc Natl Acad Sci U S A 1999; 96:552-7; PMID:9892671; http://dx.doi.org/ 10.1073/pnas.96.2.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev 2002; 16:1760-5; PMID:12130536; http://dx.doi.org/ 10.1101/gad.978002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gan B, Peng X, Nagy T, Alcaraz A, Gu H, Guan JL. Role of FIP200 in cardiac and liver development and its regulation of TNFalpha and TSC-mTOR signaling pathways. J Cell Biol 2006; 175:121-33; PMID:17015619; http://dx.doi.org/ 10.1083/jcb.200604129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, et al.. A map of the cis-regulatory sequences in the mouse genome. Nature 2012; 488:116-20; PMID:22763441; http://dx.doi.org/ 10.1038/nature11243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu W, Briones V, Lister R, McIntosh C, Han Y, Lee EY, Ren J, Terashima M, Leighty RM, Ecker JR, et al.. CG hypomethylation in Lsh-/- mouse embryonic fibroblasts is associated with de novo H3K4me1 formation and altered cellular plasticity. Proc Natl Acad Sci U S A 2014; 111:5890-5; PMID:24711395; http://dx.doi.org/ 10.1073/pnas.1320945111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev 2010; 20:527-32; PMID:20591647; http://dx.doi.org/ 10.1016/j.gde.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun 2013; 4:2889; PMID:24326934; http://dx.doi.org/ 10.1038/ncomms3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014; 17:89-96; PMID:24292232; http://dx.doi.org/ 10.1038/nn.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thirumoorthy N, Shyam Sunder A, Manisenthil Kumar K, Senthil Kumar M, Ganesh G, Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World J Surg Oncol 2011; 9:54; PMID:21599891; http://dx.doi.org/ 10.1186/1477-7819-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebrahimi-Kalan A, Roudkenar MH, Halabian R, Milan PB, Zarrintan A, Roushandeh AM. Down-regulation of metallothionein 1 and 2 after exposure to electromagnetic field in mouse testis. Iran Biomed J 2011; 15:151-6; PMID:22395140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol 2015; 4:60-73; PMID:25498968; http://dx.doi.org/ 10.1016/j.redox.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol 2008; 73:119-26; PMID:19028990; http://dx.doi.org/ 10.1101/sqb.2008.73.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev 2006; 20:2293-305; PMID:16912278; http://dx.doi.org/ 10.1101/gad.390906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puschendorf M, Terranova R, Boutsma E, Mao X, Isono K, Brykczynska U, Kolb C, Otte AP, Koseki H, Orkin SH, et al.. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat Genet 2008; 40:411-20; PMID:18311137; http://dx.doi.org/ 10.1038/ng.99 [DOI] [PubMed] [Google Scholar]
- 55.Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 2012; 48:849-62; PMID:23219530; http://dx.doi.org/ 10.1016/j.molcel.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33:9003-12; PMID:23699511; http://dx.doi.org/ 10.1523/JNEUROSCI.0914-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA 2014; 111:2200-5; PMID:24469827; http://dx.doi.org/ 10.1073/pnas.1305609111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Werken C, van der Heijden GW, Eleveld C, Teeuwssen M, Albert M, Baarends WM, Laven JS, Peters AH, Baart EB. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun 2014; 5:5868; PMID:25519718; http://dx.doi.org/ 10.1038/ncomms6868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Heijden GW, Ramos L, Baart EB, van den Berg IM, Derijck AA, van der Vlag J, Martini E, de Boer P. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Biol 2008; 8:34; PMID:18377649; http://dx.doi.org/ 10.1186/1471-213X-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furimsky A, Vuong N, Xu H, Kumarathasan P, Xu M, Weerachatyanukul W, Bou Khalil M, Kates M, Tanphaichitr N. Percoll gradient-centrifuged capacitated mouse sperm have increased fertilizing ability and higher contents of sulfogalactosylglycerolipid and docosahexaenoic acid-containing phosphatidylcholine compared to washed capacitated mouse sperm. Biol Reprod 2005; 72:574-83; PMID:15525814; http://dx.doi.org/ 10.1095/biolreprod.104.036095 [DOI] [PubMed] [Google Scholar]
- 61.Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, Tachibana M, Ogura A, Shinkai Y, Nakano T. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 2012; 486:415-9; PMID:22722204 [DOI] [PubMed] [Google Scholar]
- 62.Yu W, McIntosh C, Lister R, Zhu I, Han Y, Ren J, Landsman D, Lee E, Briones V, Terashima M, et al.. Genome-wide DNA methylation patterns in LSH mutant reveals de-repression of repeat elements and redundant epigenetic silencing pathways. Genome Res 2014; 24(10):1613-23; PMID:25170028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1-13; PMID:19033363; http://dx.doi.org/ 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 2010; 28:495-501; PMID:20436461; http://dx.doi.org/ 10.1038/nbt.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren J, Briones V, Barbour S, Yu W, Han Y, Terashima M, Muegge K. The ATP binding site of the chromatin remodeling homolog Lsh is required for nucleosome density and de novo DNA methylation at repeat sequences. Nucleic Acids Res 2015; 43:1444-55; PMID:25578963; http://dx.doi.org/ 10.1093/nar/gku1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly DM, Nettleship JE, Muraleedharan V, Sellers DJ, Brooke JC, McLaren DS, Channer KS, Jones TH. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci 2014; 109:95-103; PMID:24953607; http://dx.doi.org/ 10.1016/j.lfs.2014.06.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.