Abstract
Externally supplied protein (bovine serum albumin, BSA) affects root development of Arabidopsis, increasing root biomass, root hair length, and root thickness. While these changes in root morphology may enhance access to soil microenvironments rich in organic matter, we show here that the presence of protein in the growth medium increases the plant's resilience to the root pathogen Cylindrocladium sp.
Keywords: Arabidopsis, BSA, protein, pathogens, plant resistance
Abbreviations
- N
nitrogen
- ON
organic nitrogen
- IN
inorganic nitrogen
New evidence is emerging that large organic compounds affect root architecture. Organic molecules such as amino acids,1 DNA,2 and proteins3 increase root branching, root hair length, and overall root length, and this has been interpreted as enhancing root foraging in organic-matter rich sites.4 The observed increase in root thickness is a response specific to protein (BSA) in the growth medium3 and we assessed if it affects the plant's interaction with soil-borne fungal pathogen Cylindrocladium sp.
Axenic Arabidopsis plants were grown on 30 mL of N-free Murashige and Skoog (MS5) Basal Salt Solution (M0529, Sigma-Aldrich, Australia) supplemented with 1% sucrose, 3 mM CaCl2, 1.5 mM MgSO4 and 1.25 mM KH2PO4. The growth medium was adjusted to pH 5.5 and 0.3% phytagel (Phytotechnologies, Kansas, USA) was used as solidifying substance. Nitrogen was added as replete inorganic N (IN) as (10 mM N ammonium nitrate, 20 mM N), or low IN (4 mM N ammonium nitrate), or low IN (4 mM N ammonium nitrate) + BSA (16 mM N, to match the N concentration of the replete IN treatment). Inoculated plants were kept in a growth chamber (21°C, 16h/8h day/night, 150 μmol m−2 s−1) and grown in a vertical position for 2 weeks before being inoculated with a Cylindrocladium sp. strain.
Cylindrocladium sp BRIP52551 (Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia) was grown on potato dextrose agar (PDA, Sigma-Aldrich, Australia) plates for 1 week. Spores were prepared by irrigation of plates with distilled water containing 0.01% (v/v) Tween 20 and drained through Miracloth. Spores were collected by centrifugation and resuspended in sterile water containing 0.01% tween 20 to a final concentration of 100 spores mL−1. One mL of spore suspension was placed onto the surface of roots of 2-week-old Arabidopsis plants (see above for growth conditions). Plants were kept in the same growth conditions except that a higher temperature (28°C) was used to assist the growth of fungi. Control plants received distilled water with 0.01% tween 20. Each plate contained 5 seedlings and each treatment consisted of 3 plates. The experiment was repeated 3 times independently.
The specificity of the infection and development of Cylindrocladium sp. were visualized by lactophenol-cotton blue staining as described by Mendez-Moran et al. (2005).6 Cylindrocladium sp hyphae were observed in leaves (Fig. 1A) confirming the disease progression to shoots. Disease symptoms are typical brown-black discoloration of vascular tissues indicating Cylindrocladium sp. infection, which continues via chlorosis and leaf death. Symptoms were scored as levels of infection using a susceptibility index with 0, no evidence of disease; 1, ≤ 10% of leaf area is brown-black; 2, ≤ 20%; 3, ≤ 50%; 4, ≤ 80%; and 5, ≤ 100% of leaf area affected (Fig. 1B). Disease scoring was performed at 5, 7, 9 and 13 days post inoculation (Fig. 1C).
Figure 1.
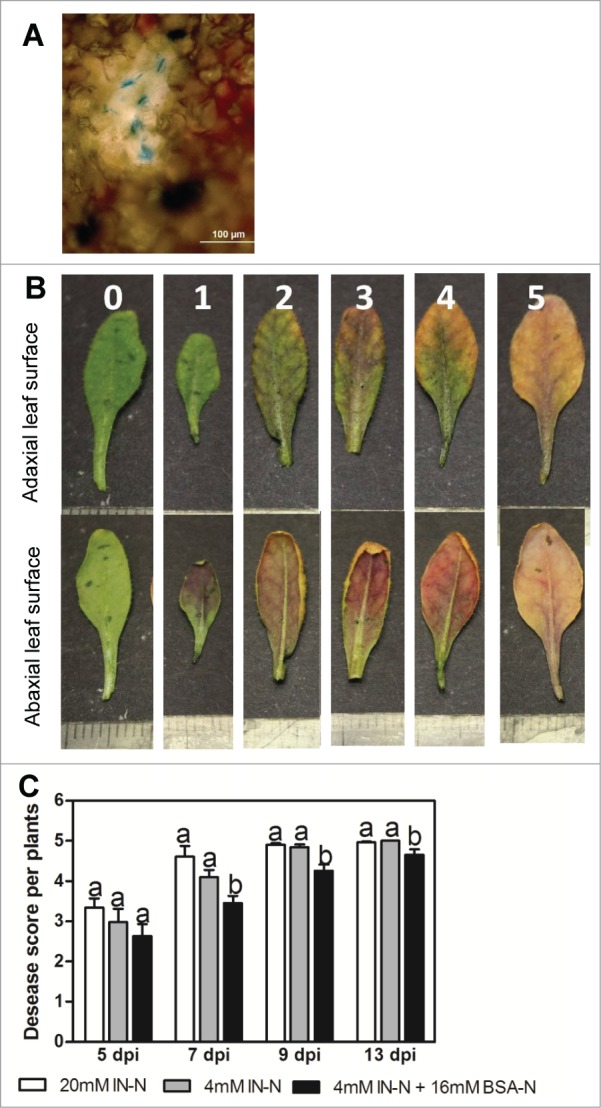
(A) Cotton blue staining of leaves confirming fungal infection (chitin-staining dye). Leaves of Arabidopsis plants after 7 days inoculation with Cylindrocladium sp. were stained and viewed under microscope with hyphae appearing in dark blue. (B) Visual disease scores for leaves of plants infected with root pathogen Cylindrocladium, 0 = no evidence of disease 1 = <10% 2 = ∼20% 3 = ∼50% 4 = ∼80% 5 = 100% of adaxial leaf area affected; scale bar divisions represent 1 mm. (C) Disease score per plant grown with IN (20 or 4 mM N) or IN+BSA (4+16 mM N). Scores were made on 4 to 6 mature leaves per plant and 12 plants, dpi (days post inoculation). Different letters indicate significant differences within each time point (dpi) at P < 0.001 (ANOVA, Neuman–Keuls post hoc test).
The results showed that compared to plants supplied with IN only, plants grown in presence of BSA were significantly more resistant to fungal infection at 7, 9 and 13 dpi. The differences between treatments observed at 5 dpi (Fig. 5C) were not statistically significant. This observation implies that the presence of BSA is associated with the increased resistance, while the amount of N in the growth medium does not affect defense responses against Cylindrocladium. There is evidence that the presence of organic nutrients may reduce soil-borne diseases (reviewed by 7-9). It is suggested that organic amendments have biological or chemical properties affecting disease agents directly or through stimulation of competitor microorganisms, thereby increasing plant survival.10,11 Our finding confirmed that addition of BSA in the medium increased Arabadopsis resistance to Cylindrocladium sp. Since experimental conditions were controlled we can rule out a possibility of suppression of microbial antagonists. The increase of root biomass and root thickness induced by protein (BSA) is presumably a factor that increases the plant's vigour, which can possibly lead to the enhanced resistance to Cylindrocladium sp. observed here. The next steps of this research are to extend the scale to soil and field testing to test the hypothesis that the use of organic compounds has multiple benefits for plant health and associated growth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr Roger Shivas for providing Cylindrocladium sp BRIP52551. Thanks to the ARC Center of Excellence for Integrative Legume Research access to research facilities.
Funding
This research was supported by Australian Research Council Discovery Grant DP0986495.
References
- 1. Cambui CA, Svennerstam H, Gruffman L, Nordin A, Ganeteg U, Näsholm T. Patterns of Plant Biomass Partitioning Depend on Nitrogen Source. PLoS ONE 2011; 6:e19211; PMID:21544211; http://dx.doi.org/ 10.1371/journal.pone.0019211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paungfoo-Lonhienne C, Lonhienne TGA, Mudge SR, Schenk PM, Christie M, Carroll BJ, Schmidt S. DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth. Plant Physiol 2010; 153:799-805; PMID:20388669; http://dx.doi.org/ 10.1104/pp.110.154963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lonhienne T, Trusov Y, Young A, Rentsch D, Näsholm T, Schmidt S, Paungfoo-Lonhienne C. Effects of externally supplied protein on root morphology and biomass allocation in Arabidopsis. Scientific Reports 2014; 4:5055; PMID:24852366; http://dx.doi.org/ 10.1038/srep05055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S Past, present and future of organic nutrients. Plant Soil 2012; 359:1-18; http://dx.doi.org/ 10.1007/s11104-012-1357-6 [DOI] [Google Scholar]
- 5. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 1962; 15:473-497; http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 6. Mendez-Moran L, Reynaga-Pena CG, Springer PS, Ruiz-Herrera J. Ustilago maydis infection of the nonnatural host Arabidopsis thaliana. Phytopathology 2005; 95:480-488; PMID:18943312; http://dx.doi.org/ 10.1094/PHYTO-95-0480 [DOI] [PubMed] [Google Scholar]
- 7. Bailey KL, Lazarovits G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Till Res 2003; 72:169-180; http://dx.doi.org/ 10.1016/S0167-1987(03)00086-2 [DOI] [Google Scholar]
- 8. Hoitink H, Boehm M. Biocontrol within the context of soil microbial communities: a substrate-dependent phenomenon. Annu Rev Phytopathol 1999; 37:427-446; PMID:11701830; http://dx.doi.org/ 10.1146/annurev.phyto.37.1.427 [DOI] [PubMed] [Google Scholar]
- 9. Janvier C, Villeneuve F, Alabouvette C, Edel-Hermann V, Mateille T, Steinberg C.. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol Biochem 2007; 39:1-23; http://dx.doi.org/ 10.1016/j.soilbio.2006.07.001 [DOI] [Google Scholar]
- 10. Ghorbani R, Wilcockson S, Leifert C. Alternative treatments for late blight control in organic potato: Antagonistic micro-organisms and compost extracts for activity against Phytophthora infestans. Potato Res 2005; 48:181-189; http://dx.doi.org/ 10.1007/BF02742375 [DOI] [Google Scholar]
- 11. Zhang W, Han DY, Dick WA, Davis KR, Hoitink HAJ. Compost and compost water extract-induced systemic acquired resistance in cucumber and Arabidopsis. Phytopathology 1998; 88:450-455; PMID:18944926; http://dx.doi.org/ 10.1094/PHYTO.1998.88.5.450 [DOI] [PubMed] [Google Scholar]


