Abstract
Background
A raised carcinoembryonic antigen (CEA) may be associated with significant pathology during the postoperative follow-up of lung adenocarcinoma.
Methods
We reviewed the medical records of 305 patients who underwent surgical resections for primary lung adenocarcinoma at a single institution between April 2006 and February 2013.
Results
Preoperative CEA levels were significantly associated with age, smoking history, pathologic stage including pT (pathologic tumor stge), pN (pathologic nodal stage) and overall pathological stage, tumor size and differentiation, pathologically positive total lymph node, N1 and N2 lymph node, N2 nodal station (0/1/2=1.83/2.94/7.21 ng/mL, p=0.019), and 5-year disease-free survival (0.591 in group with normal preoperative CEA levels vs. 0.40 in group with high preoperative CEA levels, p=0.001). Preoperative CEA levels were significantly higher than postoperative CEA levels (p<0.001, Wilcoxon signed-rank test). Postoperative CEA level was also significantly associated with disease-free survival (p<0.001). A follow-up serum CEA value of >2.57 ng/mL was found to be the appropriate cutoff value for the prediction of cancer recurrence with sensitivity and specificity of 71.4% and 72.3%, respectively. Twenty percent of patients who had recurrence of disease had a CEA level elevated above this cutoff value prior to radiographic evidence of recurrence. Postoperative CEA, pathologic stage, differentiation, vascular invasion, and neoadjuvant therapy were identified as independent predictors of 5-year disease-free survival in a multivariate analysis.
Conclusion
The follow-up CEA level can be a useful tool for detecting early recurrence undetected by postoperative imaging studies. The perioperative follow-up CEA levels may be helpful for providing personalized evaluation of lung adenocarcinoma.
Keywords: Lung adenocarcinoma, Carcinoembryonic antigen, Prognosis
INTRODUCTION
Lung cancer is one of the leading causes of death, and adenocarcinoma has been reported to be the most common subtype of lung cancer [1]. Despite having the same cell type and pathologic stages, the clinical prognosis after surgical resection for non-small cell lung cancer varies for each individual [1,2]. Therefore, personalized evaluation and management for non-small cell lung cancer is essential, and information on other factors as well as the pathologic stage is necessary to evaluate the current status and to predict the accurate prognosis [3–5]. One such factor can be serum carcinoembryonic antigen (CEA) [2,6]. The serum CEA level has been widely used as a tumor marker and as a predictor of disease progression or recurrence in various types of cancer, particularly in colorectal cancer [7,8]. Further, some studies have proposed the possibility of the CEA level as a tumor marker or as a predictor after surgical resection for early non-small cell lung cancer [2,9]. However, the National Comprehensive Cancer Network (NCCN) guidelines for non-small cell lung cancer do not include serum CEA for the evaluation and management of non-small cell lung cancer. When the preoperative or follow-up CEA level exceeds the normal range, the evaluation and management of these patients tend to be same as those of patients with a normal CEA level. The purpose of the present study is to clarify the significance of the perioperative serum CEA level in predicting the clinical course (i.e., recurrence or metastasis) of a patient with lung adenocarcinoma. We retrospectively compiled the preoperative and follow-up serum CEA levels in all cases of surgical pulmonary resection. These CEA levels were analyzed along with the resulting pathologies and the subsequent clinical courses.
METHODS
1) Materials and methods
We reviewed the medical records of 305 patients who underwent surgical resection for primary adenocarcinoma lung cancer at Seoul Saint Mary’s Hospital between April 2006 and February 2013. Because there was no definite policy or algorithm for the measurement of the serum CEA level, some of the patients did not have a record of their preoperative or follow-up CEA level. The remainder, 158 of 305 patients, had records of both preoperative and follow-up CEA. The inclusion criteria were primary lung adenocarcinoma, pre-operative and follow-up serum CEA levels, and curative and complete resection including intended limited resection. The exclusion criteria were secondary primary lung cancer cases, salvage or palliative cases, and epidermal growth factor receptor tyrosine inhibitor (EGFR TKI) intake for adjuvant therapy cases. Intended limited resection (wedge resection or segmentectomy) was performed in high-risk patients at a clinically early stage. Preoperative evaluations for staging were routinely performed with chest X-ray, chest computed tomography (CT), and positron emission tomography (PET), brain magnetic resonance imaging (MRI), or bone scan (as needed). The clinical and pathologic stages were determined according to the American Joint Committee on Cancer tumor-node-metastasis staging method (7th edition). The postoperative follow-up consisted of chest X-ray every 3 months, chest CT, PET-CT, and brain MRI (as needed) every 6 months for 5 years post-surgery. The preoperative and follow-up CEA levels were measured using the chemiluminescent enzyme immunoassay technique. All laboratory tests were carried out by standard methods, using an auto-analyzer (Hitachi 7600-210; Hitachi, Tokyo, Japan) and commercially available assay kits (Sekisui Medical Co. Ltd., Tokyo, Japan). The normal reference value used for our laboratory was a CEA level of 3 ng/mL. A serum CEA level of less than 3 ng/mL was defined as normal, and follow-up CEA levels were taken every 3 or 6 months. Because there was no definite policy or algorithm for the measurement of the CEA level, we defined each CEA level strictly according to time. The serum CEA level within 1 month before surgery was defined as ‘preoperative CEA’ and the serum CEA level within 5 months after surgery was defined as ‘postoperative CEA.’ The serum CEA level within 6 months before recurrence was defined as ‘CEA before recurrence.’ The serum CEA level at the last follow-up was defined as ‘last CEA.’ If there were more than two CEA levels in the same period, we chose the higher level. Neoadjuvant or adjuvant therapy was performed by following the NCCN guidelines with consideration of the patient condition. The clinicopathologic data including imaging studies, serum CEA level, surgical procedure, pathology and mutation studies (EGFR and k-ras), and clinical prognosis were analyzed. Since most patients were in the early lung cancer stage, the number of cancer-related deaths was small, and various factors after recurrence could influence the overall survival, we carried out a 5-year disease-free survival (DFS, also referred to as recurrence-free survival) study instead of an overall survival study by using recurrence as a primary endpoint to clarify the significance of the serum CEA level. Any recurrence or death due to any cause was included in the recurrence-free survival. Note that DFS is interchangeable with recurrence-free survival under this study design of following up on post operative patients with lung cancer. The parameters associated with DFS included age; gender; mutation study (EGFR and k-ras mutation); extent of resection; CEA levels; pathologic stage; differentiation; pleural, vascular, and lymphatic invasion; and neoadjuvant or adjuvant therapy. The approval from the institutional review board of Seoul St. Mary’s Hospital was obtained for the present study (IRB approval number: KC13RISI0290).
2) Statistical analysis
Since our data did not have a normal distribution, all data were analyzed using nonparametric statistical methods. The comparisons among subgroups were evaluated using the Mann-Whitney U test, Wilcoxon’s signed-rank test, or the Jonckheere-Terpstra test. Association studies were evaluated using Spearman’s rho test. DFS rates were calculated using the Kaplan-Meier method, and comparisons of survival curves were carried out by using the log-rank test. To determine the independent prognostic factors of survival, a multivariate analysis was conducted using the Cox proportional hazards model (backward stepwise method). Recurrence or metastasis was defined as an event. The results were analyzed using the PASW SPSS computer software program ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
RESULTS
1) Clinicopathologic characteristics
There were 158 patients (81 male and 77 female) who underwent complete resections or intended limited resections; their median age was 64.5 years (range, 33 to 85 years). Among these 158 patients, 6 (3.8%) patients received neo-adjuvant therapy and 36 patients (22.8%) received adjuvant therapy. All patients were pathologically diagnosed with adenocarcinoma or adenocarcinoma combined with another type of cancer, such as adenosquamous carcinoma and mucinous adenocarcinoma. The differentiation grades varied as follows: well, 81 (51.3%); moderate, 64 (40.5%); and poor, 13 (8.2%). The pathologic stages were as follows: IA, 94 (59.5%); IB, 35 (22.1%); IIA, 12 (7.6%); IIB, 3 (1.9%); and IIIA, 14 (8.9%). The overall clinicopathologic characteristics of these patients are summarized in Table 1.
Table 1.
Overall clinicopathologic characteristics of the patients (N=158)
| Characteristic | Value |
|---|---|
| Age (yr) | 64.5 |
| Gender | |
| Male | 81 (51.3) |
| Female | 77 (48.7) |
| Combined other cancers | |
| No | 128 (81.0) |
| Yes | 30 (19.0) |
| Pathologic stage | |
| IA | 94 (59.5) |
| IB | 35 (22.2) |
| IIA | 12 (7.6) |
| IIB | 3 (1.9) |
| IIIA | 14 (8.9) |
| Extent of resection | |
| Wedge resection | 19 (12.0) |
| Segmentectomy | 1 (0.6) |
| Lobectomy | 135 (85.4) |
| Bilobectomy | 3 (1.9) |
| Preoperative serum carcinoembryonic antigen level (ng/mL) | 1.88 |
| Normal | 113 (71.5) |
| High | 45 (28.5) |
| Differentiation | |
| Good | 81 (51.3) |
| Moderate | 64 (40.5) |
| Poor | 13 (8.2) |
| Status of follow-up | |
| Dead | 16 (10.1) |
| Alive or follow-up loss | 142 (89.9) |
| Recurrence | |
| No or follow-up loss | 103 (65.2) |
| Yes | 55 (34.8) |
| Epidermal growth factor receptor mutation | |
| No | 37 (23.4) |
| Yes | 41 (25.9) |
| Not measured | 80 (50.6) |
| k-ras mutation | |
| No | 64 (40.5) |
| Yes | 15 (9.5) |
| Not measured | 79 (50.0) |
| Neoadjuvant therapy | |
| No | 152 (96.2) |
| Yes | 6 (3.8) |
| Adjuvant therapy | |
| No | 122 (77.2) |
| Yes | 36 (22.8) |
Values are presented as number of patients (%) or median.
2) Preoperative carcinoembryonic antigen levels
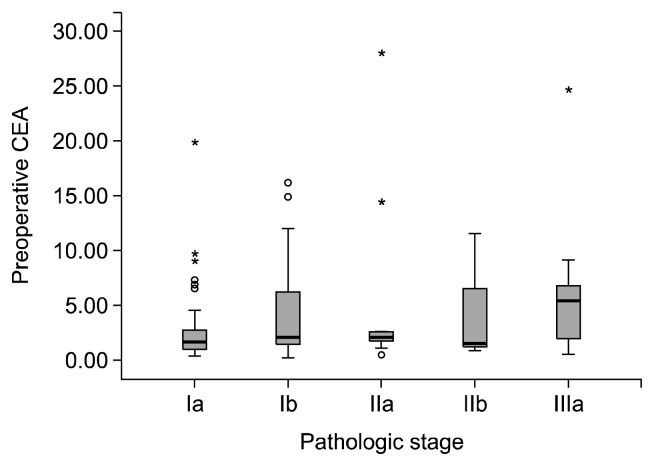
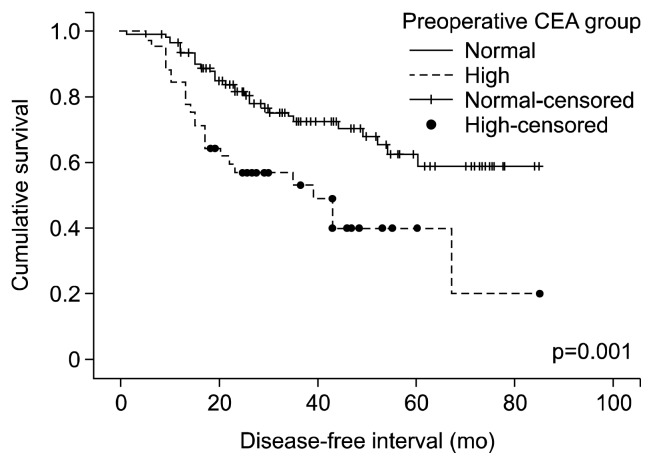
The incidence of patients with high preoperative CEA levels was 28.5%. Along with the low incidence of a high pre-operative CEA level, we found that the preoperative CEA levels were significantly associated with age; smoking history; pathologic stage including pathologic tumor (pT), pathologic nodal (pN) stage, and overall pathologic stage (IA, IB, IIA, IIB, and IIIA); tumor size; tumor grading (differentiation of cancer cells); number of pathologically positive total lymph nodes, N1 lymph nodes, N2 lymph nodes, and N2 nodal stations; and DFS rate. Preoperative CEA levels of smokers were higher than those of non-smokers (1.44 vs. 2.40 ng/mL; p=0.010; Mann-Whitney U test). Preoperative CEA levels were positively associated with age (p=0.009, Spearman’s rho test), pT stage, pN stage, and the overall pathologic stage (p<0.001, p=0.023, and p=0.001, respectively; Jonckheere-Terpstra test) (Fig. 1), tumor size (p=0.001, Spearman’s rho test), pathologically positive total lymph nodes (p=0.007, Spearman’s rho test), pathologically positive N1 and N2 lymph nodes (p=0.040 and p=0.017, respectively; Spearman’s rho test), pathologically positive N2 lymph nodal stations (p=0.019, Jonckheere–Terpstra test), and poorer differentiation well/moderate/poor=1.59/2.20/2.26 ng/mL; p=0.003; Jonckheere–Terpstra test). The DFS of patients with a normal pre-operative CEA level was significantly higher than that of patients with a high preoperative CEA level (0.591 vs. 0.40; p=0.001; log-rank test) (Fig. 2). The results of a univariate analysis of preoperative CEA are summarized in Table 2.
Fig. 1.
Preoperative serum carcinoembryonic antigen (CEA) levels according to the pathologic stage. Preoperative CEA levels were positively associated with the overall pathologic stage (p=0.001). In addition, there were more patients in the high preoperative CEA group than in the normal preoperative CEA group at the relatively high pathologic stages (p=0.017, Fisher’s exact test).
Fig. 2.
Disease-free survival (i.e., recurrence-free survival) according to preoperative carcinoembryonic antigen (CEA) levels. DFS of the normal preoperative CEA group is significantly higher than that of the high preoperative CEA group (p=0.001, log-rank test).
Table 2.
Results of a univariate analysis of preoperative CEA levels
| Variable | Relationship | p-value |
|---|---|---|
| Age | Positive correlation | 0.009 |
| Gender | Not associated | 0.684 |
| Combined with another cancer | Not associated | 0.597 |
| Smoking | Preoperative CEA levels of smokers were higher than those of non-smokers. | 0.010 |
| Overall pathologic stage | Positive correlation | 0.001 |
| Pathologic tumor stage | Positive correlation | <0.001 |
| Tumor size | Positive correlation | 0.001 |
| Pathologic nodal stage | Positive correlation | 0.023 |
| Pathologically positive LN | Positive correlation | 0.007 |
| Pathologically positive N1 LN | Positive correlation | 0.040 |
| Pathologically positive N2 LN | Positive correlation | 0.017 |
| Pathologically positive N2 nodal station | Positive correlation | 0.019 |
| Differentiation | Negative correlation | 0.003 |
| Pleural invasion | Not associated | 0.148 |
| Vascular invasion | Not associated | 0.061 |
| Lymphatic invasion | Not associated | 0.148 |
| Epidermal growth factor receptor mutation | Not associated | 0.988 |
| k-ras mutation | Not associated | 0.375 |
| Disease-free interval | Disease-free interval of the normal preoperative CEA group was longer than that of the high preoperative CEA group | 0.001 |
CEA, serum carcinoembryonic antigen; LN, lymph node.
3) Postoperative carcinoembryonic antigen levels
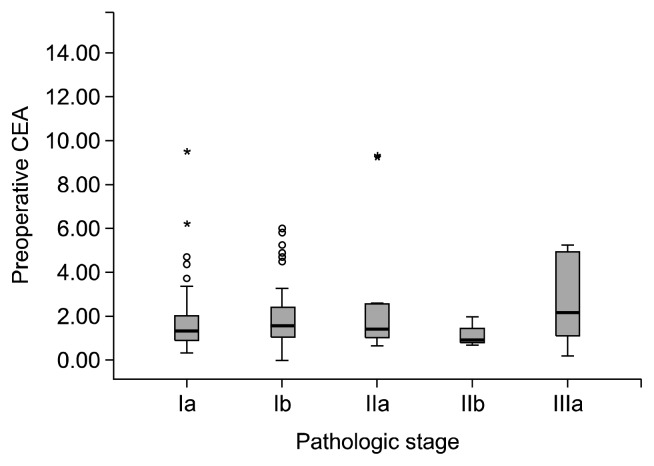
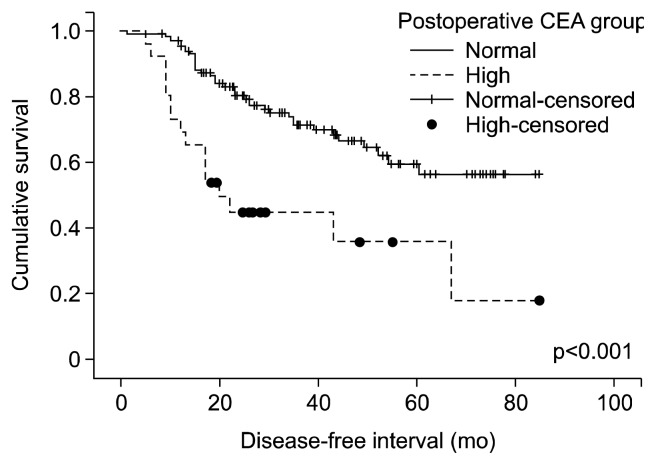
The incidence of patients with a high postoperative CEA level was 16.5%. There was no significant relationship between postoperative CEA levels and the overall pathologic stage (p=0.062, Jonckheere-Terpstra test) (Fig. 3). However, the highest postoperative CEA level had a positive association with the pathologic stage (p=0.008, Jonckheere-Terpstra test). Postoperative CEA levels in patients with recurrent disease were significantly higher than the follow-up CEA levels in disease-free patients (p=0.002, Mann–Whitney U test). Further, the patient group with a normal postoperative CEA level had a longer disease-free interval than the patient group with a high postoperative CEA level (p<0.001, log-rank test) (Fig. 4).
Fig. 3.
Postoperative carcinoembryonic antigen (CEA) levels according to pathologic stage. There was no significant relationship between postoperative CEA levels and the overall pathologic stage (p=0.062). In addition, there was no relationship between the postoperative CEA groups (normal and high) and the overall pathologic stage.
Fig. 4.
DFS according to postoperative carcinoembryonic antigen (CEA) levels. The disease-free interval in the patient group with a normal postoperative CEA level is significantly longer than that in the patient group with a high postoperative CEA level (p<0.001, log-rank test).
4) Relationship between preoperative and postoperative carcinoembryonic antigen levels
Preoperative CEA levels were positively associated with postoperative CEA levels (correlation coefficient=0.789; p< 0.001; Spearman’s rho test). Preoperative CEA levels were significantly higher than postoperative CEA levels (1.88 vs. 1.46 ng/mL; p<0.001; Wilcoxon’s signed-rank test). However, the difference between the preoperative and the postoperative CEA levels was not associated with the disease-free interval.
5) Receiver operating characteristic analysis of follow-up carcinoembryonic antigen levels
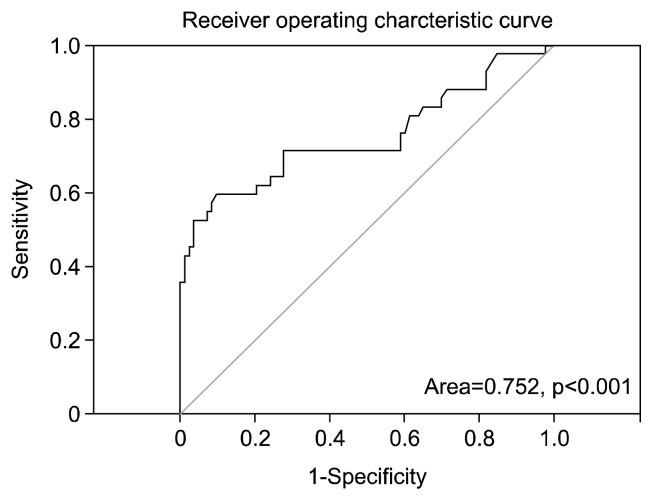
The cutoff CEA value from the Receiver operating characteristic (ROC) curve analysis of serum CEA to predict cancer recurrence is shown in Fig. 5. For the purpose of screening, a follow-up serum CEA value of >2.57 ng/mL was found to be the appropriate cutoff value for the prediction of cancer recurrence with a sensitivity of 71.4% and a specificity of 72.3%. We determined the cutoff value that provided the likelihood ratios and the sensitivity and specificity values that had the greatest clinical value in the screening of lung cancer recurrence, and we chose a higher sensitivity at the cost of lower specificity. The area under the ROC curve was 0.752 (95% confidence interval [CI], 0.650–0.854; p<0.001), which indicated the potential predictive value of cancer recurrence.
Fig. 5.
Cutoff carcinoembryonic antigen (CEA) values to predict cancer recurrence or metastasis. A follow-up serum CEA value of >2.57 ng/mL seems to be the appropriate cutoff level for the prediction of cancer recurrence or metastasis with a sensitivity of 71.4% and a specificity of 72.3%.
6) Follow-up carcinoembryonic antigen levels in groups with high or normal preoperative carcinoembryonic antigen levels
The group with high preoperative CEA levels had significantly higher follow-up CEA levels than the other group with normal preoperative CEA levels in both recurrent and disease-free cases (p<0.001; p=0.001; Mann-Whitney U test). In addition, the ROC curve analysis of the serum CEA level in the group with high preoperative CEA levels showed the appropriate cutoff value (>4.18 ng/mL) for the prediction of cancer recurrence or metastasis with a sensitivity of 85.0% and a specificity of 83.3% with an area of 0.901 (95% CI, 0.806–0.997), which suggests that follow-up postoperative CEA levels can be used as a potential predictive value for cancer recurrence or metastasis even in patients with high preoperative CEA levels.
7) Follow-up carcinoembryonic antigen levels in patients with or without recurrent disease
Patients with recurrent disease had significantly higher follow-up CEA levels at the time of recurrence than the disease-free patients (p<0.001, Mann-Whitney U test). Further, the follow-up CEA levels at recurrence were significantly higher than the follow-up CEA levels before recurrence in the same patient (p<0.001, Wilcoxon’s signed-rank test). We found that 20% of the patients who had disease recurrence had an elevated CEA level above the suggested cutoff value (>2.57 ng/mL) prior to the radiographic evidence of recurrence.
8) Univariate and multivariate prognostic analyses for 5-year disease-free survival
The mean follow-up period of the present study was 35.5 months (range, 5 to 88 months), and the overall 5-year DFS rate for the cases was 52.8%. Radiographic evaluations revealed that there was recurrence or metastasis in 55 patients (34.8%) during the follow-up and that 16 patients (10.1%) died of cancer-related causes. The 5-year DFS rate in the univariate analysis was associated with pre- and postoperative CEA levels; pathologic stage; differentiation; pleural, vascular, and lymphatic invasion; and neoadjuvant and adjuvant therapy. However, age, gender, EGFR and k-ras mutation, and extent of resection were not associated with 5-year DFS after surgical resection (since we began measuring EGFR and k-ras mutation after 2010, the effects of EGFR and k-ras mutation on 5-year DFS could not be analyzed completely because of the short duration of observation). There was no DFS difference between wedge resection and anatomic resection, nor limited resection (wedge and segmentectomy) and more extended resections. In contrast to our expectations, neoadjuvant and adjuvant therapy had negative prognostic effects on the 5-year DFS rate. The results of a univariate analysis based on the 5-year DFS rate are shown in Table 3. All variables found to be significant in the univariate analysis of the 5-year DFS were included as covariates for the multivariate analysis, and accordingly, postoperative CEA levels, pathologic stage, differentiation, vascular invasion, and neo-adjuvant therapy were identified as independent predictors of the 5-year DFS in the multivariate analysis. The results of the multivariate analysis based on the 5-year DFS rate are shown in Table 4.
Table 3.
Univariate analysis for 5-year DFS
| Variable | 5-year DFS (%) | p-value |
|---|---|---|
| Age (yr) | 0.380 | |
| ≤65 | 54.7 | |
| >65 | 55.5 | |
| Gender | 0.237 | |
| Male | 55.1 | |
| Female | 55.8 | |
| Preoperative CEA level (ng/mL) | 0.001 | |
| ≤3.0 | 62.6 | |
| >3.0 | 40.2 | |
| Postoperative CEA level (ng/mL) | <0.001 | |
| ≤3.0 | 59.6 | |
| >3.0 | 35.9 | |
| Pathologic stage | <0.001 | |
| IA | 66.0 | |
| IB | 57.6 | |
| IIA | 33.8 | |
| IIB | 0.00 | |
| IIIA | 17.9 | |
| Pleural invasion | 0.003 | |
| No | 60.8 | |
| Yes | 31.3 | |
| Vascular invasion | 0.001 | |
| No | 57.7 | |
| Yes | 40.4 | |
| Lymphatic invasion | <0.001 | |
| No | 62.1 | |
| Yes | 25.5 | |
| Epidermal growth factor receptor mutation (4-year DFS) | 0.117 | |
| No | 24.5 | |
| Yes | 53.4 | |
| k-ras mutation (5-year DFS) | 0.182 | |
| No | 19.7 | |
| Yes | 34.2 | |
| Differentiation | <0.001 | |
| Good | 70.0 | |
| Other (moderate and poor) | 34.6 | |
| Extent of resection | 0.085 | |
| Wedge resection | 41.2 | |
| Segmentectomy or greater resection | 53.7 | |
| Neoadjuvant therapy | <0.001 | |
| No | 57.9 | |
| Yes | 0.00 | |
| Adjuvant therapy | <0.001 | |
| No | 66.1 | |
| Yes | 0.00 |
DFS, disease-free survival; CEA, serum carcinoembryonic antigen.
Table 4.
Multivariate analyses for 5-year DFS
| Variable | Hazard ratio | p-value |
|---|---|---|
| Pathologic stage | ||
| IA | 0.001 | |
| IB | 0.817 | 0.613 |
| IIA | 2.659 | 0.024 |
| IIB | 6.530 | 0.003 |
| IIIA | 2.623 | 0.029 |
| Preoperative CEA | ||
| High (>3 ng/mL) | 0.732 | |
| Postoperative CEA | ||
| High (>3 ng/mL) | 2.168 | 0.032 |
| Pleural invasion | ||
| Yes | 0.211 | |
| Vascular invasion | ||
| Yes | 3.485 | 0.004 |
| Lymphatic invasion | ||
| Yes | 0.748 | |
| Differentiation | 2.253 | 0.011 |
| Neoadjuvant therapy | ||
| Yes | 4.722 | 0.004 |
| Adjuvant therapy | ||
| Yes | 0.690 | |
DFS, disease-free survival; CEA, serum carcinoembryonic antigen.
DISCUSSION
Despite having the same cell type and pathologic stages, the clinical prognosis after surgical resection for non-small cell lung cancer varies for each individual [1,2]. This may be due to inaccurate or insufficient evaluation, and heterogeneous characteristics of the patients with the same stage of disease. We routinely evaluate the preoperative cancer staging and the postoperative status through imaging studies, such as chest CT and PET-CT. However, there may be unidentified or missed metastases. Even if the staging is correct, the patients’ heterogeneity in the same stage can result in unexpected recurrence or metastases. Therefore, we need modalities for additional evaluation to improve the prognosis of these patients [10,11]. One modality can be the serum CEA levels [2,6]. Serum CEA is one of the most widely used markers in various cancers, particularly in the case of colorectal cancer [7,8]. The possibility of using serum CEA levels as a tumor marker or a predictor has been reported after the surgical resection of early non-small cell lung cancer [6,9]. Our study showed that the pre- and postoperative and follow-up CEA levels are strongly associated with the current status and prognosis of patients in all surgical stages following surgical resection. The preoperative CEA levels were generally higher with more progressive cancer before surgery. Patients with higher pre-operative CEA levels had a significantly shorter 5-year DFS than those with normal preoperative CEA levels. There was a significant decrease in the CEA level after surgery, which can reflect the reduction or disappearance of the cancer load and extent. Patients with higher postoperative CEA levels also had a significantly shorter 5-year DFS than patients with normal postoperative CEA levels, which can suggest the possibility of the cancer remaining after surgery in patients with relatively high postoperative CEA levels [12]. The follow-up CEA level at recurrence was significantly higher than both the CEA level before recurrence in the same patient and the median follow-up CEA level in the disease-free patients. Therefore, this may suggest that the possibility of recurrence or metastasis may exist in asymptomatic, average-risk patients with an elevated follow-up CEA level. In addition, we found that 20% of the patients who had recurrence of disease had an elevated CEA level prior to the radiographic evidence of recurrence, which suggests the possibility that CEA may provide earlier detection of recurrence or metastases than imaging studies in some cases. The ROC curve analysis showed that follow-up CEA levels can be a potential predictor of cancer recurrence or metastasis, and follow-up CEA levels were considered to be helpful in detecting early recurrence, which was not detected by imaging studies. At present, the serum CEA level is not included in the NCCN guidelines. Nevertheless, follow-up CEA levels, as well as preoperative CEA levels, can be helpful prognostic indicators in patients who undergo resections for lung adenocarcinoma [12,13]. Previous studies have also reported that the independent prognostic factors of the 5-year DFS after surgery are as follows: the pathologic stage, neoadjuvant and adjuvant therapy, differentiation, vascular invasion, lymphatic invasion, preoperative CEA levels, and the extent of resection and gene mutation [4,13,14]. Similarly, the present study showed that the postoperative CEA levels, the pathologic stage, differentiation, vascular invasion, and neoadjuvant therapy were the prognostic factors of the 5-year DFS in the multivariate analysis. We tentatively suggest that the CEA levels, including the pre-operative and follow-up CEA levels, should be included in the NCCN guidelines for more appropriate decision making and management. Patients with relatively high preoperative or follow-up CEA levels need to be evaluated and managed more carefully, even in patients with stage IA or IB disease [6,14]. After the completion of adjuvant therapy without any detection of recurrence or metastasis by imaging studies, additional management may be needed if a patient has elevated follow-up CEA levels [9,10]. A number of previous studies have reported that the serum CEA level was closely associated with EGFR or k-ras mutation [7,15,16]. However, our study found no association between these factors. In contrast to our expectations, neoadjuvant therapy had a negative prognostic effect on the 5-year DFS in the multivariate analysis, which may be due to the small sample size, and on patients having more advanced disease, and led to poor prognosis in patients who required neoadjuvant therapy. The extent of resection had no effect on the 5-year DFS, which may be due to the patients undergoing limited resection at the early stages, and having less invasive disease and a good prognosis.
This study has several limitations. The first is its retrospective design. The second limitation is the small sample size, not having a normal distribution of the data, and the short observation period. The most common type of lung cancer identified during the early study period was squamous cell carcinoma, and compared to the present, regular health check-ups were less common in the past. Hence, most of our data were registered in the later study period and most patients were in their early pathologic stage for lung adenocarcinoma. Considering the homogenous nature of our data, the results were statistically significant and comparable to those of the other large-scale studies even though our study enrolled a relatively small number of patients and the patients were not evenly distributed with respect to their pathologic stage (IA in 94, IB in 35, IIA in 12, IIB in 3, and IIIA in 14). A future analysis of a larger number of cases for a longer period may be able to provide more reliable results. The third limitation is the lack of strict standardization of the CEA measurement algorithm. To overcome the absence of a definite algorithm, we defined each CEA level strictly according to the time. Therefore, only 158 of 305 patients could be included in the present study. The fourth limitation is the extent of resection. There was no definite indication for limited resections, and limited resections were mainly performed in high-risk patients in their early stage, making the number of designated patients relatively small. In spite of this, the pre-operative CEA levels did not differ according to the extent of the resections in this study. Further, there was no significant difference in the prognosis between patients who underwent limited resection and lobectomy, or more extensive resection.
Nevertheless, this study shows strong evidence of associations between serum CEA levels and disease status and prognosis after surgery. Therefore, the measurement of CEA levels may improve the evaluation of the current status and prognosis after surgery for lung adenocarcinoma patients. Moreover, individualized treatment for lung adenocarcinoma can be achieved.
In conclusion, although the CEA level cannot be a pre-operative screening test for lung adenocarcinoma due to the low incidence of CEA elevation, the preoperative CEA levels and follow-up CEA levels can be used as prognostic factors for lung adenocarcinoma after surgery. The follow-up CEA level can be a useful tool for detecting early recurrence undetected by postoperative imaging studies. Therefore, pre-operative and postoperative follow-up CEA levels may be helpful for providing personalized evaluation and management.
ACKNOWLEDGMENTS
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No.KTCS04-034).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multi-disciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–43. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Takamochi K, Yoshida J, Nishimura M, et al. Prognosis and histologic features of small pulmonary adenocarcinoma based on serum carcinoembryonic antigen level and computed tomographic findings. Eur J Cardiothorac Surg. 2004;25:877–83. doi: 10.1016/j.ejcts.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Onitsuka T. Prognostic significance of preoperative serum carcinoembryonic antigen level in lung adenocarcinoma but not squamous cell carcinoma. Ann Thorac Cardiovasc Surg. 2004;10:76–80. [PubMed] [Google Scholar]
- 5.Hatzakis KD, Froudarakis ME, Bouros D, Tzanakis N, Karkavitsas N, Siafakas NM. Prognostic value of serum tumor markers in patients with lung cancer. Respiration. 2002;69:25–9. doi: 10.1159/000049366. [DOI] [PubMed] [Google Scholar]
- 6.Kawachi R, Nakazato Y, Takei H, Koshi-ishi Y, Goya T. Clinical significance of preoperative carcinoembryonic antigen level for clinical stage I non-small cell lung cancer: can preoperative carcinoembryonic antigen level predict pathological stage? Interact Cardiovasc Thorac Surg. 2009;9:199–202. doi: 10.1510/icvts.2009.206698. [DOI] [PubMed] [Google Scholar]
- 7.Lim YK, Kam MH, Eu KW. Carcinoembryonic antigen screening: how far should we go? Singapore Med J. 2009;50:862–5. [PubMed] [Google Scholar]
- 8.Hang ZQ, Zheng MF, Huang JH. Detection and diagnostic value of serum carcinoembryonic antigen and cytokeratin 19 fragment in lung cancer patients. Zhonghua Zhong Liu Za Zhi. 2011;33:847–9. [PubMed] [Google Scholar]
- 9.Sakao Y, Tomimitsu S, Takeda Y, Natsuaki M, Itoh T. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg. 2004;25:520–2. doi: 10.1016/j.ejcts.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Fukai R, Sakao Y, Sakuraba M, et al. The prognostic value of carcinoembryonic antigen in T1N1M0 and T2N1M0 non-small cell carcinoma of the lung. Eur J Cardiothorac Surg. 2007;32:440–4. doi: 10.1016/j.ejcts.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka K, Sumitomo S, Nakashima N, Nakajima D, Misaki N. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:435–9. doi: 10.1016/j.ejcts.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Nonaka M, Kataoka D, Yamamoto S, et al. Pre- and post-operative serum carcinoembryonic antigen in primary lung adenocarcinoma. Ann Thorac Cardiovasc Surg. 2004;10:281–4. [PubMed] [Google Scholar]
- 13.Igai H, Matsuura N, Tarumi S, et al. Clinicopathological study of p-T1aN0M0 non-small-cell lung cancer, as defined in the seventh edition of the TNM classification of malignant tumors. Eur J Cardiothorac Surg. 2011;39:963–7. doi: 10.1016/j.ejcts.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Igai H, Matsuura N, Tarumi S, et al. Prognostic factors in patients after lobectomy for p-T1aN0M0 adenocarcinoma. Eur J Cardiothorac Surg. 2012;41:603–6. doi: 10.1093/ejcts/ezr006. [DOI] [PubMed] [Google Scholar]
- 15.Shoji F, Yoshino I, Yano T, et al. Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung adenocarcinomas. Cancer. 2007;110:2793–8. doi: 10.1002/cncr.23101. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Lee CY, Kim DJ, Hong DJ, Lee JG, Chung KY. Pathologic correlation of serum carcinoembryonic antigen and cytokeratin 19 fragment in resected nonsmall cell lung cancer. Korean J Thorac Cardiovasc Surg. 2013;46:192–6. doi: 10.5090/kjtcs.2013.46.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]