Abstract
Breast augmentation and reconstruction mammaplasty have been in practice for decades and are highly prevalent surgeries performed worldwide. While overall patient satisfaction is high, common long-term effects include breast tissue atrophy, accelerated ptosis and inframammary fold breakdown. Increasing evidence attributes these events to the durative loading and compressive forces introduced by the breast implants. Mechanical challenges exceeding the elastic capacity of the breast tissue components, eventually lead to irreversible tissue stretching, directly proportional to the introduced mass. Thus, it is suggested that, contrary to long-standing dogmas, implant weight, rather than its volume, stands at the basis of future tissue compromise and deformation. A novel lightweight implant has been developed to address the drawbacks of traditional breast implants, which demonstrate equivalence between their size and weight. The B-Lite® breast implant (G&G Biotechnology Ltd., Haifa, Israel) design allows for a reduction in implant weight of up to 30%, while maintaining the size, form, and function of traditional breast implants. The CE-marked device can be effectively implanted using standard of care procedures and has been established safe for human use. Implantation of the B-Lite® breast implant is projected to significantly reduce the inherent strains imposed by standard implants, thereby conserving tissue stability and integrity over time. In summary, this novel, lightweight breast implant promises to reduce breast tissue compromise and deformation and subsequent reoperation, further improving patient safety and satisfaction.
Breast augmentation and reconstruction mammaplasty have been performed for decades and boast high patient satisfaction rates.1,2 Women of all age groups3 opt for cosmetic improvement or restoration of breast volume and symmetry by means of breast augmentation. Statistics compiled by the American Society for Aesthetic Plastic Surgery ranked breast augmentation the second-most-common surgical cosmetic procedure in the United States in 2013.3 A similar trend has been observed in the United Kingdom, where breast augmentation was the leading cosmetic procedure performed in 2014.4 Breast reconstruction has become an important means of improving quality of life following mastectomy; in the present day, an estimated 20% of the 5 to 10 million women with breast implants5 have previously had breast cancer.6
Despite their widespread use and well-established safety profile,2 procedures involving breast implants are associated with a gamut of postoperative complications,6 affecting a larger number of recipients as the time from the procedure increases.7,8 Maxwell et al and others have reported long-term and often permanent tissue deformities, with adverse events including breast tissue atrophy, accelerated ptosis, sensory loss, and inframammary fold breakdown.9,10 Vegas and Martin del Yerro postulate that all these effects are directly related to the biomechanical (viscoelastic) properties of soft tissues and their response to loading and compressive forces. Moreover, secondary mastopexy and related complications of revisional surgery chiefly stem from the augmentation-induced modifications of the breast tissue.11 This article presents a literature based exposition of the role of breast implant weight in long-term breast augmentation complications. The discourse is hinged upon fundamental laws of physics and materials, and is further strengthened by the primary author's clinical experience performing more than 15,000 breast augmentation over more than three decades.
Breast Composition and Response to Mechanical Forces
The female breast is composed of various tissue types, the relative ratios of which are influenced by both endogenous and exogenous elements and shift with age, hormonal status, lactation, and body mass index.12-15 More specifically, during pregnancy, the breast reaches maximal maturity and contains a high glandular-to-fat ratio.16,17 In contrast, in response to reduced estrogen levels, the glandular mammary tissue fully atrophies throughout menopause, significantly altering the makeup of the breast.17 Each tissue component features a characteristic elasticity profile that bears a unique capacity to respond to both naturally occurring and externally exerted stresses, such as weight-induced mechanical strain. The fibrous tissue elements demonstrate the highest strain-dependent modulus, in sharp contrast to the fat component, which bears a relatively constant modulus over a range of applied strain levels.18
A spring-mass model provides a simple, yet effective, approximation of elastic systems such as breast tissue. As Hooke's Law dictates, the degree of tissue stretching will be proportional to its mass and inversely proportional to its elasticity (Figure 1). Thus, gravitational forces, aggravated by accelerative forces on the breast, are directly proportional to tissue and implant weight and inversely proportional to their elasticity. When maintaining an upright, static posture, the pull of gravity on the breast is constant and unidirectional. In dynamic states such as walking, descending stairs, and running, accelerative forces result in significant breast movement and tissue impact. In breast kinematic measurements made on a treadmill, all kinematic variables significantly increased with breast size. Vertical bare-breasted displacements increased on average from 4.2 cm for an A-cup-sized breast to 9.9 cm for a G-cup-sized breast. In addition, measured velocities and accelerations increased more than three-fold between these breast sizes. In their scaling models, Scurr et al found that breast mass was the only anthropometric measure to consistently explain differences in breast kinematics between cup sizes.19
Figure 1.
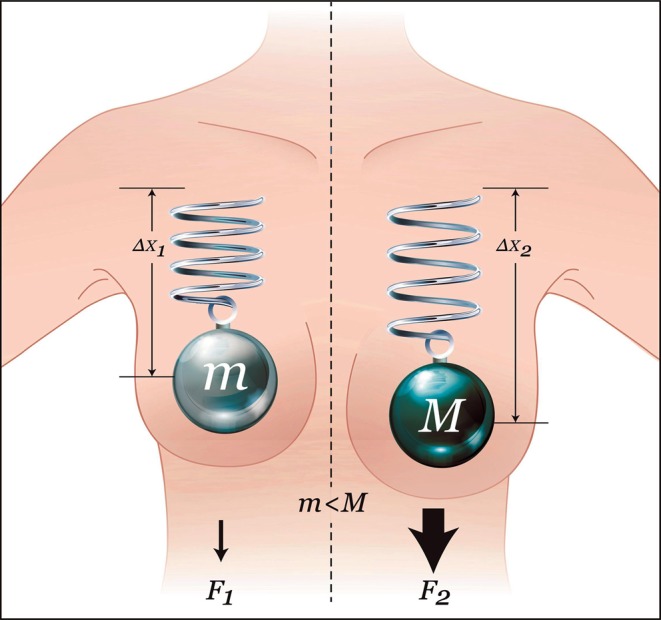
Hooke's law and breast tissue responses. The elastic tissue of the breast is symbolized by a spring with constant K. In a static, upright posture, the weight of an implant will displace the breast downwards with a force proportional to the weight of the implant, as described by the following formula: 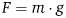 , where F is the force, m is the mass, and g is the standard gravity constant. The tissue's stretch is linear (within the elasticity limits of the tissue), and, therefore, tissue displacement will increase in direct correlation with implant weight. The displacement is described as
, where F is the force, m is the mass, and g is the standard gravity constant. The tissue's stretch is linear (within the elasticity limits of the tissue), and, therefore, tissue displacement will increase in direct correlation with implant weight. The displacement is described as 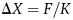 , where
, where 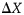 is the displacement, F is the force applied, and K is the spring constant. A heavier implant will result in increased forces and consequential stretch of the breast, as compared with a lighter implant. Therefore, as can be seen in Figure 1, F1 < F2 and
is the displacement, F is the force applied, and K is the spring constant. A heavier implant will result in increased forces and consequential stretch of the breast, as compared with a lighter implant. Therefore, as can be seen in Figure 1, F1 < F2 and 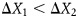 . Reprinted with permission from G&G Biotechnology Ltd., Haifa, Israel.
. Reprinted with permission from G&G Biotechnology Ltd., Haifa, Israel.
Repetitive, accumulated forces expose breast tissue components and their underlying support structures to static and dynamic stress.20 Thus, the breast tissue's response to such stress will be a function of its overall makeup as well as the precise location, intensity, and duration of the applied stress and the regional tissue components. The health problems secondary to macromastia, including poor blood circulation, impaired breathing, shoulder indentation, chronic back or neck pain, and ulnar paresthesia, further attest to the long-term adverse effects of breast weight on proximal tissues and organs.17 Resection of 256 g breast tissue has been shown to be sufficient to significantly alleviate the physical symptoms of macromastia and was shown to be as effective as resection of tissue weight exceeding 1000 g.21,22 Taken together, even a slight reduction in breast tissue weight can adequately reduce gravitational forces on the tissue, diminishing their long-term detrimental effects and associated symptoms.
Breast Augmentation and Long-term Mechanical Stress
Tissue expanders, employed as part of a variety of plastic surgery and reconstructive procedures, impart an initial stress on breast tissues, leading to cellular growth and tissue regeneration, which only cease when the tissue achieves full relaxation.23 In contrast, extensive and durative compression, which directly correlates to the implant's weight, induces irreversible tissue derangement. In other words, the extending forces’ impact on tissues is transient and weight-induced mechanical stress is persistent and unrelenting. Age- and/or hormone-related changes in support structures within the breast tissue will dictate the tissue's overall response to its augmented postimplantation weight.10 Upon subjection to persistent compression loading, the tissue's inherent vulnerability is eventually translated to dynamic creep deformation and consequential ptosis and thinning. Such effects are suggested to be more prevalent with heavy implants (>350-400 g) and among women with reduced breast support tissue, causing increased stretching and thinning of the breast envelope and parenchymal atrophy, uncorrectable chest wall deformities, and compromised tissue vascularity.24 For this reason, in his High Five™ system, Tebbetts ranks breast implant weight second in priority among the five most critical preimplantation decisions surgeons must make.25 Considering such issues preoperatively has been shown to reap clinical benefits,26-28 lowering reoperation rates by five-fold or more.29,30
It is broadly recognized that multiple factors – notably, tissue integration – dictate the long-term effects of breast implants on the surrounding tissue.31 However, the static and dynamic forces that impact breast tissue, defined by the implant's mass, are exerted independent of the other factors. Although further investigation and modeling will be necessary to better characterize the effect of tissue integration on implant-induced stress, it is safe to assume that lower implant weight will result in smaller forces and stress on the tissues, regardless of the degree of tissue integration.
In summary, contrary to long-standing precepts that are largely based on the equivalence of the weight and volume of conventional silicone and saline breast implants, the breast implant's weight, rather than its volume, is the cause of many postoperative complications and side effects. Long-term stability of the augmented breast directly correlates to the resilience of breast tissue and implant weight. Implant weights exceeding tissue support capacities will ultimately lead to tissue atrophy and breast deformation.
A Lightweight Solution
Since their introduction, saline and silicone breast implant volume and weight have been synonymous (specific gravity: 1.0 g/mL and 0.97 g/mL, respectively). Recognition of the weight-dependent impact of mechanical forces on breast tissue integrity has prompted the design of the novel, fifth-generation form-stable, silicone gel B-Lite® Lightweight Breast Implant (LWBI) (G&G Biotechnology Ltd., Haifa, Israel). The round or anatomical implant (Figure 2) contains a continuous phase, reinforced, medical-grade silicone gel filler enriched with inert, high-purity, hollow, borosilicate glass microspheres chemically bound to and encapsulated by the gel network. Microsphere spatial positioning is fixed by means of a proprietary curing process. Furthermore, the microspheres are surface-treated to increase their hydrophobicity and ensure that they remain affixed within the silicone gel. The resulting high-strength bond between the microspheres and the gel is estimated to significantly exceed that of the cohesion forces within the gel, effectively creating gel-covered microspheres and obviating the risk of both free microspheres leaking from the encasing shell and/or separating from the gel. In the unlikely event of shell degradation or rupture, the gel-microsphere bond ensures bodily exposure to gel material only, as would be the case with rupture of a conventional silicone gel implant. Taken together, the unbreakable nature of the gel, the microspheres, and the microsphere-gel interactions result in an implant with a marked mechanical gel strength that is instrumental in maintaining the form and structure of the implant, resisting dispersion even in case of rupture (Figure 3). Furthermore, the microspheres are size-selected to achieve the desired spatial distribution and concentration. The strictly enforced particle size limitation (>30 µm) stymies leaching and migration through the intact shell. Likewise, in the unlikely event of the implant's rupture and direct contact of its contents with bodily tissues, the microspheres are too large to undergo phagocytosis or drainage through the lymphatic system and would, at most, only elicit a local foreign body reaction.
Figure 2.

Round and anatomical B-Lite® Lightweight Breast Implants (LWBIs). The current B-Lite® catalogue includes over 350 styles and sizes. Reprinted with permission from G&G Biotechnology Ltd., Haifa, Israel.
Figure 3.
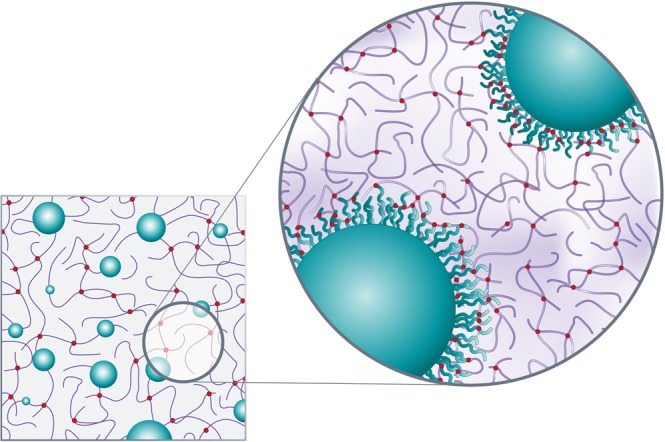
Microsphere-enhanced silicone gel. Integration of silicone gel cross-linked to borosilicate microspheres yields a reinforced gel. The magnified segment shows the high cross-linking density at the surface of the microspheres, resulting in a microsphere-gel adhesion strength exceeding the gel's internal strength of cohesion. Even in the unlikely event of rupture, microspheres will remain bound within the gel and will not disperse. Reprinted with permission from G&G Biotechnology Ltd., Haifa, Israel.
Utilizing microspheres enables a substantial reduction of the implant's weight, up to 30%, when compared with conventional silicone-filled implants of equal size. Simultaneously, their presence reduces the volume of gel required to fill the implant, further lowering its silicone content. The gel is confined within a standard silicone elastomer shell with an internal barrier layer, which is sealed with a patch composed of the same silicone elastomer and barrier layer. The shell, which is prepared in a high-quality manufacturing process, has been in clinical use for years as part of an array of commercially available implants that have been implanted safely in hundreds of thousands of women. The implant's final shape is dictated by the outer shell, which is available in both smooth and textured forms, and is identified by shape, volume, profile, and projection measures. The B-Lite® material is available in volumes ranging from 45 to 920 cc and is implanted into the submuscular or subglandular planes using standard procedures.
Microspheres in Clinical Applications
Both resorbable and permanent microspheres, alone or as composite agents, have many clinical applications in the fields of aesthetics, orthopedics, oncology, urology, dentistry, dermatology, gastroenterology, and cosmetics (among others) for close to a century and have a remarkable safety record. Microspheres are also integrated into an array of products, such as injectable dermal fillers and bulking agents for aesthetic use, soft tissue bulking agents, hard tissue fillers, embolotherapy agents, drug delivery systems, dental fillers, vaccine delivery systems, topical dermal lubricants, and reflective agents in cosmetic applications as well as for imaging purposes. Borosilicate glass, in particular, is an insoluble, inert,32 low-alkali, corrosion-resistant, and biocompatible substance that has been approved for pharmaceutical, cosmetic, and tissue regeneration applications, following establishment of the absence of carcinogenic, genotoxic, and oral toxic effects of both silica33-35 and elemental boron and borates below a specific dose range.36 Furthermore, in a study in which borosilcate glass pellets were implanted into rabbit tibiae, boron release into the subject animals was well below toxic levels.37 Borosilicate glass is ubiquitous in clinically applied materials and is reportedly a part of over 750 cosmetic products.35 Its high silicate concentration renders it nonreactive and elicits little, if any, host tissue responses,32 making it an ideal reference agent for biomaterial biocompatibility tests.38,39
In summary, borosilicate microspheres exhibit superior biological, mechanical, and chemical properties, such as crush resistance, biocompatibility, inertness, and chemical resistance, making them a preferred biomaterial for demanding medical applications. It is for this reason that borosilicate microspheres were selected as an integral weight-reduction component of the LWBI.
Preclinical Validation of the B-Lite® LWBI
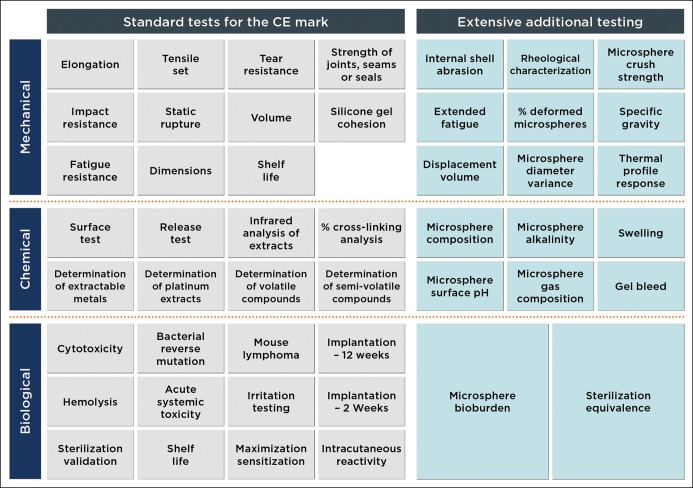
The various elements of the LWBI design were extensively vetted in preclinical testing and validation phases. An exhaustive battery of stringent biocompatibility, mechanical, and chemical tests were performed to establish the safety of B-Lite® for human use and its equivalency with or superiority to currently marketed silicone breast implants (Figure 4). Tests were conducted at leading laboratories under good laboratory practice conditions according to applicable international standards, including ISO 10993 and ISO 14607, and based on an in-depth risk analysis performed by G&G Biotechnology Ltd. All tests simulated worst-case scenarios, involving exposures to the highest concentration of microspheres and implant filler material, to assess the maximal untoward effects of potential implant rupture. The tests demonstrated that the cohesion of the silicone gel filler is as required by the standards, as were the implant's resistance to fatigue, impact and static rupture.
Figure 4.
Preclinical test matrix. Extensive testing was performed by independent test laboratories under good laboratory practice conditions. Worst-case conditions were simulated for every test. The B-Lite® LWBI passed all the conducted tests successfully.
After multiple fatigue resistance tests, the gel remained intact. Following simulated gel exposure to bodily fluids, by means of submersing B-Lite® gel fragments in saline for an extended period of time, no visible separation, swelling, or deterioration of the gel and no microsphere “escape” was observed. Moreover, in this test, chemical characterization of LWBI extracts proved its equivalence to standard silicone gel implants, and no microsphere leaching was detected. Crush strength tests demonstrated that the borosilicate microspheres in B-Lite® can withstand pressures of over 200 psi (>13 atm), far beyond the pressure ranges women are predicted to be exposed to following implantation, including during flight and recreational diving.
Biological testing of the device, including short- and long-term implantation, simulated worst-case scenarios by evaluating implants cut in half, a significantly more severe condition than would occur in a natural rupture, but one which allowed for direct contact between the filler and the tissues. The tested filler had either an equivalent or higher concentration of microspheres as compared with the product in clinical use. The B-Lite® implants successfully passed all tests, and no migration of microspheres was observed, demonstrating the strong chemical bond between the microspheres and the silicone gel. In addition, no granulomas, irritation, or migration of the gel or the microspheres were noted in the cut implants. The LWBI has also been determined to be compatible with common breast imaging methodologies, as well as magnetic resonance imaging (MRI)-compatible and MRI-safe. Overall, the B-Lite® LWBI is considered to be biocompatible with human tissues and mechanically sound under both normal and worst-case conditions.
Breast implants are a Class III device under European regulations and, accordingly, must comply with a long list of standards and directives. Approval for such devices is obtained from a Notified Body, which, for B-Lite®, is the German MDC (Medical Device Certification GmbH), an organization specifically authorized to approve breast implants. The Notified Body reviews the Design Dossier (Technical File) of the device from technical, preclinical, and clinical perspectives and ensures that both the company and the product are in compliance with all necessary directives and standards. The LWBI was granted a CE-mark in Europe in 2013. The device's status under US Food and Drug Administration (FDA) regulations is considered proprietary commercial information until that information is formally published.
As this paper was designed to provide theoretical background in support of lightweight breast implants, the clinical outcomes of ongoing clinical studies lie beyond the scope of the article and will be described in follow-up publications. Thus far, clinical experiences with B-Lite LWBI have been positive.
CONCLUSION
In conclusion, breast implant weight is a critical determinant of long-term clinical outcomes. The B-Lite® LWBI was designed to be a lightweight alternative to conventional silicone breast implants that would maintain the form and function of currently marketed products, but would reduce the incidence of those products’ untoward weight-related effects. All mechanical, chemical and biological tests, simulating worse-case scenarios, demonstrated LWBI resilience and safety for human use. Preliminary clinical results confirmed its safety profile and efficacy.
Considering soft tissue's responses to an implant as part of surgical planning promises to significantly reduce adverse effects on the tissue. The addition of B-Lite® to implants is expected to enable the surgeon to achieve the patient's desired breast shape without jeopardizing long-term tissue stability or integrity. Such avoidance of tissue compromise and deformation as well as, ultimately, reoperation is projected to further improve both patient satisfaction with and the long-term outcomes of the world's most popular aesthetic surgical procedure.
Disclosures
Dr Govrin-Yehudain and Mr Govreen-Segal are co-founders of G&G Biotechnology Ltd., the company that developed the implant discussed in this article. Dr Dvir is a current employee and Dr Preise is an ex-employee of G&G Biotechnology Ltd.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.Cunningham BL, Lokeh A, Gutowski KA. Saline-filled breast implant safety and efficacy: a multicenter retrospective review. Plast Reconstr Surg. 2000;1056:2143-2149. [DOI] [PubMed] [Google Scholar]
- 2.Spear SL, Murphy DK, Slicton A, Walker PS; Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S-16S. [DOI] [PubMed] [Google Scholar]
- 3.Cosmetic Surgery National Data Bank: Statistics 2013. Aesthet Surg J. 2014;34(1 suppl):1S-22S. [DOI] [PubMed] [Google Scholar]
- 4.Tweak not tuck. http://baaps.org.uk/about-us/audit/2040-auto-generate-from-title Accessed March 3, 2015.
- 5.FDA Update on the Safety of Silicone Gel-Filled Breast Implants. http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM260090.pdf Accessed January 26, 2015.
- 6.Bondurant S, Ernster V, Herdman R, eds. Safety of Silicone Breast Implants. Washington, DC: National Academies Press, 1999. [PubMed] [Google Scholar]
- 7.Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;1173:757-767. [DOI] [PubMed] [Google Scholar]
- 8.Breast Implant Complications Booklet. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm259296.htm Accessed January 26, 2015.
- 9.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;326:709-717. [DOI] [PubMed] [Google Scholar]
- 10.Vegas MR, Martin del Yerro JL. Stiffness, compliance, resilience, and creep deformation: understanding implant-soft tissue dynamics in the augmented breast: fundamentals based on materials science. Aesthetic Plast Surg. 2013;375:922-930. [DOI] [PubMed] [Google Scholar]
- 11.Handel N. Secondary mastopexy in the augmented patient: a recipe for disaster. Plast Reconstr Surg. 2006;118(7 Suppl):152S-163S. [DOI] [PubMed] [Google Scholar]
- 12.Hussain Z, Roberts N, Whitehouse GH, García-Fiñana M, Percy D. Estimation of breast volume and its variation during the menstrual cycle using MRI and stereology. Br J Radiol. 1999;72855:236-245. [DOI] [PubMed] [Google Scholar]
- 13.Rzymski P, Skórzewska A, Skibińska-Zielińska M, Opala T. Factors influencing breast elasticity measured by the ultrasound Shear Wave elastography – preliminary results. Arch Med Sci. 2011;71:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzen J, Sinkus R, Biesterfeldt M, Adam G. Menstrual-cycle dependence of breast parenchyma elasticity: estimation with magnetic resonance elastography of breast tissue during the menstrual cycle. Invest Radiol. 2003;384:236-240. [DOI] [PubMed] [Google Scholar]
- 15.Gasperoni C, Salgarello M. Rationale of subdermal superficial liposuction related to the anatomy of subcutaneous fat and the superficial fascial system. Aesthetic Plast Surg. 1995;191:13-20. [DOI] [PubMed] [Google Scholar]
- 16.Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res Treat. 1992;233:211-218. [DOI] [PubMed] [Google Scholar]
- 17.Smith M, Kent K. Breast concerns and lifestyles of women. Clin Obstet Gynecol. 2002;454:1129-1139. [DOI] [PubMed] [Google Scholar]
- 18.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;204:260-274. [DOI] [PubMed] [Google Scholar]
- 19.Scurr JC, White JL, Hedger W. Supported and unsupported breast displacement in three dimensions across treadmill activity levels. J Sports Sci. 2011;291:55-61. [DOI] [PubMed] [Google Scholar]
- 20.Gefen A, Dilmoney B. Mechanics of the normal woman's breast. Technol Health Care. 2007;154:259-271. [PubMed] [Google Scholar]
- 21.Chadbourne EB, Zhang S, Gordon MJ, et al. Clinical outcomes in reduction mammaplasty: a systematic review and meta-analysis of published studies. Mayo Clin Proc. 2001;765:503-510. [DOI] [PubMed] [Google Scholar]
- 22.Thoma A, Sprague S, Veltri K, Duku E, Furlong W. A prospective study of patients undergoing breast reduction surgery: health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;1201:13-26. [DOI] [PubMed] [Google Scholar]
- 23.De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;1097:2450-2462. [DOI] [PubMed] [Google Scholar]
- 24.Tebbetts JB, Teitelbaum S. High- and extra-high-projection breast implants: potential consequences for patients. Plast Reconstr Surg. 2010;1266:2150-2159. [DOI] [PubMed] [Google Scholar]
- 25.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2006;118(7 Suppl):35S-45S. [DOI] [PubMed] [Google Scholar]
- 26.Bracaglia R, Fortunato AR, Gentileschi S. A simple way to choose the right implant volume in breast augmentation. Aesthetic Plast Surg. 2005;295:407-408. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo DA, Spector JA. Preoperative sizing in breast augmentation. Plast Reconstr Surg. 2010;1256:1781-1787. [DOI] [PubMed] [Google Scholar]
- 28.Prado AC, Castillo PF. Tape measure used as a simple method for breast implant selection. Plast Reconstr Surg. 2003;1116:2114-2116. [DOI] [PubMed] [Google Scholar]
- 29.Adams WP. The High Five Process: tissue-based planning for breast augmentation. Plast Surg Nurs. 2007;274:197-201. [DOI] [PubMed] [Google Scholar]
- 30.Tebbetts JB. Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study. Plast Reconstr Surg. 2006;1186:1453-1457. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell GP, Scheflan M, Spear S, Nava MB, Hedén P. Benefits and Limitations of Macrotextured Breast Implants and Consensus Recommendations for Optimizing Their Effectiveness. Aesthet Surg J. 2014;346:876-881. [DOI] [PubMed] [Google Scholar]
- 32.Hench LL, Best S. Ceramics, glasses, and glass-ceramics. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, eds. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed San Diego, CA: Elsevier Academic Press; 2004:153-169. [Google Scholar]
- 33.Takizawa Y, Hirasawa F, Noritomi E, Aida M, Tsunoda H, Uesugi S. Oral ingestion of SYLOID to mice and rats and its chronic toxicity and carcinogenicity. Acta Med Biol. 1988;36:27-56. [Google Scholar]
- 34.Pennington JA. Silicon in foods and diets. Food Addit Contam. 1991;81:97-118. [DOI] [PubMed] [Google Scholar]
- 35.Becker LC, Bergfeld WF, Belsito DV, et al. Safety assessment of borosilicate glasses as used in cosmetics. Int J Toxicol. 2013;32(5 Suppl):65S-72S. [DOI] [PubMed] [Google Scholar]
- 36.United Nations Environment Programme, International Labour Organisation, World Health Organization: International Programme on Chemical Safety. http://www.inchem.org/documents/ehc/ehc/ehc204.htm Accessed January 26, 2015.
- 37.Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;76:2355-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silver IA, Deas J, Erecinska M. Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass(R), and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomater. 2001;22:175-185. [DOI] [PubMed] [Google Scholar]
- 39.Price RL, Gutwein LG, Kaledin L, et al. Osteoblast function on nanophase alumina materials: Influence of chemistry, phase, and topography. J Biomed Mater Res. 2003;67A:1284-1293. [DOI] [PubMed] [Google Scholar]