Abstract
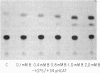
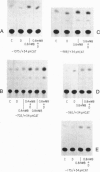
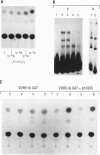
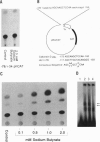
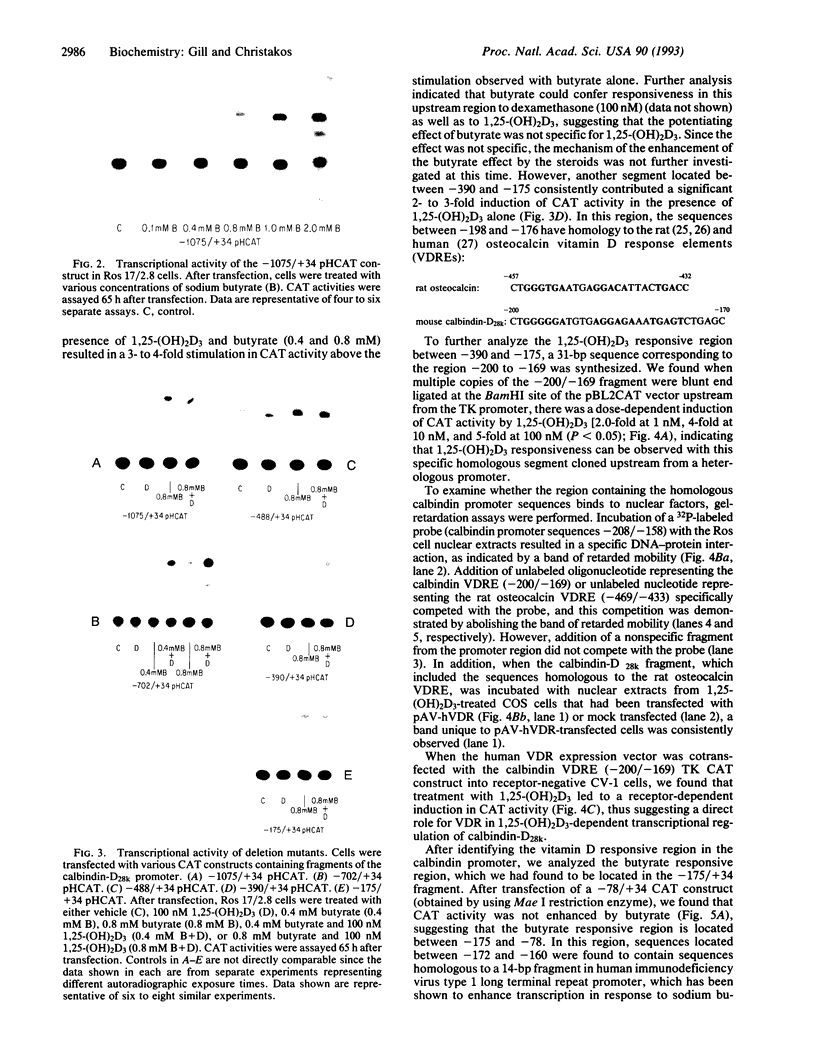
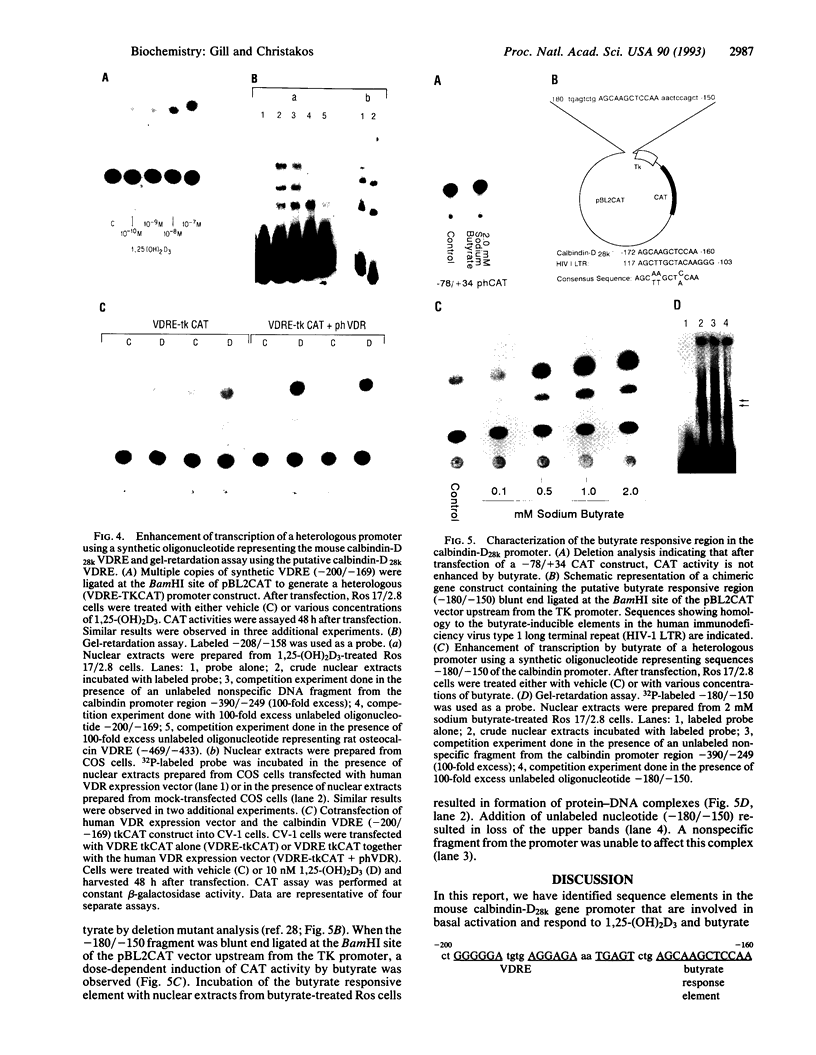
We have examined the 5' flanking region of the mouse calbindin-D28k gene and identified a 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3]-responsive element by deletion mutant analysis of the native promoter as well as by studies with a heterologous thymidine kinase (TK) promoter. The segment between residues -200 and -169 was found to confer a dose-dependent 1,25-(OH)2D3 responsiveness through the TK promoter in Ros 17/2.8 cells as well as in CV-1 cells cotransfected with pAV-hVDR (human vitamin D receptor expression vector). This region contains sequences homologous to the rat osteocalcin vitamin D response element (VDRE). Incubation of this element with nuclear extracts from 1,25-(OH)2D3-treated Ros 17/2.8 cells or from 1,25-(OH)2D3-treated COS cells that had been transfected with pAV-hVDR resulted in a specific protein-DNA interaction. In addition to 1,25-(OH)2D3, sodium butyrate, a differentiating agent, has also been found to modulate expression of calbindin-D28k. Deletion analysis of the mouse calbindin-D28k promoter as well as studies with a heterologous TK promoter resulted in identification of a butyrate-responsive element between -180 and -150 that was found to bind specifically to nuclear factors from butyrate-treated Ros 17/2.8 cells. This butyrate-responsive element may represent a genetic element acted upon by enhancer binding proteins. In summary, the 5' flanking region of the mouse calbindin-D28k gene contains responsive elements that interact with nuclear factors and may mediate, at least in part, the enhanced expression of this gene by 1,25-(OH)2D3 and butyrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birren B. W., Herschman H. R. Regulation of the rat metallothionein-I gene by sodium butyrate. Nucleic Acids Res. 1986 Jan 24;14(2):853–867. doi: 10.1093/nar/14.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan C. A., Robinson R. A., Luciw P. A., Srinivasan A. Mutational analysis of sodium butyrate inducible elements in the human immunodeficiency virus type I long terminal repeat. Virology. 1989 Oct;172(2):573–583. doi: 10.1016/0042-6822(89)90200-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronner F., Stein W. D. CaBPr facilitates intracellular diffusion for Ca pumping in distal convoluted tubule. Am J Physiol. 1988 Sep;255(3 Pt 2):F558–F562. doi: 10.1152/ajprenal.1988.255.3.F558. [DOI] [PubMed] [Google Scholar]
- Christakos S., Gabrielides C., Rhoten W. B. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989 Feb;10(1):3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- Darwish H. M., DeLuca H. F. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5'-flanking region of the rat calbindin D-9k gene. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):603–607. doi: 10.1073/pnas.89.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demay M. B., Roth D. A., Kronenberg H. M. Regions of the rat osteocalcin gene which mediate the effect of 1,25-dihydroxyvitamin D3 on gene transcription. J Biol Chem. 1989 Feb 5;264(4):2279–2282. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret J. M., Brun P., Perret C., Lomri N., Thomasset M., Cuisinier-Gleizes P. Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987 Dec 5;262(34):16553–16557. [PubMed] [Google Scholar]
- Feher J. J. Facilitated calcium diffusion by intestinal calcium-binding protein. Am J Physiol. 1983 Mar;244(3):C303–C307. doi: 10.1152/ajpcell.1983.244.3.C303. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ghosh M. K., Cox R. P. Production of human chorionic gonadotropin in HeLa cell cultures. Nature. 1976 Feb 5;259(5542):416–417. doi: 10.1038/259416a0. [DOI] [PubMed] [Google Scholar]
- Holthuis J., Owen T. A., van Wijnen A. J., Wright K. L., Ramsey-Ewing A., Kennedy M. B., Carter R., Cosenza S. C., Soprano K. J., Lian J. B. Tumor cells exhibit deregulation of the cell cycle histone gene promoter factor HiNF-D. Science. 1990 Mar 23;247(4949 Pt 1):1454–1457. doi: 10.1126/science.247.4949.1454. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Schrickel S. Rat brain calbindin D28: six domain structure and extensive amino acid homology with chicken calbindin D28. Mol Endocrinol. 1988 May;2(5):465–473. doi: 10.1210/mend-2-5-465. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Liao J., Ozono K., Sone T., McDonnell D. P., Pike J. W. Vitamin D receptor interaction with specific DNA requires a nuclear protein and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiyar A. C., Minghetti P. P., Norman A. W. Transfection of avian vitamin D-dependent calbindin-D28K 5' flanking promoter sequence in primary chick kidney cells. Mol Cell Endocrinol. 1991 Jun;78(1-2):127–135. doi: 10.1016/0303-7207(91)90193-v. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Markose E. R., Stein J. L., Stein G. S., Lian J. B. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1701–1705. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Scott R. A., Kerner S. A., O'Malley B. W., Pike J. W. Functional domains of the human vitamin D3 receptor regulate osteocalcin gene expression. Mol Endocrinol. 1989 Apr;3(4):635–644. doi: 10.1210/mend-3-4-635. [DOI] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman L. A., Saunders T. J., Bruns D. E., Boyd J. C., Mills S. E., Bruns M. E. Estrogen inhibits calbindin-D28k expression in mouse uterus. Endocrinology. 1992 Mar;130(3):1728–1735. doi: 10.1210/endo.130.3.1537319. [DOI] [PubMed] [Google Scholar]
- Pfahl M., Tzukerman M., Zhang X. K., Lehmann J. M., Hermann T., Wills K. N., Graupner G. Nuclear retinoic acid receptors: cloning, analysis, and function. Methods Enzymol. 1990;189:256–270. doi: 10.1016/0076-6879(90)89297-u. [DOI] [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Chick W. L., Habener J. F. Transcriptional regulation of genes encoding insulin, glucagon, and angiotensinogen by sodium butyrate in a rat islet cell line. Mol Cell Biol. 1987 Jan;7(1):560–563. doi: 10.1128/mcb.7.1.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Theofan G., Nguyen A. P., Norman A. W. Regulation of calbindin-D28K gene expression by 1,25-dihydroxyvitamin D3 is correlated to receptor occupancy. J Biol Chem. 1986 Dec 25;261(36):16943–16947. [PubMed] [Google Scholar]
- Varghese S., Deaven L. L., Huang Y. C., Gill R. K., Iacopino A. M., Christakos S. Transcriptional regulation and chromosomal assignment of the mammalian calbindin-D28k gene. Mol Endocrinol. 1989 Mar;3(3):495–502. doi: 10.1210/mend-3-3-495. [DOI] [PubMed] [Google Scholar]
- Wood C., Tonegawa S. Diversity and joining segments of mouse immunoglobulin heavy chain genes are closely linked and in the same orientation: implications for the joining mechanism. Proc Natl Acad Sci U S A. 1983 May;80(10):3030–3034. doi: 10.1073/pnas.80.10.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. L., Kobayashi Y., Frantz G., Varghese S., Christakos S., Tobin A. J. Molecular cloning of mammalian 28,000 Mr vitamin D-dependent calcium binding protein (calbindin-D28K): expression of calbindin-D28K RNAs in rodent brain and kidney. DNA. 1988 Nov;7(9):585–593. doi: 10.1089/dna.1988.7.585. [DOI] [PubMed] [Google Scholar]
- Yamakuni T., Kuwano R., Odani S., Miki N., Yamaguchi Y., Takahashi Y. Nucleotide sequence of cDNA to mRNA for a cerebellar Ca-binding protein, spot 35 protein. Nucleic Acids Res. 1986 Aug 26;14(16):6768–6768. doi: 10.1093/nar/14.16.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]