Abstract
Primary testicular B-lymphoblastic lymphoma is a rare entity. Primary testicular Ph-positive B lymphoblastic lymphoma was not reported before. We reported a 27-year-old man with primary testicular Ph-positive B lymphoblastic lymphoma, for which fluorescent in-situ hybridization (FISH) for the Philadelphia chromosome was not performed at the initial hospitalization. The patient showed manifestation of Ph-positive acute lymphoblastic leukemia at relapse. In this report, we reviewed the current literature about primary testicular B-lymphoblastic lymphoma, Ph-positive lymphoma and Ph-positive clone evolution. This report has 3 meanings. First: This is first report on primary testicular Ph-positive B lymphoblastic lymphoma. Second: This shows the importance of cytogenics for lymphoma treatment. Third: This implies Philadelphia-positive subclone evolution.
Keywords: B lymphoblastic lymphoma, Ph chromosome, Primary, subclone evolution, testicular
Introduction
Acute lymphoblastic leukemia/lymphoblastic lymphoma is hematologic neoplasm related to the lymphocyte lineage. The term lymphoblastic lymphoma is used when the process is confined to a mass lesion with no or minimal evidence of peripheral blood and of bone marrow. With extensive BM and PB involvement, acute lymphoblastic leukemia becomes the appropriate term.1 Leukemic cells can infiltrate extramedullary tissues during disease progression.2 Testicular infiltration occurs more commonly later during disease progression and relapse.3 It is rare to find testicular infiltration of lymphoma cells at the beginning of diagnosis. Ph-positive acute lymphoblastic leukemia is common among patients of adult acute lymphoblastic leukemia, occurred in approximately 25% of adult cases.4 The case of Ph-positive lymphoblastic lymphoma has rarely been reported, although detection of t (9; 22) is recommended by NCCN guidelines of lymphoblastic lymphoma. Here we present a case of Ph-positive lymphoblastic lymphoma with swollen testis as the first presentation.
Case report
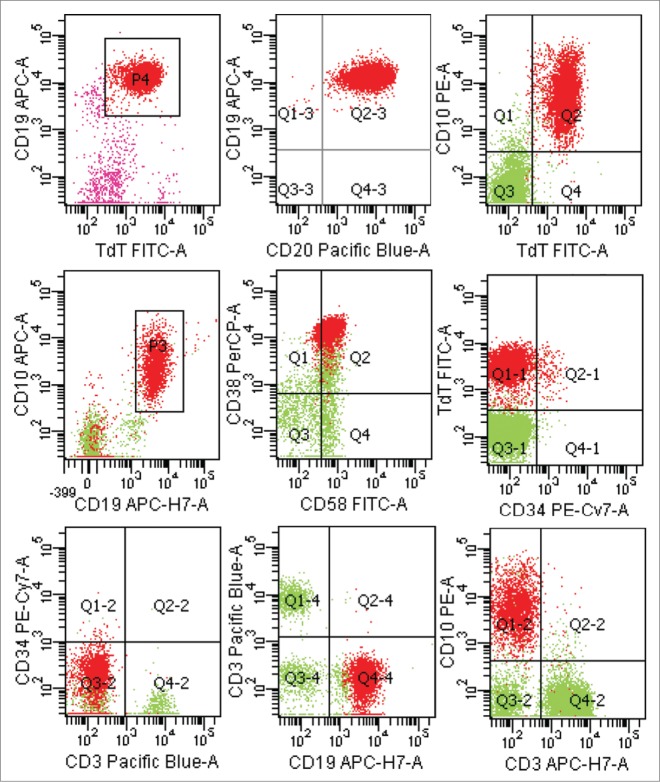
A previously healthy 27-year-old Chinese man presented to the department of urinary surgery in our hospital with mildly painful bilateral testicular swelling for one months' duration on October 10, 2013. There was no history of trauma or fever. The result of physical examination showed that there was no superficial lymph node enlargement except for the bilateral testis palpable enlargement. Ultrasound showed asymmetrically enlarged bilateral testicles with increased vascularity and without focal mass. Sonography-guided core needle biopsy in left intumescent testicle showed lymphoblastic lymphoma of B-cell origin(Fig. 1A–I). The patient was transferred to our hematology department. We examined the hemogram and blood biochemistry including serum lactate dehydrohydroxygenase (LDH) and β2-microglobulin for the patient, but there were no abnormal findings. Chest CT scans and abdominal ultrasound did not indicate mediastinal and retroperitoneal lymph node. Test of EBV and HIV displayed negative EBV-DNA. The result from cerebrospinal fluid showed that there was no lymphoma cell infiltration. Bone marrow examination using multicolor flow cytometric approach showed that there was 2.5% lymphoma cells infiltration in spite of normal bone marrow morphology examination. Immunophenotypic characteristic on lymphoma cells was CD20+ CD10+ CD19+ TdT+ CD58+ CD38+ CD34− (Fig. 2). He was diagnosed with B lymphoblastic lymphoma, Ann Arbor stage IV. He received the combined chemotherapy, and the treatment was a standard regimen of hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone ( hyper-CVAD ) alternated with high-dose methotrexate and cytarabine ( MA ). He underwent 4 cycles of the hyper-CVAD regimen and 4 cycles of the MA regimen. After that, the multicolor flow cytometric examination of bone marrow and cerebrospinal fluid showed no positive findings, and the ultrasound showed normal in testes on April, 2014, then the patient left off treatment.
Figure 1.
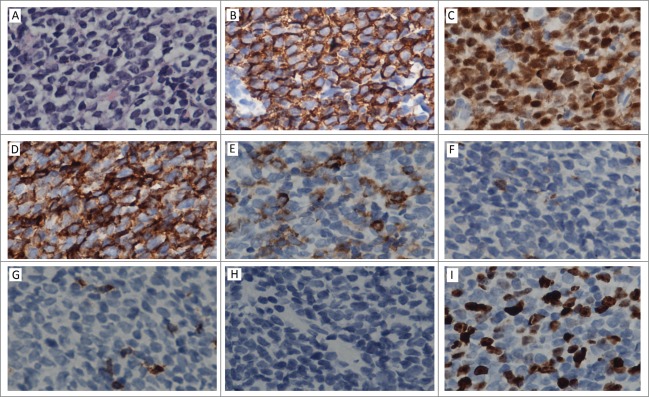
Histopathology of testis. (A) Section from testis shows diffuse lymphoblasts with high nuclear/cytoplasm ratio, irregular shaped nuclei and indistinct nucleoli, obvious mitosis(H&E). (B, C, and D) positivity for CD20, TdT and CD43. E: part positive staining for CD34. (F, G, and H) negativity for CD3,CD7, MPO. I: About 30% of the neoplastic cells display nuclear Ki67 staining.
Figure 2.
Lymphoblastic lymphoma cells with a characteristic phenotype by a multicolor flow cytometric approach at initial diagnosis.
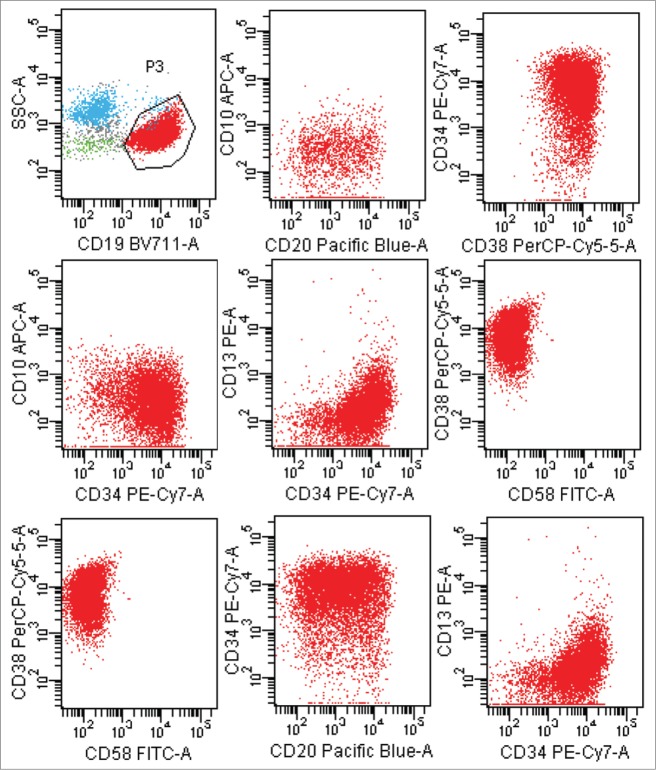
The patient was again admitted to our department on Jun 3, 2014 for gingival bleeding. The laboratory data showed an increased level of WBC [256 × 109/L (normal: 4–10 × 109/L)], and appearance of blast cells (86%) on peripheral blood analysis associated with decreased level of HB [100 g/L (normal: 120–160)] and of PLT [51 × 109/L (normal: 100–300 × 109/L)]. Levels of LDH and β2-microglobulin were increased [LDH 1654U/L, (normal: 135–225), β2-microglobulin 5.33 mg/L (normal: 0.8–2.2)] respectively. bone marrow aspiration revealed hypercellularity with 90% lymphoid blasts, which had scanty cytoplasm, and with inconspicuous nucleoli. Flow cytometric analysis of bone marrow revealed that 84.30% nucleated cells were CD19 positive B lymphocytes whose immunophenotypic characteristic was CD38+ CD34+ CD20± CD10− CD13− CD58− (Fig. 3). The immunophenotypic characteristic was not in conformity with that of lymphoma cells before. The ultrasound showed that his testes results were normal. RT-PCR detection for bcr-abl fusion gene showed positive result in bone marrow (Fig. 4B). Then we detected t(9;22)by FISH in his biopsies testicular tissue and got positive result (Fig. 4A). He was diagnosed with acute Ph-positive B lymphoblastic leukemia. The patient gave up intensive chemotherapy because he was not willing to endure torture from intensive chemotherapy, and he received the treatment of tyrosine kinase inhibitor imatinib and low dose chemotherapy (vincristine, prednisone).Two month later, he committed suicide because he could not bear headache and vomiting, from which we guessed he had central nervous system leukemia.
Figure 3.
Lymphoblastic leukemia cells with a characteristic phenotype by a multicolor flow cytometric approach during the disease process.
Figure 4.
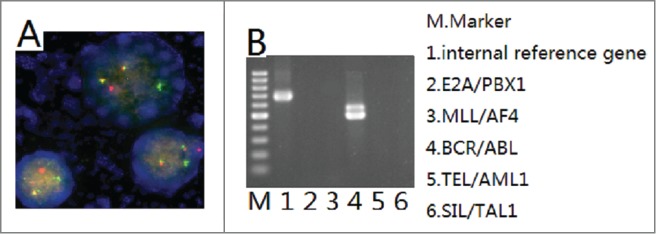
Detection of bcr/abl. A: t(9;22)detected by FISH in testes biopsies. B: bcr/abl fusion gene detected by RT-PCR in bone marrow.
Discussion
Testicular infiltration of B lymphoblastic leukemia/lymphoblastic lymphoma more commonly occurs during disease progression and relapse.3 Although leukemic cell infiltration can be seen in extramedullary tissues, it is rare to find testicular infiltration during diagnosis. There were some reports about primary testicular precursor B-lymphoblastic leukemia/lymphoma.5-7 After searching PubMed, we startlingly found there was no case report of Ph-positive lymphoblastic lymphoma. There were some cases of progressive chronic myeloid leukemia (CML) manifesting as lymphoma.8-12 But we didn't find case report of primary testicular Ph-positive lymphoblastic lymphoma. We report a case of primary testicular Ph-positive precursor B-lymphoblastic lymphoma. The initial symptom of the patient was bilateral testicular swelling, and the diagnosis was determined by sonography guided core needle biopsy. We ignored the possibilities of Ph-positive lymphoblastic lymphoma because there were no reports of Ph-positive primary testicular lymphoblastic lymphoma before. Ph chromosome was not detected at his first admission to our hospital: the patient lost a chance of tyrosine kinase inhibitor therapy. After the patient received combined chemotherapy treatment, the clinical features showed difference during disease progression. The patient showed manifestation of acute lymphoblastic leukemia. Flow cytometric analysis revealed that there was an obvious contrast between the immunophenotypic characteristic of lymphoma cells and that of leukemia cells. We suspected that lymphoma cells and leukemia cells were different clones, so we checked t(9;22) by FISH in his testes biopsies and get Ph-positive result. We corrected the diagnosis as primary testicular Ph-positive B cell lymphoblastic lymphoma. But the treatment of tyrosine kinase inhibitor was delayed for 8 months, which might influence the patient outcome. Cytogenetics analysis for MYC, t(9;22) and t(8;14) is recommended by NCCN guidelines of lymphoblastic lymphoma, but these detections are not routine clinical examinations in many hospitals in developing countries. This case showed the importance and necessity of cytogenetics analysis.
Some advances have shown that inter-tumor heterogeneity between tumors of the same histopathological subtype13 and the trunk-branch model of tumor gives a reasonable explanation about tumor heterogeneity and about subclone evolution.14 Early somatic mutations in early clonal progenitors are represented within the “trunk” of the tumor. Such trunk somatic mutations are likely to be ubiquitous events during the progression of disease. By contrast, later somatic aberrations as branch aberrations represent heterogeneous events, which illustrate subclone evolution. As the subclone evolution, clinical manifestations may be diverse. In 2011, Anderson approved the evolution of recurrent gene alterations occurring in different subclones of the same patient through multiplexing FISH analyses of ALL single cells. Gene alterations are independently and capriciously acquired in subclones of individual patients in initiating diagnosis and in relapse.15 There are some reports about Philadelphia-positive minor subclone in Philadelphia-positive hematologic malignancies.16–18 For CML, surveillance of subclone-evolution can be used for optimised management of patients.19
Tumor heterogeneity manifests as cell morphology heterogeneity, as immunophenotype disparity and as different tissue tropisms. Cell morphological heterogeneity was described with the term anaplasia by David Von Hansemann in 1890. Tumor morphological heterogeneity is observed from the difference in necrosis and differentiation between microscopy fiends. Immunophenotype difference is considered as feature of inter-tumor heterogeneity. Tumor subclone evolution is accompanied with immunophenotypic change (losses or gains). Immunophenotypic characteristic on lymphoma cells in bone marrow at initial diagnosis was CD20+ CD10+ CD19+ TdT+ CD58+ CD38+ CD34-. B-ALL with Philadelphia-positive is typically CD10+CD19+ and TdT+.1,20,21 Immunophenotypic characteristic on lymphoma cells in bone marrow at initial diagnosis is in accord with immunophenotypic characteristic of Philadelphia-positive ALL. Immunophenotypic characteristic on leukemia cell in bone marrow at relapse was CD38+ CD34+ CD20± CD10− CD13− CD58− . By comparing, we found that gain of expression of CD34 and losses of expression of CD10 and of CD58 implied subclone evolution. Inter-tumor subclones developing in a branched evolutionary pattern gave rise to metastatic disease. Metastatic subclones were distinct from non-metastatic subclones.22 Gene instability occurred early in cancer and contributed to on-going tumor evolution at metastatic sites. Different subclones showed different organ specific metastases.23 Different affected sites at initial diagnosis and at relapse gave the evidence of subclone-evolution in the case we reported. Unfortunately, we could not confirm cytogenetic changes because of sample insufficiency.
In a word, this is first report about primary testicular Ph-positive precursor B-lymphoblastic lymphoma and this case implies Philadelphia-positive subclone evolution. This case shows the importance of cytogenetics analysis for lymphoblastic lymphoma treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Grant from the National Natural Science Foundation of China (No 81370658).
References
- 1.Sherdlow SH, Campo E, Harris NL. World Health Organization classification of tumors of haematopoietic and lymphoid tissues IARC; 2008; Lyon: 186. [Google Scholar]
- 2.Hustu HO, Aur RJ. Extramedullary leukaemia. Clin Haematol. 1978; 7:313-337; PMID:354833; http://dx.doi.org/ 10.1016/S0308-2261(78)80008-3 [DOI] [PubMed] [Google Scholar]
- 3.Eden OB, Rankin A, Kay HEM. Isolated testicular relapse in acute lymphoblastic leukaemia of childhood. Arch Dis Child. 1983; 58:128-131; PMID:6572494; http://dx.doi.org/ 10.1136/adc.58.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004. April 8; 350(15):1535-48; http://dx.doi.org/ 10.1056/NEJMra023001 [DOI] [PubMed] [Google Scholar]
- 5.Biswas A, Sahana PK. A case of Acute Lymphoblastic Leukemia presenting with Macroorchidismin a fourteen-year-old boy: A rare presentation. Int J Hematol. 2009. 1(1):5 [Google Scholar]
- 6.Garcia AV1, Alobeid B, Traina JM, Chen SS, Weiner MA, Middlesworth W. Isolated primary testicular B lymphoblastic lymphoma: an unusual presentation. J Pediatr Hematol Oncol. 2013. March; 35(2):e88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahni C, Desai S. Primary testicular precursor B-lymphoblastic lymphoma: a rare entity. Leuk Lymphoma. 2007. October; 48(10):2060-2; http://dx.doi.org/ 10.1080/10428190701535496 [DOI] [PubMed] [Google Scholar]
- 8.Wei J1, Huang M, Wang Y, Zhou J. Sudden extramedullary blast crisis of chronic myeloid leukemia manifesting as T-cell lymphoblastic lymphoma. Onkologie. 2013; 36(3):119-22; PMID:23486000; http://dx.doi.org/ 10.1159/000348681 [DOI] [PubMed] [Google Scholar]
- 9.Jin GN1, Zou P, Chen WX, Ding ZY, Zhou H. Fluorescent in situ hybridization diagnosis of extramedullary nodal blast crisis. Diagn Cytopathol. 2013. March; 41(3):253-6. [DOI] [PubMed] [Google Scholar]
- 10.Găman AM1, Dobrea C, Rotaru I.A case of non-Hodgkin lymphoma in a patient with chronic myeloid leukemia. Rom J Morphol Embryol. 2013; 54(4):1141-5; PMID:24399014 [PubMed] [Google Scholar]
- 11.Chen X1, Rutledge JC, Wu D, Fang M, Opheim KE, Xu M. Chronic myelogenous leukemia presenting in blast phase with nodal, bilineal myeloid sarcoma and T-lymphoblastic lymphoma in a child. Pediatr Dev Pathol. 2013. Mar-Apr; 16(2):91-6. [DOI] [PubMed] [Google Scholar]
- 12.Ye CC1, Echeverri C, Anderson JE, Smith JL, Glassman A, Gulley ML, Claxton D, Craig FET-cell blast crisis of chronic myelogenous leukemia manifesting as a large mediastinal tumor. Hum Pathol. 2002. July; 33(7):770-3; http://dx.doi.org/ 10.1053/hupa.2002.126190 [DOI] [PubMed] [Google Scholar]
- 13.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012. October 1;72(19):4875-82; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap T, Gerlinger M, Futreal A, Pustzai L, Swanton C. Intratumour Heterogeneity: Seeing the wood for the trees. Science Translational Medicine. 2012. March 28; 4(127):127ps10. [DOI] [PubMed] [Google Scholar]
- 15.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J, et al.. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011; 469(7330):356-61; PMID:21160474; http://dx.doi.org/ 10.1038/nature09650 [DOI] [PubMed] [Google Scholar]
- 16.Ney-Garcia DR, Vieira TP, Liehr T, Bhatt S, de Souza MT, de Figueiredo AF, Ribeiro RC, Silva ML. A case of childhood T cell acute lymphoblastic leukemia with a complex t(9;9) and homozygous deletion of CDKN2A gene associated with a Philadelphia-positive minor subclone. Blood Cells Mol Dis. 2013. February; 50(2):131-3; http://dx.doi.org/ 10.1016/j.bcmd.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 17.La Starza R1, Vitale A, Serra A, Saglio G, Fioritoni G, Falzetti D, Martelli MF, Foà R, Mecucci C. Philadelphia-positive acute lymphoblastic leukemia with multiple subclones including duplication of the Philadelphia chromosome and Abelson oncogene. Cancer Genet Cytogenet. 2002. January 1;132(1):46-50; http://dx.doi.org/ 10.1016/S0165-4608(01)00507-6 [DOI] [PubMed] [Google Scholar]
- 18.Pedraza MA, Mason D, Doslu FA, Marsh RA, Boblett JP. Cell population analysis of a heterogeneous blast cell crisis of chronic myelogenous leukemia. Diagn Immunol. 1984; 2(1):19-24; PMID:6594209 [PubMed] [Google Scholar]
- 19.Preuner S1, Mitterbauer G, Mannhalter C, Herndlhofer S, Sperr WR, Valent P, Lion T. Quantitative monitoring of BCR/ABL1 mutants for surveillance of subclone-evolution, -expansion, and -depletion in chronic myeloid leukaemia. Eur J Cancer. 2012 Jan;48(2):233-6; PMID:21955823; http://dx.doi.org/ 10.1016/j.ejca.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 20.Tabernero MD1, Bortoluci AM, Alaejos I, López-Berges MC, Rasillo A, García-Sanz R, García M, Sayagués JM, González M, Mateo G, et al.. Adult precursor B-ALL with BCR/ABL gene rearrangements displays a unique immunophenotype based on the pattern of CD10, CD34, CD13 and CD38 expresssion. Leukemia. 2001. March; 15(3):406-14. [DOI] [PubMed] [Google Scholar]
- 21.Primo D, Tabernero MD, Perez JJ, Rasillo A, Sayagués JM, Espinosa AB, Lopez-Berges MC, García-Sanz R, Gutierrez NC, Hernandez JM, et al.. Genetic heterogeneity of BCR/ABL+ adult B-cell precursor acute lymphoblastic leukemia: impact on the clinical, biological and immunophenotypical disease characteristics. Leukemia. 2005. May; 19(5):713-20; PMID:15789066; http://dx.doi.org/ 10.1038/sj.leu.2403714 [DOI] [PubMed] [Google Scholar]
- 22.Yachida S1, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al.. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010. October 28; 467(7319):1114-7; PMID:20981102; http://dx.doi.org/ 10.1038/nature09515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, et al.. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010. October 28; 467(7319):1109-13; PMID:20981101; http://dx.doi.org/ 10.1038/nature09460 [DOI] [PMC free article] [PubMed] [Google Scholar]