Abstract
In Arabidopsis and many other plant species, anthocyanin pigments accumulate only after light exposure and not in darkness. Excess light of very high fluence rates leads to a further, very strong increase in anthocyanin levels. How excess light is sensed is not well understood. Here, we show that mutations in the key repressor of light signaling, the COP1/SPA complex, cause a strong hyperaccumulation of anthocyanins not only under normal light but also under excess, high light conditions. Hence, normal light signaling via COP1/SPA is required to prevent hyperaccumulation of anthocyanins under these high light conditions. However, since cop1 and spa mutants show a similar high-light responsiveness of anthocyanin accumulation as the wild type it remains to be resolved whether COP1/SPA is directly involved in the high-light response itself.
Keywords: anthocyanin, COP1/SPA, high light, light, photomorphogenesis, signaling, stress
Abbreviations
- COP1
constitutive photomorphogenic 1
- CRY1
cryptochrome 1
- HY5
elongated hypocotyl 5
- PAP
production of anthocyanin pigment
- SPA
supressor of phytochrome A-105
Anthocyanins are secondary metabolites belonging to the class of flavonoids. They range in color from red to blue and provide a visual cue to flowers and fruits to help attract animals for pollination or seed dispersal.1 In vegetative tissues anthocyanins are involved in processes such as protection of the photosynthetic apparatus, herbivory and free-radical scavenging.2 Anthocyanin biosynthesis increases in response to environmental stresses such as nutrient deficiency, drought, low temperature, pathogen infection and high light.3 Regarding the protection of the photosynthetic apparatus, it was proposed already decades ago that anthocyanins shade the photosynthetic apparatus from excess light and therefore reduce susceptibility to photo-inhibition.4 Excess light was shown to damage the photosynthetic apparatus at least in part by reactive oxygen species (ROS) generated by excitation energy and electrons leaking from the photochemical reactions and electron transport system.5
In Arabidopsis and many other species, anthocyanin production is a feature of light-grown plants and does not occur in darkness. The biosynthesis of anthocyanins is controlled by transcription factors which induce the expression of structural genes in the anthocyanin biosynthesis pathway. Anthocyanin biosynthesis in response to light involves the transcription factor ELONGATED HYPOCOTYL 5 (HY5) and a protein complex consisting of MYB-bHLH-WD40 transcription factors (MBW complex).6-10 Both, HY5 and the MYB transcription factors PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1, also called MYB75) and PAP2 (also called MYB90) as members of the MBW complex are regulated by light on the transcriptional and posttranslational levels.11-14 Posttranslationally, HY5, PAP1 and PAP2 proteins are ubiquitinated in dark-grown seedlings via the CONSTITUTIVE PHOTOMORPHOGENIC 1/ SUPPRESSOR OF PHYTOCHROME A-105 (COP1/SPA) E3 ubiquitin ligase and subsequently degraded in the 26S proteasome.11,12 When seedlings or plants are exposed to light, the light-activated photoreceptors inhibit the activity of the COP1/SPA ubiquitin ligase, thereby causing stabilization of the HY5, PAP1 and PAP2 proteins. The COP1/SPA complex consists of COP1 and members of the 4-member SPA protein family (SPA1-SPA4). Defects in COP1 or SPA1 genes cause constitutive photomorphogenesis in darkness with seedlings showing features of light-grown seedlings in complete darkness.15-18
We have recently shown that the constitutive production of anthocyanins in dark-grown cop1 and spa mutants involves the failure to degrade PAP1 and PAP2 proteins in darkness.12 In these experiments, we had observed that cop1–4 mutants – besides producing anthocyanin in darkness – were hyperresponsive to light, accumulating much higher anthocyanin levels in light-grown seedlings when compared to the wild type. Hence, cop1–4 mutant seedlings were not truly constitutively photomorphogenic but retained considerable light responsiveness, suggesting that light did not fully inactivate the COP1/SPA complex under the light conditions used (40 μmol m−2 s−1 white light). To further investigate this phenomenon we grew cop1, spa triple and spa quadruple mutants under higher fluence rates of light.
Figure 1A shows that dark-grown cop1 and spa mutants - here in particular cop1–6 and the spa quadruple mutant – accumulated anthocyanins while the wild type did not. This phenotype reflects the constitutive photomorphogenesis of cop1 and spa mutants as mentioned above. When grown in white light up to 125 μmol m−2 s−1, cop1 and spa mutant seedlings accumulated higher levels of anthocyanin than the wild type. This was particularly pronounced at low fluence rates (5 and 20 μmol m−2 s−1) at which wild-type seedlings accumulated only very low levels of anthocyanin when compared to cop1 and spa mutants or wild-type seedlings grown at higher fluence rates. These results confirm that cop1 and spa mutations affect anthocyanin levels not only in darkness but also in the light. However, the light-response of cop1–4 and spa mutants appeared to saturate at 40 μmol m−2 s−1, implying that there may be COP1/SPA-independent mechanisms controlling anthocyanin production under high light. On the other hand, the cop1–6 mutant showed a non-saturating further increase in anthocyanin production over the fluence rates used.
Figure 1.
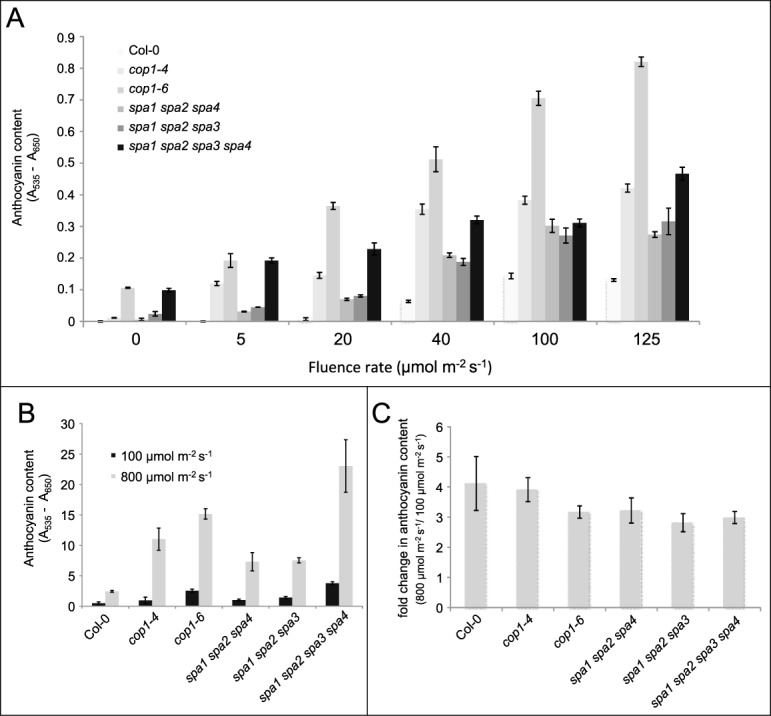
Anthocyanin content in cop1 and spa mutants grown in darkness, low light or high light. Arabidopsis seeds were surface-sterilized and cold-treated as described before.15 Seeds were plated on 1x Murashige and Skoog (MS) medium supplemented with 1% sucrose in all experiments. Cold-treated seeds were exposed to white light for 3 h and then transferred to continuous white light or continuous darkness at 21°C for 4 d or as indicated. For experiments with high light treatment, seeds were germinated on soil and grown in white light (100 μmol m−2 s−1) for 10 d and subsequently transferred to high light (800 μmol m−2 s−1 from high pressure sodium lamps) or kept in white light for 12 h. Anthocyanin content was measured as described before.20 (A) Wild-type (Col-0) and the indicated mutant seedlings were grown in increasing fluence rates of white light. Anthocyanin content is provided per 50 seedlings. (B) Wild type (Col-0) and the indicated mutants were grown in 100 μmol m−2 s−1 white light for 10 d and then transferred to high light (800 μmol m−2 s−1) for 12 h. Anthocyanin content is provided per g fresh weight. (C) Change in anthocyanin content in plants exposed to high light (800 μmol m−2 s−1 for 12 h) relative to plants kept in low light (100 μmol m−2 s−1). Mean of 3 independent experiments. All error bars indicate the standard error of the mean.
To further analyze the Arabidopsis high-light response we grew Arabidopsis wild type, cop1 and spa mutants under true high light fluence rates of 800 μmol m−2 s−1. Since germinating seedlings did not tolerate such excess light, we exposed 10-day-old rosette-stage plants grown in normal light (100 μmol m−2 s−1) to high light for 12 h. All genotypes responded to high-light treatment with a strong increase in anthocyanin accumulation (Fig. 1B). Again, cop1–6 and spa quadruple mutants showed approx. 6 or 9-fold higher anthocyanin levels, respectively, than the wild type (Fig. 1B). cop1–4 and the two spa triple mutants exhibited a less severe phenotype. Hence, defects in the COP1/SPA E3 ligase cause hyperaccumulation of anthocyanin when compared to the wild type also under these high-light conditions. When analyzing the responsiveness to high light, the wild type and all cop1 or spa mutants exhibited a similar 3-4-fold increase in anthocyanin levels in high light relative to normal light (Fig. 1C). Hence, while cop1 and spa mutants exhibited much higher absolute anthocyanin levels than the wild type in high light, the responsiveness of these mutants to high light was not significantly different from that of the wild type. Therefore, the responsiveness of anthocyanin accumulation due to high light exposure may be COP1/SPA-independent, with COP1/SPA mainly being a prerequisite for a high-light response but not being directly involved in the high light response itself. This conclusion would be consistent with the idea that photoreceptor activities are thought to saturate at fluence rates much below 800 μmol m−2 s−1. On the other hand, the photoreceptor cry1 was shown to be required for normal high-light responsiveness in Arabidopsis. Most importantly, cry1 mutants showed a strongly reduced high light-induced expression of PAP1, PAP2 as well as of structural genes in the anthocyanin biosynthesis pathway.19 Together, the data suggest that cryptochrome signaling via COP1/SPA is required for normal accumulation of anthocyanins under high light conditions. Consistent with this idea, a downstream component of COP1/SPA signaling, HY5, was shown to be required for the accumulation of anthocyanins under high light.19 Whether and, if so, how photoreceptors and other mechanisms sensing excess light, e.g. the redox state of the electron transport chain in the chloroplast, co-act in the regulation of gene expression in response to high light remains to be resolved.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 635) to U.H.
References
- 1. Zhang Y, Butelli E, Martin C. Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol 2014; 19c:81-90; http://dx.doi.org/ 10.1016/j.pbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 2. Gould KS. Nature's Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J Biomed Biotechnol 2004; 2004:314-20; PMID:15577195; http://dx.doi.org/ 10.1155/S1110724304406147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 1999; 70:1-9; http://dx.doi.org/ 10.1111/j.1751-1097.1999.tb01944.x [DOI] [Google Scholar]
- 4. Hatier JH, Gould KS. Anthocyanin function in vegetative organs. In: Gould KD K.S., Winefield C., ed. Anthocyanins: biosynthesis, functions, and applications. New York: Springer, 2008:1-19. [Google Scholar]
- 5. Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 1999; 50:333-59; PMID:15012213; http://dx.doi.org/ 10.1146/annurev.arplant.50.1.333 [DOI] [PubMed] [Google Scholar]
- 6. Ang L-H, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng X-W. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1998; 1:213-22; PMID:9659918; http://dx.doi.org/ 10.1016/S1097-2765(00)80022-2 [DOI] [PubMed] [Google Scholar]
- 7. Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 2005; 10:236-42; PMID:15882656; http://dx.doi.org/ 10.1016/j.tplants.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 8. Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007; 19:731-49; PMID:17337630; http://dx.doi.org/ 10.1105/tpc.106.047688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin J, Park E, Choi G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 2007; 49:981-94; PMID:17319847; http://dx.doi.org/ 10.1111/j.1365-313X.2006.03021.x [DOI] [PubMed] [Google Scholar]
- 10. Feller A, Machemer K, Braun EL, Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J 2011; 66:94-116; PMID:21443626; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04459.x [DOI] [PubMed] [Google Scholar]
- 11. Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 2000; 405:462-6; PMID:10839542; http://dx.doi.org/ 10.1038/35013076 [DOI] [PubMed] [Google Scholar]
- 12. Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hulskamp M, Hoecker U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J 2013; 74:638-51; PMID:23425305; http://dx.doi.org/ 10.1111/tpj.12153 [DOI] [PubMed] [Google Scholar]
- 13. Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GI, Tonelli C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 2008; 165:886-94; PMID:17766004; http://dx.doi.org/ 10.1016/j.jplph.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 14. Shin DH, Choi M, Kim K, Bang G, Cho M, Choi SB, Choi G, Park YI. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett 2013; 587:1543-7; PMID:23583450; http://dx.doi.org/ 10.1016/j.febslet.2013.03.037 [DOI] [PubMed] [Google Scholar]
- 15. Laubinger S, Fittinghoff K, Hoecker U. The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in Arabidopsis. Plant Cell 2004; 16:2293-306; PMID:15308756; http://dx.doi.org/ 10.1105/tpc.104.024216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng X-W, Caspar T, Quail PH. cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 1991; 5:1172-82; PMID:2065972; http://dx.doi.org/ 10.1101/gad.5.7.1172 [DOI] [PubMed] [Google Scholar]
- 17. Hoecker U. Regulated proteolysis in light signaling. Curr Opin Plant Biol 2005; 8:469-76; PMID:16039154; http://dx.doi.org/ 10.1016/j.pbi.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 18. Lau OS, Deng XW. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 2012; PMID:22705257 [DOI] [PubMed] [Google Scholar]
- 19. Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand A. Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 2007; 144:1391-406; PMID:17478635; http://dx.doi.org/ 10.1104/pp.107.098293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baumgardt RL, Oliverio KA, Casal JJ, Hoecker U. SPA1, a component of phytochrome A signal transduction, regulates the light signaling current. Planta 2002; 215:745-53; PMID:12244439; http://dx.doi.org/ 10.1007/s00425-002-0801-x [DOI] [PubMed] [Google Scholar]


