Abstract
The role of ions in the generation and mechanism of propagation of variation potential (VP) has been widely investigated. It is likely that Ca2+ influx via calcium channels is an initial stage of VP; however, development of long-term membrane depolarization requires prolonged open times of calcium channels. We investigated depolarization time in the present study. It was shown that local burning induced VP in wheat seedling and the electrical response was suppressed under EGTA presence. Depolarization formation, which may indicate open time of calcium channels at VP generation, was observed up to 30 s after reaction induction when calcium ions were added to initially calcium-free medium. Long-term calcium channel open time may be the reason for long membrane depolarization at VP and may also be connected with the type of channels participating in wound reaction propagation.
Keywords: calcium channels, microelectrode technique, open time, variation potential, wheat
Abbreviations
- AP
action potential
- APW
artificial pond water
- EGTA
ethylene glycol tetraacetic acid
- Em
transmembrane electrical potential
- VP
variation potential
Introduction
Coordination of plant responses to external wounding is based on activation of a number of signaling systems. The electrophysiological system plays a leading role in the coordination of wound reactions; however, little is known about its functioning. In particular, the process of variation potential generation (VP), defined as slow transitional depolarization of cell membranes extending out to the zone of local damaging irritation1-3, is poorly understood. According to the literature, variation potential is not a self-propagating signal, rather it is a local electrical reaction, induced by signal transmission from the wounded area1. The signal can be either a hydraulic wave or a chemical agent4-7. The ionic nature of changes in membrane potential at VP remains almost unknown. The main hypothesis about the mechanism of the wound-induced electric reaction generation is that it is caused by a temporary inactivation of the plasma membrane's proton pump.6,8-10 Passive fluxes of Ca2+, Cl− and K+ may also play a role in variation potential formation11-13. It is probable that calcium ion influx is a key component in the process of VP generation in cells. Increase in the intracellular concentration of free calcium leads to inactivation of the proton pump and activation of anionic channels.8,11-13 The role of calcium ions as inductors of electric reaction development was also shown for another type of the plant cell electric signal – the action potential (AP).14 In comparison to AP, cell depolarization at VP is much longer, most likely caused by a more lengthy duration of plasma membrane proton pump inactivation. The reason for the long-term inactivation of H+-ATPase may be based on long-term increase in the content of calcium in cytosol that probably is connected with the open times of calcium channels.
Accordingly, the objective of this study was to elucidate the participation of calcium ions’ fluxes in VP initiation and to estimate the open time of calcium channels in the electrical reaction system.
Results and Discussion
Electrical activity was registered in parenchymal cells of wheat leaves after wounding. Leaf tip burning led to generation of variation potential with amplitude of 75 ± 4 mV (Fig. 1). Parameters of VP measured in wheat leaf cells were comparable with VPs registered in other higher plants.2,8,10-11,15-16
Figure 1.
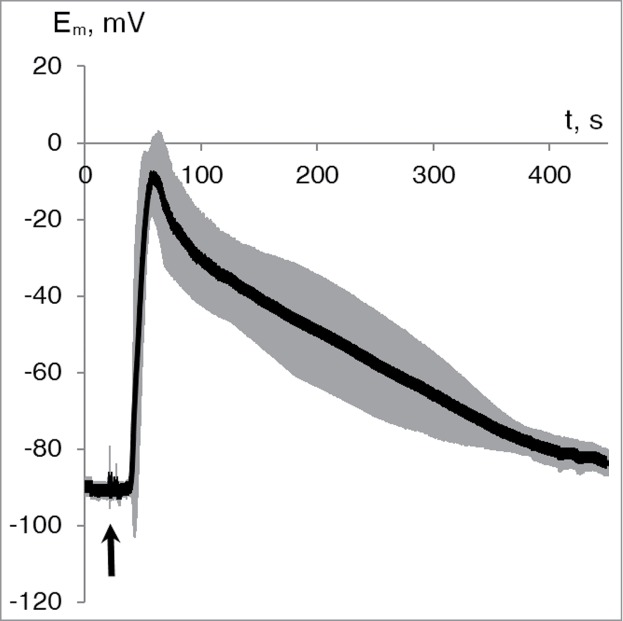
Average dynamics of the variation potential in wheat leaf cells (n = 10). Gray shade indicates standard error of the values. The distance between wounding zone and Em measurement was approximately 5 cm. Initiation of the burn wound is indicated by the black arrow.
In calcium-free medium, VP amplitude decreased considerably, equaling 13 ± 8% of the control value (Fig. 2a). VP generation was also suppressed in the presence of La3+ (Fig. 2b) or Gd3+ (Fig. 2c). Depolarization amplitudes were 4 ± 1% and 14 ± 1% (at La3+ and Gd3+, respectively). These results show that VP generation required Ca2+ influx from external medium and that VP is probably connected with activation of calcium channels.
Figure 2.
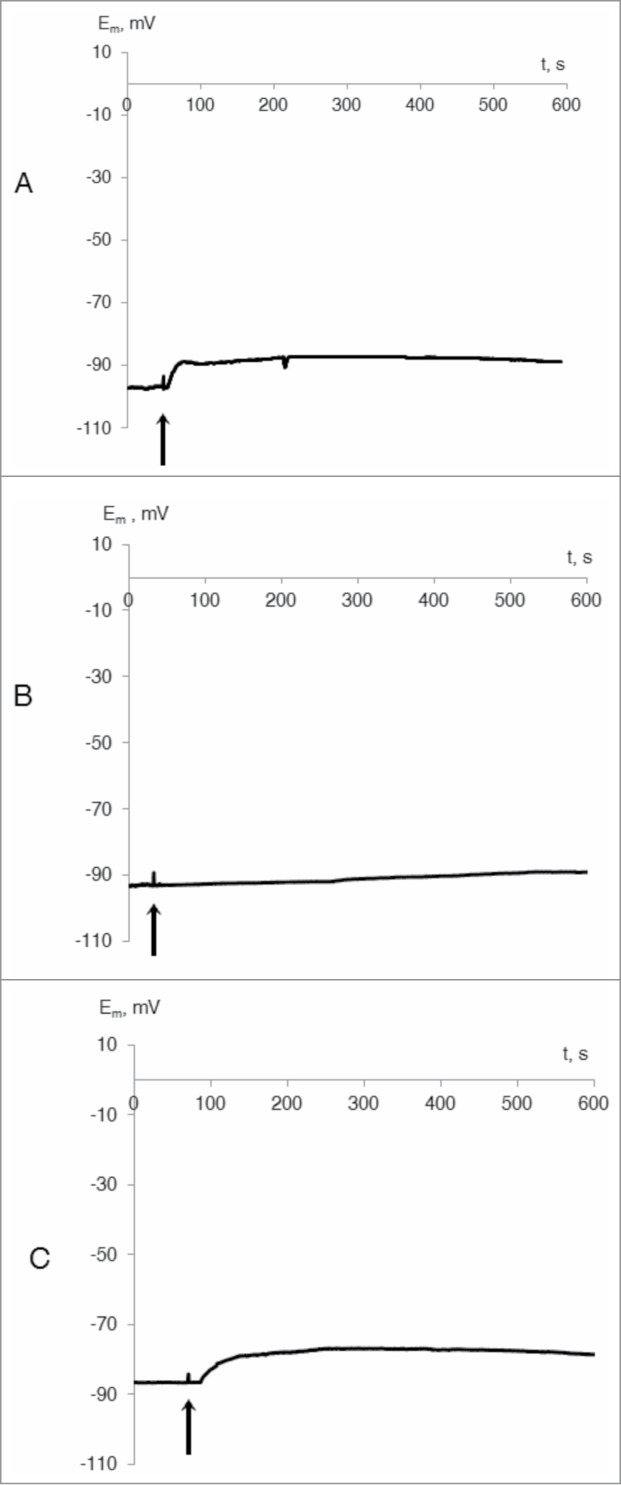
Typical records of changes of membrane potential of wheat leaf cells at the leaf tip burn in presence of EGTA (1 mM) (A) and calcium channel blockers: lanthanum chloride (5 mM) (B) and gadolinium chloride (5 mM) (C) (n = 5–7). The distance between wounding zone and Em registration was approximately 5 cm. Initiation of the burn wound is indicated by the black arrow.
Our results are in agreement with data from the literature that showed that blocking of Ca2+-channels by gadolinium or by lanthanum led to the decrease in amplitude of the electric reaction following wounding in different plants.2,8 Participation of calcium influx in VP generation was also shown in experiments with EGTA, where incubation of plant seedlings in the presence of calcium chelator led to a decrease in the amplitude of VP induced by a burn.8,11-12 Replacement of calcium ions with magnesium ions in the solution surrounding tomato stems caused reduction in the quantity of fast electrical reaction impulses following a burn wound.17
We conclude that calcium influx from extracellular spaces is necessary for VP development. Calcium ions serve as the inductor of VP formation, activating anionic channels and inactivating proton pumps.8,13 A similar role for calcium has been shown for the formation of action potential, another type of electrical reaction. The long-term duration of membrane depolarization at VP in comparison with AP is connected to the long inactivation of H+ -ATPase, which can be caused by elongated prolonged entrance of calcium ions into cells, i.e., long open time of calcium channels.
The classical way to analyze characteristics of ionic channels is with a patch-clamp method.18 However, use of whole plants as the objects of the research has a number of methodological difficulties, including the presence of cell walls, small size cells and their integration in the symplast. In this regard, the indirect way of an assessment of the open time of calcium channels was offered. Electric reaction was induced by a leaf burn in the calcium-free medium and then Ca2+ was added to the solution at various time intervals after the irritation.
In the absence of the burn wound stimulus, increasing at calcium concentration increasing in the washing solution to standard values (10−3 M) membrane depolarization was not observed; on the contrary, there was a slight hyperpolarization (8 ± 4 mV) (Fig. 3).
Figure 3.
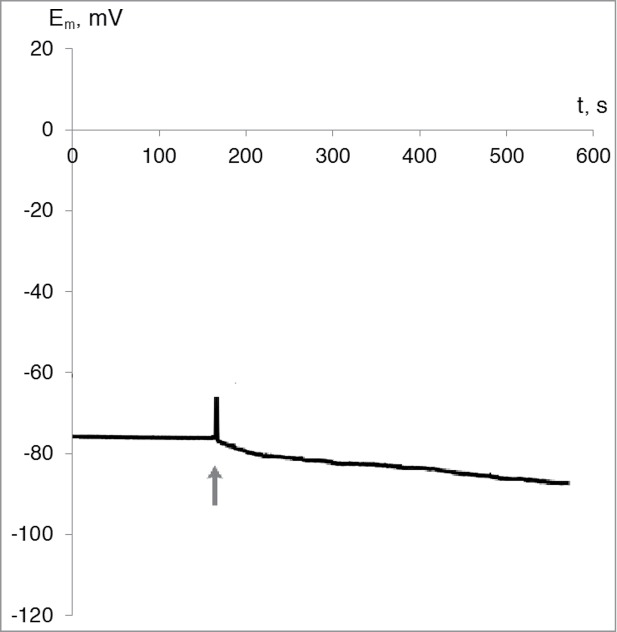
Typical record of changes of membrane potential of wheat leaf cells upon addition of calcium ions to the initially calcium-free medium. Final concentration of the calcium ions in the washing solution was 10−3 M (n = 4). The moment of calcium ions addition is indicated by the gray arrow.
Calcium ions were added to the medium after 4 different time intervals after VP induction and led to the development of membrane depolarization (Fig. 4). This effect was observed with addition of calcium ions at 10, 20, and 30 s after wounding. At 40 s, calcium addition to the medium did not lead to noticeable changes in electric activity of cells. It should also be noted that VP amplitude depended on the time between reaction induction and calcium addition, i.e., increased time interval led to decreased VP amplitude.
Figure 4.
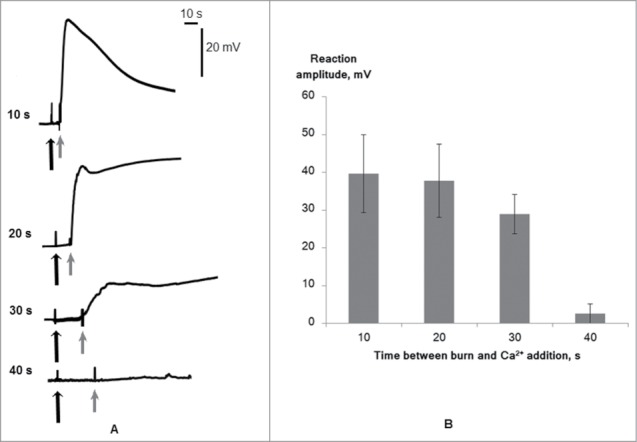
Changes of membrane potential of wheat leaf cells induced by leaf tip burn in the calcium-free medium followed by addition of calcium ions in the washing solution on different time intervals after wounding. (A) Typical records. (B) Average magnitudes (n = 4–6). The distance between the wounding zone and Em measurement was approximately 5 cm. Initiation of the wound is indicated by the black arrow. The moment of addition of calcium ions is indicated by the gray arrow.
These results indicate long-term (at least 30 s) influx of calcium ions to the cytoplasm at VP. The long-term influx can be caused by large open time of calcium channels participating in VP generation. However, we cannot exclude an alternative explanation for our results, in which long-term Ca2+ influx reflects prolonged action of VP-induced signals and repeated activation of calcium channels. This signal could be a hydraulic wave or caused by chemical agent propagation.4-7 If the signal is hydraulic, long-term Ca2+ influx could be caused by prolonged mechanical deformation, which activates mechanosensitive channels. If it has a chemical basis, the long-term influx reflects prolonged increase of the chemical agent concentration, which can activate ligand-dependent channels.
Conclusion
We conclude from our results that there is evidence showing that the prolonged depolarization of a membrane at VP, in contrast to a membrane at AP, is caused by the long open time of calcium channels and, as a result, concomitant increase in cytosol calcium concentration. The duration (about 30 s) of calcium channels being open at VP can be connected with participation of ligand-activated or mechanosensitive ionic channels in the reaction development. This is in contrast to fast potential-dependent calcium channels, in which activation takes place with AP formation.
Materials and Methods
Seedlings of wheat (Triticum aestivum L.) were cultivated hydroponically in a Binder KBW 240 (Binder GmbH, Tuttlingen, Germany) plant growth chamber at 24°C under a 16/8 h (light/dark) photoperiod. Seedlings used in experiments were 14–21 d-old with a second leaf length of 15–20 cm.
The microelectrode technique was used for intracellular membrane potential (Em) measurements (microscope Olympus BX51); automatic manipulators Luigs and Neumann; amplifier Multiclamp 700B (Axon Inst., Molecular Devices, Sunnyvale, CA, USA); PC).12 Data acquisition speed was 3 points/s and low pass filter frequency was 10 Hz. The plant was placed beside the microscope and the leaf was fixed horizontally. Part of the leaf was placed in a cuvette filled with artificial pond water (APW), containing 0.1 mМ NaCl, 1 mМ KCl, and 0.5 mМ CaCl2 in distilled water (pH = 6–7). In separate experiments, APW in the cuvette was changed to solutions with calcium chelator or calcium channel blockers as described below.
Micropipettes were fabricated from 1.2 mm (O.D.) borosilicate glass tubing (Sutter Instruments, Novato, CA, USA). Capillaries were pulled in a horizontal pipette puller and filled with 100 mM KCl. The tip diameter was approximately 0.5 μm. The electrode was moved by motorized manipulators, with its location controlled visually on the microscope. Electrical measurements were carried out in parenchymal cells in the 2nd–3rd layer below the epidermis. The reference electrode was immersed into cuvette with APW. The VP was induced by open-flame burning of the leaf tip (apical 2–3 mm) for 2 s.7 Distance between the registration zone and wounded area was 5 cm.12
Measurements of VP parameter changes after exposure to calcium chelator (EGTA) (1 mM) and calcium channel blockers lanthanum chloride (5 mM) and gadolinium chloride (5 mM) were used for estimation of calcium ion participation in VP generation.8,11,12 Plants were incubated in the presence of the inhibitor for at least 1 h prior to wounding.
For estimation of calcium channel open time at VP generation, the seedling was incubated in the presence of calcium chelator (1 mM) for 1 h. VP was induced in the calcium-free media and then at different time intervals—10, 20, 30 and 40 s—after wounding, calcium ions (CaCl2, 10−3 M) were added to the cuvette.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Russian Scientific Fund (Project No. 14-26-00098).
References
- 1. Stankovik B, Zawadzki T, Davies E. Characterization of the Variation Potential in Sunflower. Plant Physiology 1997; 115:1083-88; PMID:12223859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zimmermann MR, Felle HH. Dissection of heat-induced systemic signals: superiority of ion fluxes to voltage changes in substomatal cavities. Planta 2009; 229:539-47; PMID:19011895; http://dx.doi.org/ 10.1007/s00425-008-0850-x [DOI] [PubMed] [Google Scholar]
- 3. Gallé A, Lautner S, Flexas J, Fromm J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environmental and Experimental Botany 2014; http://dx.doi.org/ 10.1016/j.envexpbot.2014.06.013 [DOI] [Google Scholar]
- 4. Rhodes JD, Thain JF, Wildon DC. Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Annals of Botany 1990; 84: 109-16; http://www.sciencedirect.com/science/article/pii/S0098847214001634 [Google Scholar]
- 5. Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust J Plant Physiol 1999; 26: 55-61; http://dx.doi.org/ 10.1071/PP98098 [DOI] [Google Scholar]
- 6. Stahlberg R, Cleland RE, Volkenburgh E. Slow wave potentials — a propagating electrical signal unique to higher plants. In: Communication in Plants. Baluška F., Mancuso S. and Volkmann D. (ed.) Springer Berlin Heidelberg; 2006; 291-308. [Google Scholar]
- 7. Vodeneev V, Orlova A, Morozova E, Orlova L, Akinchits E, Orlova O, Sukhov V. The mechanism of propagation of variation potentials in wheat leaves. J Plant Physiol 2012; 169: 949-54; PMID:22533926; http://dx.doi.org/ 10.1016/j.jplph.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 8. Julien JL, Desbiez MO, De Jaegher G, Frachisse JM. Characteristics of the wave of depolarization induced by wounding in Bidens pilosa L. J Exp Bot 1991; 42: 131-7. [Google Scholar]
- 9. Zimmermann M, Mithöfer A. Electrical long-distance signaling in plants. In: Long-Distance Systemic Signaling and Communication in Baluška Plants. F. (ed.) Springer Berlin Heidelberg; 2013; 291-308. [Google Scholar]
- 10. Sukhov V, Sherstneva O, Surova L, Katicheva L, Vodeneev V. Proton cellular influx as a probable mechanism of variation potential influence on photosynthesis in pea. Plant Cell Envir 2014; 37(11), 2532-41; PMID:24635649 [DOI] [PubMed] [Google Scholar]
- 11. Vodeneev VA, Akinchits EK, Orlova LA, Sukhov VS. The role of Ca2+, H+, and Cl– ions in generation of variation potential in pumpkin plants. Russ J Plant Physiol 2011; 58: 974-81; http://dx.doi.org/ 10.1134/S1021443711050256 [DOI] [Google Scholar]
- 12. Katicheva L, Sukhov V, Akinchits E, Vodeneev V. Ionic nature of burn-induced variation potential in wheat leaves. Plant Cell Physiol. 2014; 55(8): 1511-9; PMID:24928219; http://dx.doi.org/ 10.1093/pcp/pcu082 [DOI] [PubMed] [Google Scholar]
- 13. Sukhov V, Akinchits E, Katicheva L, Vodeneev V. Simulation of Variation Potential in Higher Plant Cells. J Membrane Biol 2013; 246: 287-96; PMID:23417063 [DOI] [PubMed] [Google Scholar]
- 14. Felle H, Zimmermann M. Systemic signaling in barley through action potentials. Planta 2007; 226: 203-14; PMID:17226028; http://dx.doi.org/ 10.1007/s00425-006-0458-y [DOI] [PubMed] [Google Scholar]
- 15. Malone M, StankoviĆ B. Surface potentials and hydraulic signals in wheat leaves following localized wounding by heat. Plant Cell Environ. 1991; 14: 431-6; http://dx.doi.org/ 10.1111/j.1365-3040.1991.tb00953.x [DOI] [Google Scholar]
- 16. Stahlberg R, Cleland RE, Van Volkenburgh E. Decrement and amplification of slow wave potentials during their propagation in Helianthus annuus L. shoots. Planta 2005; 220: 550-8; PMID:15365838 [DOI] [PubMed] [Google Scholar]
- 17. Rousset M, De Roo M, Le Guennec JY, Pichon O. Electrophysiological characterization of tomato hypocotyl putative action potentials induced by cotyledon heating. Physiol. Plant. 2002; 115: 197-203; PMID:12060236; http://dx.doi.org/ 10.1034/j.1399-3054.2002.1150204.x [DOI] [PubMed] [Google Scholar]
- 18. Elzenga J. Theo M. Patch clamp techniques for plant cells. In: Plant Electrophysiology: Methods and Cell Electrophysiology. Volkov Alexander G. (ed.) Springer Berlin Heidelberg; 2012; 225-43 [Google Scholar]


