Emerging cancer therapeutics target the immune system, stimulating host antitumor response. Tumor cells generate an immunosuppressive milieu with multiple mechanisms to evade immune destruction, including disruption of effective antigen presentation, reduction of effector T-cell function, and upregulation of pathways that promote tolerance and T-cell anergy.1 The programmed death (PD) -1/PD ligand-1 (PD-L1) pathway is a critical component of tumor-mediated immunosuppression. Antibodies to PD-1 and PD-L1 have shown potential clinical benefit in advanced solid tumors.2 The US Food and Drug Administration approved the PD-1 inhibitors pembrolizumab and nivolumab for metastatic melanoma and also recently approved nivolumab for the treatment of metastatic squamous non–small-cell lung cancer. The US Food and Drug Administration has also designated the PD-L1 inhibitor MPDL3280A as a breakthrough therapy for bladder cancer and non–small-cell lung cancer. These drugs and additional immune checkpoint inhibitors are currently under investigation in multiple clinical trials as single-agent therapy and also in combination with other agents.
As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs—the accurate determination of clinical efficacy. Historically, the WHO and the RECIST Group have provided standard guidelines to define tumor response to therapy.3,4 Although imperfect, the RECIST criteria are an accepted platform for defining the moment of disease progression and have guided clinician determination of tumor response and driven subsequent drug approval for years.5 By RECIST criteria, a significant increase in the size of tumor lesions and the development of new lesions are considered unequivocal disease progression. Oncologists in the community routinely use RECIST criteria as operational thresholds in clinical decision making. Patients undergo scheduled restaging scans and radiographic measurements of tumor lesions to determine the extent of change in tumor size. Current therapy is discontinued and alternative treatments are initiated when patients meet parameters for disease progression. Significant tumor growth on therapy has traditionally been considered equivalent to treatment failure.
Some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria; however, distinct immune-related patterns of response have also been observed. Some patients with melanoma treated with ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte–associated antigen-4, experienced initial increased size of tumor lesions, confirmed by biopsy as inflammatory cell infiltrates or necrosis, with subsequent decreased tumor burden.6 Immune-related response patterns have been observed in clinical trials of ipilimumab, including development of new lesions associated with edema and infiltrates of immune cells and transient increases in baseline tumor lesions. Delayed clinical responses were also observed in studies of immunotherapeutic agents, such that an increase in total tumor burden was later followed by tumor regression. These findings of pseudoprogression would have been classified prematurely as progressive disease by historic WHO or RECIST criteria and have prompted the development of the immune-related response criteria.7
The initial report of immune-related response criteria in patients who received ipilimumab for treatment of melanoma found that 9.7% of patients (22 of 227 patients) had clinical responses (partial response and stable disease) that would have been misclassified as disease progression by WHO criteria.7 Patients who had responses consistent with both WHO and immune criteria had a reported median survival of 31.2 months (95% CI, 27.8 to 31.2 months), whereas the median overall survival in patients with responses consistent with immune criteria only have not been reached (95% CI, 13.5 months to not reached), and these patients had improved survival profiles compared with nonresponders.7
Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice. Recent studies have evaluated the role of immune-related response criteria in patients with melanoma. One study of patients with metastatic melanoma treated with nivolumab reported that 10% (11 of 107 patients) experienced distinct immune-related responses.8 Data from another clinical trial of the anti–PD-1 monoclonal antibody pembrolizumab in patients with advanced melanoma found that 3.6% (seven of 192 patients) experienced RECIST progressive disease at first assessment, followed by clinical response at second assessment. An additional 3.1% of patients (six of 192 patients) on this study had RECIST progressive disease followed by delayed clinical response at later clinical assessment, for a total of 6.7% of patients (13 of 192 patients) with pseudoprogression. Furthermore, Hodi et al9 conducted a study-wide analysis and found that 12% of patients (51 of 411 patients) with melanoma treated with pembrolizumab were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST. This patient cohort had improved overall survival compared with the patients who met criteria of progressive disease by both immune response criteria and RECIST criteria.9
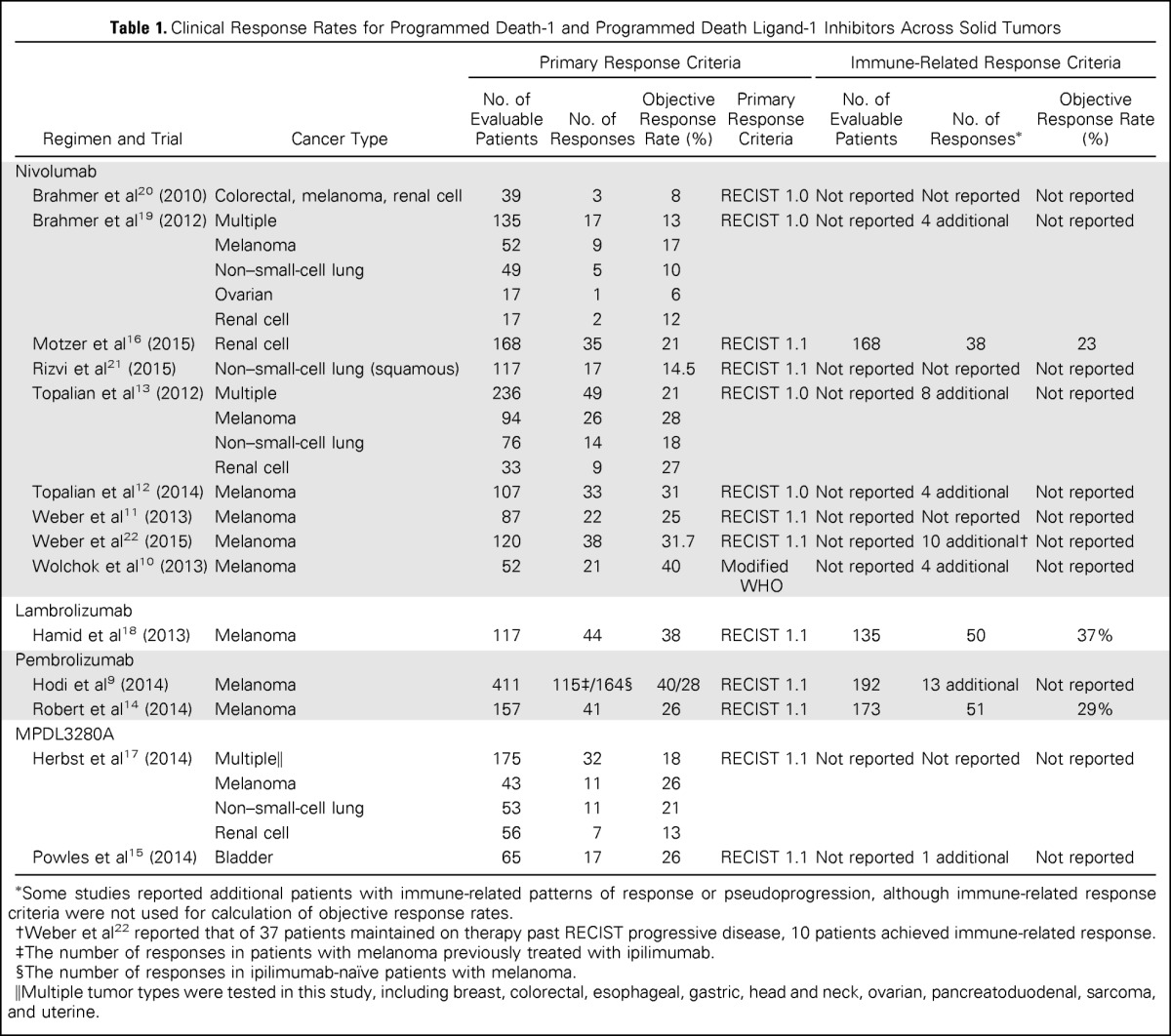
Multiple recent clinical trials using antibodies to PD-1 and PD-L1 in the treatment of advanced solid tumors have been completed and published,10–22 enabling broader evaluation of pseudoprogression across solid tumors. The majority of these clinical trials evaluated the safety and efficacy of immune checkpoint blockade in the treatment of patients with melanoma. However, additional studies were conducted in patients with bladder cancer, breast cancer, colorectal cancer, esophageal cancer, gastric cancer, head and neck cancer, lung cancer, pancreatoduodenal cancer, ovarian cancer, renal cell cancer, sarcoma, and uterine cancer. The observed incidence of distinct immune responses across different solid tumor types is provided in Table 1.
Table 1.
Clinical Response Rates for Programmed Death-1 and Programmed Death Ligand-1 Inhibitors Across Solid Tumors
| Regimen and Trial | Cancer Type | Primary Response Criteria |
Immune-Related Response Criteria |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Evaluable Patients | No. of Responses | Objective Response Rate (%) | Primary Response Criteria | No. of Evaluable Patients | No. of Responses* | Objective Response Rate (%) | ||
| Nivolumab | ||||||||
| Brahmer et al20 (2010) | Colorectal, melanoma, renal cell | 39 | 3 | 8 | RECIST 1.0 | Not reported | Not reported | Not reported |
| Brahmer et al19 (2012) | Multiple | 135 | 17 | 13 | RECIST 1.0 | Not reported | 4 additional | Not reported |
| Melanoma | 52 | 9 | 17 | |||||
| Non–small-cell lung | 49 | 5 | 10 | |||||
| Ovarian | 17 | 1 | 6 | |||||
| Renal cell | 17 | 2 | 12 | |||||
| Motzer et al16 (2015) | Renal cell | 168 | 35 | 21 | RECIST 1.1 | 168 | 38 | 23 |
| Rizvi et al21 (2015) | Non–small-cell lung (squamous) | 117 | 17 | 14.5 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Topalian et al13 (2012) | Multiple | 236 | 49 | 21 | RECIST 1.0 | Not reported | 8 additional | Not reported |
| Melanoma | 94 | 26 | 28 | |||||
| Non–small-cell lung | 76 | 14 | 18 | |||||
| Renal cell | 33 | 9 | 27 | |||||
| Topalian et al12 (2014) | Melanoma | 107 | 33 | 31 | RECIST 1.0 | Not reported | 4 additional | Not reported |
| Weber et al11 (2013) | Melanoma | 87 | 22 | 25 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Weber et al22 (2015) | Melanoma | 120 | 38 | 31.7 | RECIST 1.1 | Not reported | 10 additional† | Not reported |
| Wolchok et al10 (2013) | Melanoma | 52 | 21 | 40 | Modified WHO | Not reported | 4 additional | Not reported |
| Lambrolizumab | ||||||||
| Hamid et al18 (2013) | Melanoma | 117 | 44 | 38 | RECIST 1.1 | 135 | 50 | 37% |
| Pembrolizumab | ||||||||
| Hodi et al9 (2014) | Melanoma | 411 | 115‡/164§ | 40/28 | RECIST 1.1 | 192 | 13 additional | Not reported |
| Robert et al14 (2014) | Melanoma | 157 | 41 | 26 | RECIST 1.1 | 173 | 51 | 29% |
| MPDL3280A | ||||||||
| Herbst et al17 (2014) | Multiple‖ | 175 | 32 | 18 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Melanoma | 43 | 11 | 26 | |||||
| Non–small-cell lung | 53 | 11 | 21 | |||||
| Renal cell | 56 | 7 | 13 | |||||
| Powles et al15 (2014) | Bladder | 65 | 17 | 26 | RECIST 1.1 | Not reported | 1 additional | Not reported |
Some studies reported additional patients with immune-related patterns of response or pseudoprogression, although immune-related response criteria were not used for calculation of objective response rates.
Weber et al22 reported that of 37 patients maintained on therapy past RECIST progressive disease, 10 patients achieved immune-related response.
The number of responses in patients with melanoma previously treated with ipilimumab.
The number of responses in ipilimumab-naïve patients with melanoma.
Multiple tumor types were tested in this study, including breast, colorectal, esophageal, gastric, head and neck, ovarian, pancreatoduodenal, sarcoma, and uterine.
In these studies, tumor assessments included physical examination and radiologic assessment with computed tomography and/or magnetic resonance imaging. Tumor assessments were confirmed with repeat imaging studies or by an independent radiology review. Primary response criteria for the studies included RECIST 1.0, RECIST 1.1, and modified WHO criteria. The majority of existing trials used immune response criteria determined by investigator as a corollary to the RECIST criteria. For example, these criteria were referenced for clinical decision making in the event of mixed response, such as decrease in target lesions and development of new nontarget lesions. In other trials, the immune-related response criteria were used as an exploratory end point. Immune-related response criteria were not used in the reporting of objective response rates in 71% of the studies (10 of 14 studies).
Immune-related responses distinct from RECIST responses have been reported in recently published studies of immune checkpoint blockade. Half of the clinical trials reported the presence of a few additional patients with distinct immune-related patterns of response that did not meet RECIST criteria (44 of 1,126 total patients; an approximate overall incidence of 4%). This incidence calculation may be an underestimation because immune-related response criteria were not evaluated across all patients in these studies. In some studies, there was limited anecdotal reporting of patients meeting immune-related response criteria. The most common pattern reported was a decrease in target tumor lesions in the presence of new lesions. Cases of initial tumor enlargement with delayed shrinkage were also reported. Immune response distinct from RECIST response was reported in multiple patients with melanoma (6.6%; 31 of 471 patients). However, there were isolated occurrences of immune response not captured by RECIST response reported in patients with bladder cancer (1.5%; one of 65 patients), renal cell cancer (1.8%; three of 168 patients), and lung cancer (unquantified; reported in a study with multiple malignancies). Head-to-head comparison of RECIST criteria and immune-related response criteria was performed in less than a third of the studies (four of 14 studies), with similar response rates.
Pseudoprogression and immune-related patterns of mixed response pose a growing clinical challenge for practitioners and patients. Increasing numbers of patients with cancer will have opportunities to receive immunotherapy through experimental trials and recent drug approvals. Patients may continue treatment in the presence of tumor enlargement or new tumor lesions on imaging scans when informed of potential pseudoprogression. However, some of these patients have true disease progression and may consider transitioning to alternative treatment options. The overall reported incidence of pseudoprogression in solid tumors is low. Additional information is necessary for oncologists to use the immune response criteria in the context of treatment decisions and to counsel patients about the incidence of immune-related responses in their tumor types.
Given the current evidence published in clinical trials and supplemental data, a small percentage of patients achieve immune-related responses that are not captured by RECIST criteria. This low reported percentage may be related in part to the unique mechanism of action of immunotherapeutics. Immune agents impact host antitumor response and may require additional time to achieve measurable or sustained clinical effects compared with traditional cytotoxic chemotherapy. It remains unclear whether these response patterns reported in patients with melanoma occur within the same time frame and to the same extent in patients with other solid tumors. Increased reporting of immune-related response phenomena in ongoing trials is necessary to determine whether pseudoprogression is a surrogate for clinical benefit and increased survival and to further elucidate the complex dynamics of tumor interactions with the immune system.
Acknowledgment
Supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pseudoprogression and Immune-Related Response in Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Victoria L. Chiou
No relationship to disclose
Mauricio Burotto
No relationship to disclose
REFERENCES
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Fojo AT, Noonan A. Why RECIST works and why it should stay: Counterpoint. Cancer Res. 2012;72:5151–5157. doi: 10.1158/0008-5472.CAN-12-0733. [DOI] [PubMed] [Google Scholar]
- 6.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, Sznol M, Kluger HM, et al. Long term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. J Clin Oncol. 2014;(suppl 15s):32. abstr 9002. [Google Scholar]
- 9.Hodi FS, Ribas A, Daud A, et al. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;(suppl 15s):32. abstr 3006. [Google Scholar]
- 10.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]


