Abstract
Purpose
Survival rates for individuals diagnosed with retinoblastoma (RB) exceed 95% in the United States; however, little is known about the long-term psychosocial outcomes of these survivors.
Patients and Methods
Adult RB survivors, diagnosed from 1932 to 1994 and treated in New York, completed a comprehensive questionnaire adapted from the Childhood Cancer Survivor Study (CCSS), by mail or telephone. Psychosocial outcomes included psychological distress, anxiety, depression, somatization, fear of cancer recurrence, satisfaction with facial appearance, post-traumatic growth, and post-traumatic stress symptoms; noncancer CCSS siblings served as a comparison group.
Results
A total of 470 RB survivors (53.6% with bilateral RB; 52.1% female) and 2,820 CCSS siblings were 43.3 (standard deviation [SD], 11) years and 33.2 (SD, 8.4) years old at the time of study, respectively. After adjusting for sociodemographic factors, RB survivors did not have significantly higher rates of depression, somatization, distress, or anxiety compared with CCSS siblings. Although RB survivors were more likely to report post-traumatic stress symptoms of avoidance and/or hyperarousal (both P < .01), only five (1.1%) of 470 met criteria for post-traumatic stress disorder. Among survivors, having a chronic medical condition did not increase the likelihood of psychological problems. Bilateral RB survivors were more likely than unilateral RB survivors to experience fears of cancer recurrence (P < .01) and worry about their children being diagnosed with RB (P < .01). However, bilateral RB survivors were no more likely to report depression, anxiety, or somatic complaints than unilateral survivors.
Conclusion
Most RB survivors do not have poorer psychosocial functioning compared with a noncancer sample. In addition, bilateral and unilateral RB survivors seem similar with respect to their psychological symptoms.
INTRODUCTION
Approximately 350 children are diagnosed with retinoblastoma (RB) each year in the United States. Survival rates in the United States and higher-income countries now exceed 95%.1 Although much has been published regarding RB survivors' increased lifelong risk for second malignancies,2–6 little is known about their long-term psychosocial functioning.
The few psychosocial studies focused on long-term outcomes of RB survivors have demonstrated that these survivors have an overall good quality of life, lower educational attainment, and some impairment in daily living because of visual difficulties.7–13 Survivors' reproductive behaviors have also been influenced by the perceived risk of having a child with RB.7–13 However, interpretation of these studies has generally been limited by small sample sizes and lack of comparison populations.
The purpose of our exploratory study was to characterize the long-term psychosocial outcomes among adult survivors of RB and to compare survivors with unilateral versus bilateral disease. We also sought to compare RB survivors' psychosocial outcomes with those of a noncancer cohort, siblings from the Childhood Cancer Survivor Study (CCSS). We modeled our questionnaires on those used in the CCSS and focused on the psychosocial areas of depression, anxiety, post-traumatic stress and growth, fears and worries (eg, of recurrence and passing RB to potential offspring), health status, and satisfaction with facial appearance. These outcomes were chosen on the basis of the existing literature on childhood cancer survivors and the unique characteristics of RB survivors, such as the young age at diagnosis, genetic predisposition of RB for potential offspring, and the effects of the disease and treatment on long-term vision, satisfaction with facial appearance, and psychosocial well-being.
PATIENTS AND METHODS
Participants
Eligible participants were defined as RB survivors, treated in the New York area, currently at least 18 years of age and alive at the time of study. Potential participants were identified via the Memorial Sloan Kettering Cancer Center and National Cancer Institute (NCI) RB databases (n = 987). The study was approved by the Memorial Sloan Kettering Cancer Center and NCI Institutional Review Boards.
All eligible participants were sent an initial mailing that included a letter of invitation, informed consent, and the study survey. Participants were contacted by telephone approximately 2 weeks after receipt of the letter. Interested participants provided consent and completed the assessment by mail or telephone interview.
Among the 987 identified RB survivors, 290 (29.3%) were lost to follow-up. An additional 46 survivors were ineligible for a variety of reasons, such as death or inability to provide consent because of cognitive limitations. Among the remaining 651 survivors we were able to contact, 62 refused and 470 (50%) consented and completed the questionnaire. Therefore, 72.2% of eligible survivors who were successfully contacted participated in the study (Appendix Fig A1, online only).
The current study was modeled after the CCSS, a questionnaire-based, retrospective cohort study investigating the long-term health outcomes of more than 14,000 survivors of pediatric and adolescent cancer diagnosed between 1970 and 1986, which has been extensively described elsewhere.14,15 The CCSS includes survivors of a wide range of pediatric cancers; however, RB survivors are not included in the cohort.
Our comparison group was derived from the CCSS sibling cohort,14 a random sample of CCSS participants' nearest age, living siblings, none of whom were siblings of our RB survivor cohort. To provide a noncancer comparison group, data from CCSS siblings aged 18 years or older, without a history of cancer, who completed the follow-up 2003 CCSS questionnaire (to most closely mirror our RB psychosocial survey), were used for comparison purposes for the current study. Self-reported data from 2,820 siblings were used for purposes of comparison.
Survey
The survey used in this study was adapted from the CCSS questionnaires.15,16 We included questions about sociodemographic variables, chronic medical conditions, cancer history, and various psychosocial measures of depression, somatization, anxiety, post-traumatic stress, fears of recurrence, and post-traumatic growth. Study surveys can be viewed at http://ccss.stjude.org.
The Brief Symptom Inventory-18 (BSI-18) is a reliable and valid standardized self-report screen for depression, somatization, and anxiety in medical and community cohorts.17 It has 18 five-point Likert scale items (from 0 [“not at all”] to 4 [“extremely”]) exploring the degrees to which particular problems distressed or bothered the participant during the past 7 days, with a higher score indicating more symptoms. Age- and sex-corrected T scores are used, with clinically significant distress defined as a T score greater than or equal to 63 on the Global Severity Index (GSI).
The Impact of Events Scale (IES) was used to measure post-traumatic symptoms. The IES is a 15-item self-report measure focusing on intrusive thoughts and avoidance associated with a stressor, in this case having had RB, with a higher score indicating more post-traumatic symptoms.18 Participants rate on a 4-point Likert type scale how true each statement is for them, and Intrusion and Avoidance subscale scores are computed. The IES has good internal consistency (Cronbach's α for intrusion = .87; avoidance = .86) and has been used extensively with high cancer risk and patient populations with cancer.18–22 The Post-Traumatic Growth Inventory23 is a 21-item instrument that assesses positive outcomes reported by people who have experienced traumatic events, with a higher score indicating greater positive outcomes reported. It has good convergent and construct validity as well as good reliability (overall α = .90 and factors ranged from .67 to .85). Fear of cancer recurrence was assessed using the Fear of Recurrence Questionnaire,24,25 which specifically assesses fear of cancer recurrence, with higher scores indicating greater fear, and has demonstrated high internal consistency (Cronbach's α = .92).
Severity of self-reported chronic conditions was coded using the NCI's Common Terminology Criteria for Adverse Events (version 4.03),26 a scoring system used to grade acute and chronic conditions in patients with cancer and survivors. Conditions were graded as mild (grade 1), moderate (grade 2), severe/disabling (grade 3), or life-threatening (grade 4). The chronic conditions were graded according to the algorithms established by the CCSS and were overseen by two experienced survivorship clinicians (K.C.O. and C.A.S.).27,28 There were no fatal or grade 5 conditions because all participants were alive at the time of survey completion.
Statistical Analysis
Frequency distributions of patient characteristics between unilateral and bilateral RB survivors, as well as between survivors and the comparison group of CCSS siblings, were summarized. Differences in characteristics between groups were examined by Wilcoxon rank-sum test for continuous variables or Fisher's exact test for categorical variables.
Distributions of health status, satisfaction with facial appearance, and fears and worries by disease laterality were also summarized among the RB survivors. A Fisher's exact test was used to examine the differences between unilateral and bilateral RB survivors.
Bivariate analyses were used to compare scores of survivors and siblings on the GSI and three subscales (depression, somatization, and anxiety) of the BSI-18. Respondents' raw scores were converted to T scores on the basis of a linear transformation that standardizes scores while adjusting for sex differences observed in the normative population.17 This procedure enabled a comparison of survivor and sibling scores to community norms. By definition, the standardized population distributions of T scores for the GSI and three subscales have means of 50 and a standard deviation of 10. Once T scores were obtained for RB survivors and siblings, comparison with population norms entailed running a one-sample t-test with the null hypothesis that the sample mean would be 50. This process was conducted for the GSI and for each of the subscales. Two-sample t-tests were used to compare scores between RB survivors and CCSS siblings. χ2 Tests were used to compare significant distress between RB survivors and CCSS siblings. Distress above a clinically meaningful threshold, following the standard definition, was defined as a T score ≥ 63 on the GSI or T scores ≥ 63 on any two of the following subscales: depression, anxiety, and somatization.17 Post-traumatic stress disorder was defined, using Diagnostic and Statistical Manual of Mental Disorders-IV criteria, as having at least one reexperiencing symptom on the IES, three avoidance symptoms, and two arousal symptoms and significant distress on the BSI, as has been used previously.29
A Wilcoxon rank-sum test was used to compare the IES and Post-Traumatic Growth Inventory between RB survivors and the noncancer comparison group as well as between unilateral and bilateral RB survivors. Multivariable linear regression models were used to compare continuous psychosocial outcomes between RB survivors and CCSS siblings, adjusting for age at study, race/ethnicity, household income, and highest educational level. Multivariable linear regression models were used to compare psychosocial outcomes between unilateral and bilateral RB survivors, adjusting for age at study, age at diagnosis, family history of cancer, and RB-directed chemotherapy and radiation treatment exposures. Because of differences between age at study between RB survivors and CCSS siblings, we also conducted frequency-matched analyses. We selected a frequency-matched sensitivity sample of the CCSS sibling participants (n = 2,820) who matched to the RB survivors in a 2:1 ratio on age (categories: 18 to 34, 35 to 43, 44 to 50, and 51 to 77 years) and race/ethnicity. Using frequency-matched analyses, our results were not substantively different from analyses, which adjusted for sociodemograhpic differences, thereby indicating that our results were not because of demographic differences between survivors and CCSS siblings. Therefore, we only present analyses adjusting for age at study, race/ethnicity, highest educational level, and household income, to retain our entire study sample.
Multivariable linear regression models were also used to evaluate the effect of having had a severe/life-threatening chronic health condition on psychosocial outcomes among RB survivors. Analyses were adjusted for laterality, age at study, age at diagnosis, family history of cancer, and RB-directed chemotherapy and radiation exposure. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC), and two-sided statistical inferences were used throughout the exploratory analyses. We did not perform adjustments for multiple statistical testing, but believed it was justified given the exploratory and descriptive nature of the study.
RESULTS
Characteristics of RB Survivors and CCSS Siblings
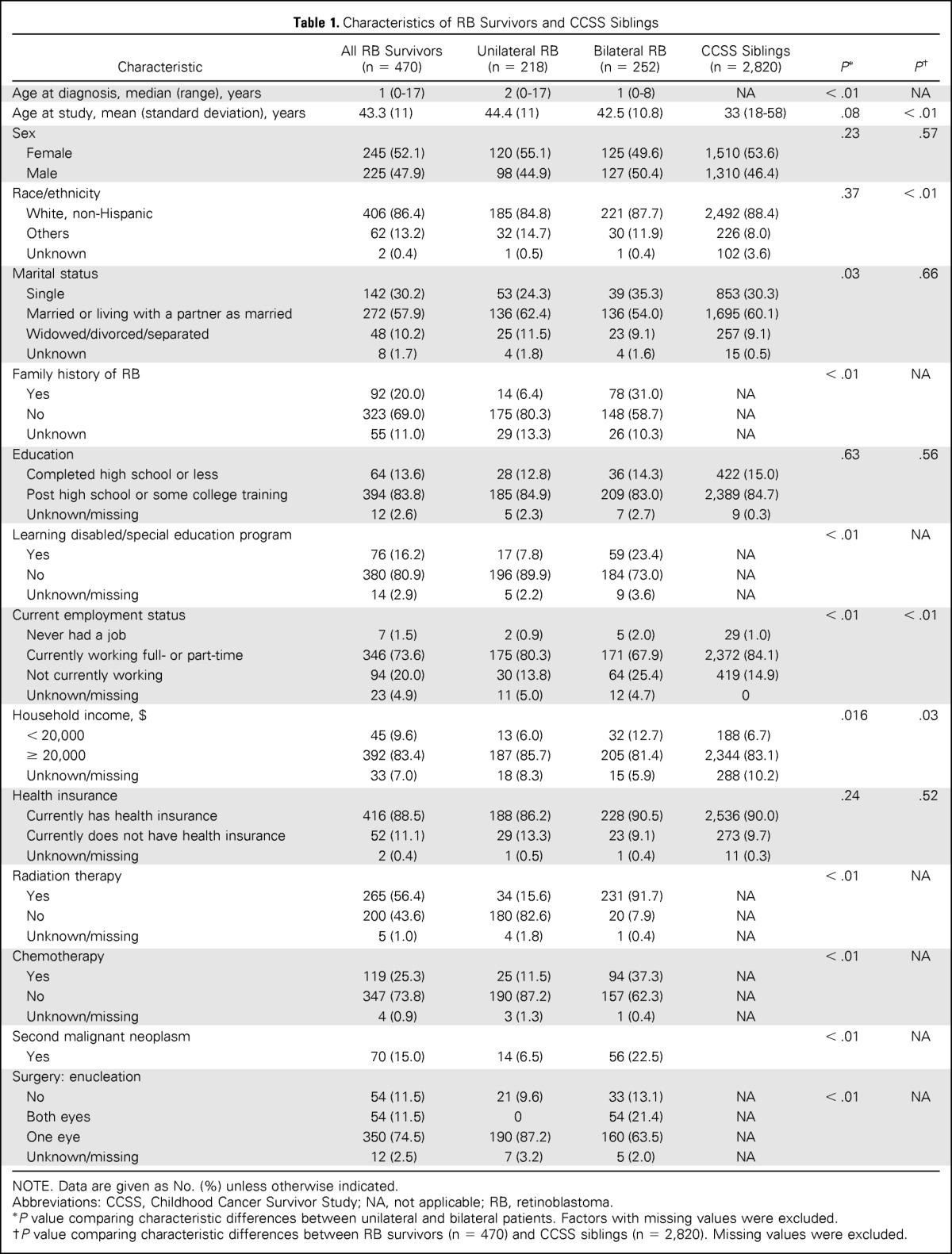
Among 470 RB survivors, 252 (53.6%) had bilateral disease (Table 1). The mean age at study for survivors was 43.3 years (SD, 11 years); 52.1% of participants were female. Most were white, non-Hispanic (86.4%), married or living with a partner (57.9%), had some post high school or college education (83.8%), and were currently working (73.6%). Among survivors, 25.3% were treated with chemotherapy and 56.4% were treated with radiation therapy, with 21.7% receiving both. A greater proportion of survivors of bilateral disease were not married, had a lower income, had been in a special education program, had higher rates of second malignant neoplasms, were diagnosed at younger ages, and received radiation and/or chemotherapy, compared with unilateral RB survivors.
Table 1.
Characteristics of RB Survivors and CCSS Siblings
| Characteristic | All RB Survivors (n = 470) | Unilateral RB (n = 218) | Bilateral RB (n = 252) | CCSS Siblings (n = 2,820) | P* | P† |
|---|---|---|---|---|---|---|
| Age at diagnosis, median (range), years | 1 (0-17) | 2 (0-17) | 1 (0-8) | NA | < .01 | NA |
| Age at study, mean (standard deviation), years | 43.3 (11) | 44.4 (11) | 42.5 (10.8) | 33 (18-58) | .08 | < .01 |
| Sex | .23 | .57 | ||||
| Female | 245 (52.1) | 120 (55.1) | 125 (49.6) | 1,510 (53.6) | ||
| Male | 225 (47.9) | 98 (44.9) | 127 (50.4) | 1,310 (46.4) | ||
| Race/ethnicity | .37 | < .01 | ||||
| White, non-Hispanic | 406 (86.4) | 185 (84.8) | 221 (87.7) | 2,492 (88.4) | ||
| Others | 62 (13.2) | 32 (14.7) | 30 (11.9) | 226 (8.0) | ||
| Unknown | 2 (0.4) | 1 (0.5) | 1 (0.4) | 102 (3.6) | ||
| Marital status | .03 | .66 | ||||
| Single | 142 (30.2) | 53 (24.3) | 39 (35.3) | 853 (30.3) | ||
| Married or living with a partner as married | 272 (57.9) | 136 (62.4) | 136 (54.0) | 1,695 (60.1) | ||
| Widowed/divorced/separated | 48 (10.2) | 25 (11.5) | 23 (9.1) | 257 (9.1) | ||
| Unknown | 8 (1.7) | 4 (1.8) | 4 (1.6) | 15 (0.5) | ||
| Family history of RB | < .01 | NA | ||||
| Yes | 92 (20.0) | 14 (6.4) | 78 (31.0) | NA | ||
| No | 323 (69.0) | 175 (80.3) | 148 (58.7) | NA | ||
| Unknown | 55 (11.0) | 29 (13.3) | 26 (10.3) | NA | ||
| Education | .63 | .56 | ||||
| Completed high school or less | 64 (13.6) | 28 (12.8) | 36 (14.3) | 422 (15.0) | ||
| Post high school or some college training | 394 (83.8) | 185 (84.9) | 209 (83.0) | 2,389 (84.7) | ||
| Unknown/missing | 12 (2.6) | 5 (2.3) | 7 (2.7) | 9 (0.3) | ||
| Learning disabled/special education program | < .01 | NA | ||||
| Yes | 76 (16.2) | 17 (7.8) | 59 (23.4) | NA | ||
| No | 380 (80.9) | 196 (89.9) | 184 (73.0) | NA | ||
| Unknown/missing | 14 (2.9) | 5 (2.2) | 9 (3.6) | NA | ||
| Current employment status | < .01 | < .01 | ||||
| Never had a job | 7 (1.5) | 2 (0.9) | 5 (2.0) | 29 (1.0) | ||
| Currently working full- or part-time | 346 (73.6) | 175 (80.3) | 171 (67.9) | 2,372 (84.1) | ||
| Not currently working | 94 (20.0) | 30 (13.8) | 64 (25.4) | 419 (14.9) | ||
| Unknown/missing | 23 (4.9) | 11 (5.0) | 12 (4.7) | 0 | ||
| Household income, $ | .016 | .03 | ||||
| < 20,000 | 45 (9.6) | 13 (6.0) | 32 (12.7) | 188 (6.7) | ||
| ≥ 20,000 | 392 (83.4) | 187 (85.7) | 205 (81.4) | 2,344 (83.1) | ||
| Unknown/missing | 33 (7.0) | 18 (8.3) | 15 (5.9) | 288 (10.2) | ||
| Health insurance | .24 | .52 | ||||
| Currently has health insurance | 416 (88.5) | 188 (86.2) | 228 (90.5) | 2,536 (90.0) | ||
| Currently does not have health insurance | 52 (11.1) | 29 (13.3) | 23 (9.1) | 273 (9.7) | ||
| Unknown/missing | 2 (0.4) | 1 (0.5) | 1 (0.4) | 11 (0.3) | ||
| Radiation therapy | < .01 | NA | ||||
| Yes | 265 (56.4) | 34 (15.6) | 231 (91.7) | NA | ||
| No | 200 (43.6) | 180 (82.6) | 20 (7.9) | NA | ||
| Unknown/missing | 5 (1.0) | 4 (1.8) | 1 (0.4) | NA | ||
| Chemotherapy | < .01 | NA | ||||
| Yes | 119 (25.3) | 25 (11.5) | 94 (37.3) | NA | ||
| No | 347 (73.8) | 190 (87.2) | 157 (62.3) | NA | ||
| Unknown/missing | 4 (0.9) | 3 (1.3) | 1 (0.4) | NA | ||
| Second malignant neoplasm | < .01 | NA | ||||
| Yes | 70 (15.0) | 14 (6.5) | 56 (22.5) | |||
| Surgery: enucleation | ||||||
| No | 54 (11.5) | 21 (9.6) | 33 (13.1) | NA | < .01 | NA |
| Both eyes | 54 (11.5) | 0 | 54 (21.4) | NA | ||
| One eye | 350 (74.5) | 190 (87.2) | 160 (63.5) | NA | ||
| Unknown/missing | 12 (2.5) | 7 (3.2) | 5 (2.0) | NA |
NOTE. Data are given as No. (%) unless otherwise indicated.
Abbreviations: CCSS, Childhood Cancer Survivor Study; NA, not applicable; RB, retinoblastoma.
P value comparing characteristic differences between unilateral and bilateral patients. Factors with missing values were excluded.
P value comparing characteristic differences between RB survivors (n = 470) and CCSS siblings (n = 2,820). Missing values were excluded.
Among the 2,820 CCSS siblings who served as our comparison group, approximately half were female (53.6%), most were white non-Hispanic (88.4%), had some post high school or college education (84.7%), were married or living with a partner (60.1%), and were currently employed (84.1%). Compared with survivors, the CCSS siblings were, on average, significantly younger at the time of the survey, more likely to be white, non-Hispanic, currently employed, and to have an income above $20,000.
Unilateral Versus Bilateral RB Survivors
There were no significant differences between unilateral and bilateral RB survivors on any of the BSI-18 subscale scores after adjusting for age at study, age at diagnosis, family history of cancer, RB-directed chemotherapy, and radiation exposure. Unilateral and bilateral RB survivors also did not differ on whether they had ever been diagnosed with depression, anxiety, or other psychiatric conditions and/or whether they had been prescribed antidepressants.
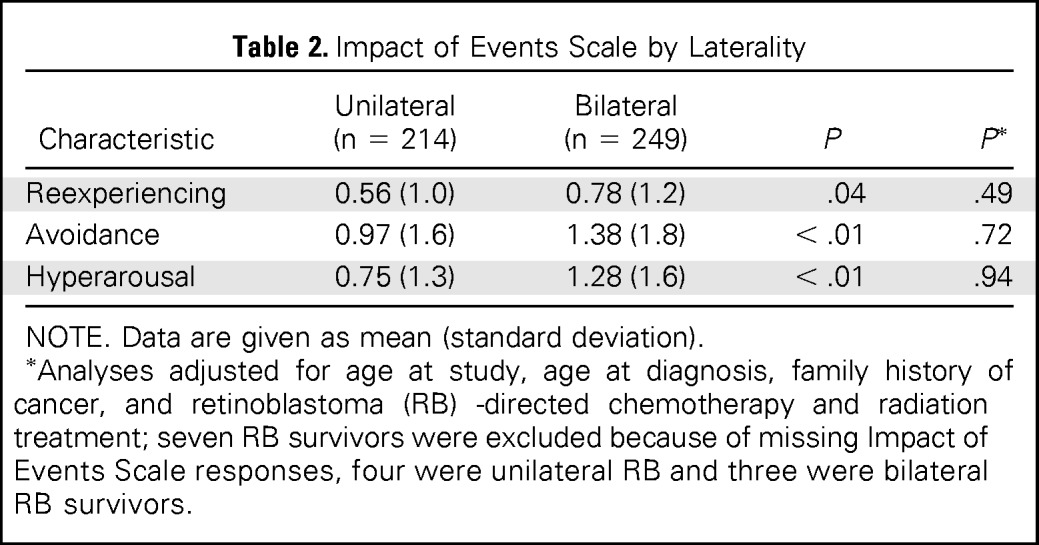
On the IES, a measure of post-traumatic stress symptoms, bilateral survivors were not more likely to report symptoms of reexperiencing, avoidance, and hyperarousal, compared with survivors with unilateral disease, after adjusting for age at study, age at diagnosis, family history of cancer, and treatment exposures. Only five survivors met criteria for post-traumatic stress disorder, as defined by the Diagnostic and Statistical Manual of Mental Disorders-IV (three unilateral survivors and two bilateral survivors; Table 2).
Table 2.
Impact of Events Scale by Laterality
| Characteristic | Unilateral (n = 214) | Bilateral (n = 249) | P | P* |
|---|---|---|---|---|
| Reexperiencing | 0.56 (1.0) | 0.78 (1.2) | .04 | .49 |
| Avoidance | 0.97 (1.6) | 1.38 (1.8) | < .01 | .72 |
| Hyperarousal | 0.75 (1.3) | 1.28 (1.6) | < .01 | .94 |
NOTE. Data are given as mean (standard deviation).
Analyses adjusted for age at study, age at diagnosis, family history of cancer, and retinoblastoma (RB) -directed chemotherapy and radiation treatment; seven RB survivors were excluded because of missing Impact of Events Scale responses, four were unilateral RB and three were bilateral RB survivors.
After adjusting for age at study, age at diagnosis, family history of disease, radiation therapy and/or chemotherapy, bilateral RB survivors endorsed significantly more positive new possibilities in life compared with unilateral RB survivors (mean, 9.7 [SD, 7.0] v mean, 5.4 [SD, 6.5]; P < .01).
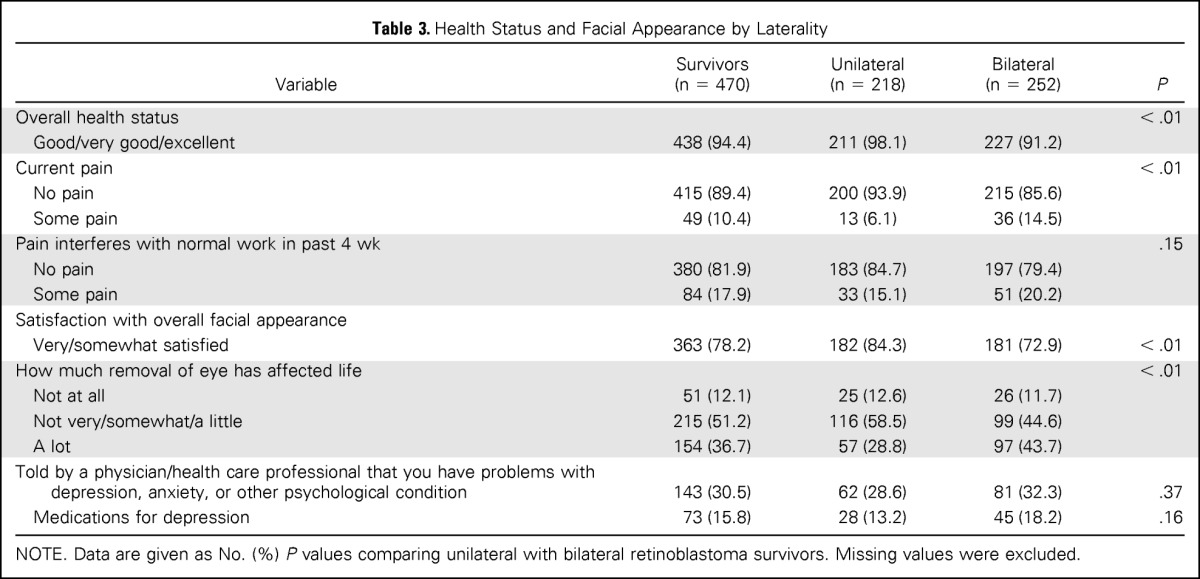
We also investigated health status and satisfaction with facial appearance among RB survivors (Table 3). Unilateral and bilateral RB survivors overwhelmingly reported good to excellent overall health status. Significantly more unilateral survivors reported good to excellent overall health and experiencing no pain compared with survivors with bilateral disease. When asked about their appearance, three-quarters of all survivors were satisfied with their appearance; however, significantly more survivors with unilateral disease reported satisfaction (84.3% unilateral v 72.9% bilateral; P < .01). Overall, 12% of RB survivors reported that the removal of an eye had not affected their life at all; this percentage did not differ between those with a history of unilateral versus bilateral disease.
Table 3.
Health Status and Facial Appearance by Laterality
| Variable | Survivors (n = 470) | Unilateral (n = 218) | Bilateral (n = 252) | P |
|---|---|---|---|---|
| Overall health status | < .01 | |||
| Good/very good/excellent | 438 (94.4) | 211 (98.1) | 227 (91.2) | |
| Current pain | < .01 | |||
| No pain | 415 (89.4) | 200 (93.9) | 215 (85.6) | |
| Some pain | 49 (10.4) | 13 (6.1) | 36 (14.5) | |
| Pain interferes with normal work in past 4 wk | .15 | |||
| No pain | 380 (81.9) | 183 (84.7) | 197 (79.4) | |
| Some pain | 84 (17.9) | 33 (15.1) | 51 (20.2) | |
| Satisfaction with overall facial appearance | ||||
| Very/somewhat satisfied | 363 (78.2) | 182 (84.3) | 181 (72.9) | < .01 |
| How much removal of eye has affected life | < .01 | |||
| Not at all | 51 (12.1) | 25 (12.6) | 26 (11.7) | |
| Not very/somewhat/a little | 215 (51.2) | 116 (58.5) | 99 (44.6) | |
| A lot | 154 (36.7) | 57 (28.8) | 97 (43.7) | |
| Told by a physician/health care professional that you have problems with depression, anxiety, or other psychological condition | 143 (30.5) | 62 (28.6) | 81 (32.3) | .37 |
| Medications for depression | 73 (15.8) | 28 (13.2) | 45 (18.2) | .16 |
NOTE. Data are given as No. (%) P values comparing unilateral with bilateral retinoblastoma survivors. Missing values were excluded.
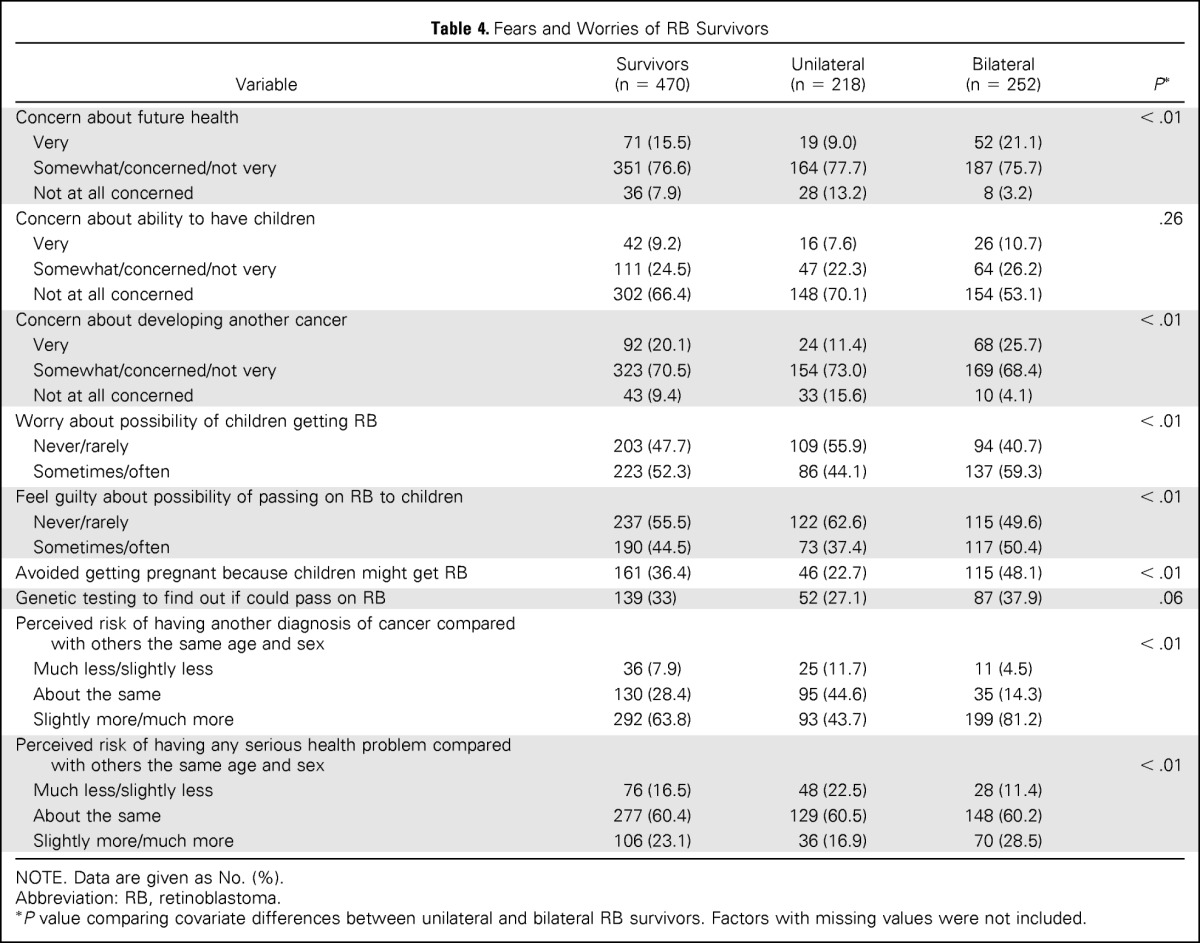
Survivors of unilateral RB had significantly lower mean scores on fears of recurrence (mean, 42.8; SD, 10.6) compared with survivors of bilateral RB (mean, 50.2; SD, 10.8; P < .01). In addition, significantly more bilateral RB survivors worried about the possibility of their children (or future children) being diagnosed with RB (59.3% v 44.1%; P < .01), felt guilty about the possibility of passing RB on to their children (50.4% v 37.4%; P < .01), and/or avoided getting pregnant because of this worry (48.1% v 22.7%; P < .01), compared with unilateral RB survivors (Table 4).
Table 4.
Fears and Worries of RB Survivors
| Variable | Survivors (n = 470) | Unilateral (n = 218) | Bilateral (n = 252) | P* |
|---|---|---|---|---|
| Concern about future health | < .01 | |||
| Very | 71 (15.5) | 19 (9.0) | 52 (21.1) | |
| Somewhat/concerned/not very | 351 (76.6) | 164 (77.7) | 187 (75.7) | |
| Not at all concerned | 36 (7.9) | 28 (13.2) | 8 (3.2) | |
| Concern about ability to have children | .26 | |||
| Very | 42 (9.2) | 16 (7.6) | 26 (10.7) | |
| Somewhat/concerned/not very | 111 (24.5) | 47 (22.3) | 64 (26.2) | |
| Not at all concerned | 302 (66.4) | 148 (70.1) | 154 (53.1) | |
| Concern about developing another cancer | < .01 | |||
| Very | 92 (20.1) | 24 (11.4) | 68 (25.7) | |
| Somewhat/concerned/not very | 323 (70.5) | 154 (73.0) | 169 (68.4) | |
| Not at all concerned | 43 (9.4) | 33 (15.6) | 10 (4.1) | |
| Worry about possibility of children getting RB | < .01 | |||
| Never/rarely | 203 (47.7) | 109 (55.9) | 94 (40.7) | |
| Sometimes/often | 223 (52.3) | 86 (44.1) | 137 (59.3) | |
| Feel guilty about possibility of passing on RB to children | < .01 | |||
| Never/rarely | 237 (55.5) | 122 (62.6) | 115 (49.6) | |
| Sometimes/often | 190 (44.5) | 73 (37.4) | 117 (50.4) | |
| Avoided getting pregnant because children might get RB | 161 (36.4) | 46 (22.7) | 115 (48.1) | < .01 |
| Genetic testing to find out if could pass on RB | 139 (33) | 52 (27.1) | 87 (37.9) | .06 |
| Perceived risk of having another diagnosis of cancer compared with others the same age and sex | < .01 | |||
| Much less/slightly less | 36 (7.9) | 25 (11.7) | 11 (4.5) | |
| About the same | 130 (28.4) | 95 (44.6) | 35 (14.3) | |
| Slightly more/much more | 292 (63.8) | 93 (43.7) | 199 (81.2) | |
| Perceived risk of having any serious health problem compared with others the same age and sex | < .01 | |||
| Much less/slightly less | 76 (16.5) | 48 (22.5) | 28 (11.4) | |
| About the same | 277 (60.4) | 129 (60.5) | 148 (60.2) | |
| Slightly more/much more | 106 (23.1) | 36 (16.9) | 70 (28.5) |
NOTE. Data are given as No. (%).
Abbreviation: RB, retinoblastoma.
P value comparing covariate differences between unilateral and bilateral RB survivors. Factors with missing values were not included.
Among survivors, 71% had a grade 3 or 4 chronic health condition. After adjusting for disease laterality, age at study, age at diagnosis, family history of cancer, and chemotherapy and radiation exposure, there were no significant differences between those survivors with at least one grade 3 or 4 condition versus those with grade 0 to 2 chronic conditions on global symptoms on the BSI, somatization, anxiety, depression, or fears of recurrence.
RB Survivors Compared With CCSS Siblings
On the BSI, RB survivors were significantly less likely to report global symptoms (P < .01), depression (P = .02), and somatic distress (P < .01) compared with CCSS siblings, after adjusting for age at study, race/ethnicity, household income, and educational level (Table 5). The multivariable analyses also indicated that RB survivors reported less anxiety compared with CCSS siblings (P < .01). The T scores for survivors and siblings were lower than the standardized community normative scores of 50, indicating that both cohorts reported lower levels of global distress (P < .01 for both cohorts) as well as fewer symptoms of depression, anxiety, and somatization than community norms (all P < .01). Fewer RB survivors (2.8%) reported significant distress compared with CCSS siblings (6.0%; P < .01).
Table 5.
Brief Symptom Inventory-18: RB Survivors Versus CCSS Siblings
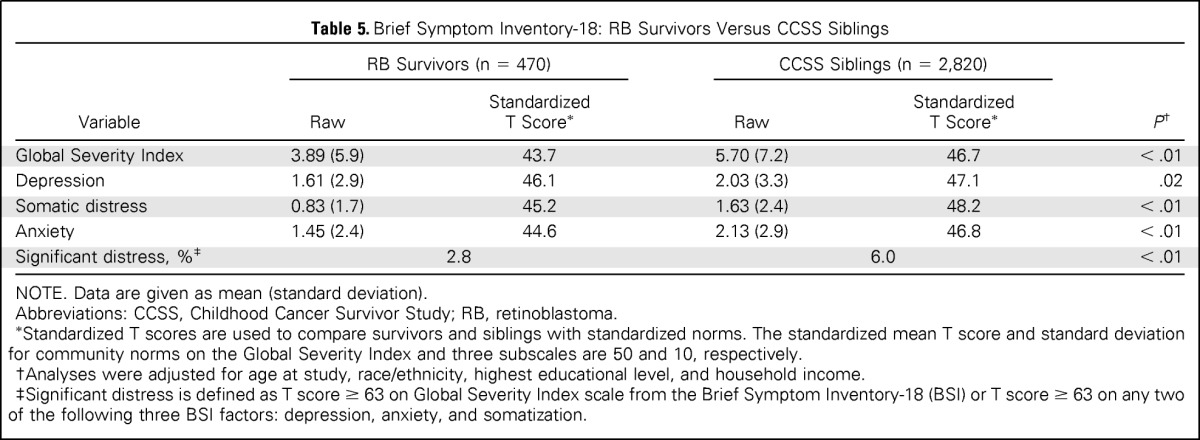
| Variable | RB Survivors (n = 470) |
CCSS Siblings (n = 2,820) |
P† | ||
|---|---|---|---|---|---|
| Raw | StandardizedT Score* | Raw | StandardizedT Score* | ||
| Global Severity Index | 3.89 (5.9) | 43.7 | 5.70 (7.2) | 46.7 | < .01 |
| Depression | 1.61 (2.9) | 46.1 | 2.03 (3.3) | 47.1 | .02 |
| Somatic distress | 0.83 (1.7) | 45.2 | 1.63 (2.4) | 48.2 | < .01 |
| Anxiety | 1.45 (2.4) | 44.6 | 2.13 (2.9) | 46.8 | < .01 |
| Significant distress, %‡ | 2.8 | 6.0 | < .01 | ||
NOTE. Data are given as mean (standard deviation).
Abbreviations: CCSS, Childhood Cancer Survivor Study; RB, retinoblastoma.
Standardized T scores are used to compare survivors and siblings with standardized norms. The standardized mean T score and standard deviation for community norms on the Global Severity Index and three subscales are 50 and 10, respectively.
Analyses were adjusted for age at study, race/ethnicity, highest educational level, and household income.
Significant distress is defined as T score ≥ 63 on Global Severity Index scale from the Brief Symptom Inventory-18 (BSI) or T score ≥ 63 on any two of the following three BSI factors: depression, anxiety, and somatization.
On the IES, RB survivors were significantly more likely to report symptoms of avoidance (mean, 1.19) and hyperarousal (mean, 1.03) compared with CCSS siblings (means, 0.73 and 0.63, respectively) after adjusting for age, race, household income, and highest educational level. However, they did not differ from CCSS siblings on symptoms of reexperiencing and intrusive thinking (P = .55; Table 6). A comparison of symptoms of post-traumatic growth between RB survivors and CCSS siblings demonstrated no significant differences on any of the subscales, including relating to others, new possibilities, personal strength, spirituality, and appreciation for life after adjusting for age at study, race/ethnicity, household income, and education.
Table 6.
Impact of Events Scale: RB Survivors Versus CCSS Siblings

| Variable | RB Survivors (n = 463) | CCSS Siblings (n = 383) | P | P* |
|---|---|---|---|---|
| Reexperiencing | 0.68 (1.1) | 0.62 (1.2) | .38 | .55 |
| Avoidance | 1.19 (1.7) | 0.73 (1.4) | < .01 | < .01 |
| Hyperarousal | 1.03 (1.5) | 0.63 (1.2) | < .01 | < .01 |
NOTE. Data are given as mean (standard deviation).
Abbreviations: CCSS, Childhood Cancer Survivor Study; RB, retinoblastoma.
Analyses were adjusted for age at study, race/ethnicity, highest educational level, and household income; seven RB survivors were excluded because all Impact of Events items were missing.
DISCUSSION
As advances in pediatric oncology have increased survival rates, attention has increasingly shifted to the long-term complications of treatment and the well-being of survivors, including their psychosocial functioning. This is the first large study of psychosocial functioning among adult RB survivors. Overall, RB survivors reported lower levels of depressive, anxious, and somatic symptoms than have been reported in the literature among non-RB childhood cancer survivors.30,31 Moreover, this is the first investigation to demonstrate that bilateral RB survivors do not experience worse psychosocial functioning compared with unilateral RB survivors.
Most previous studies have investigated quality of life9,10,13 but have not examined more specific psychosocial functioning among RB survivors. Our results suggest that adult survivors of RB are coping and functioning well as adults, even among the group that experience ongoing chronic health conditions. Although survivors of bilateral RB endorsed having greater health-related fears and had greater worries about passing along the disease to their children, this likely reflects an accurate and realistic understanding of the genetic risks among those with bilateral RB (ie, 50% chance passing along a germline RB1 mutation to their offspring). This is similar to increased worry influencing reproductive behavior, as reported in smaller studies of RB survivors.7,11
Our findings characterize RB survivors as a fairly healthy and resilient group but highlight some potential areas for future research and clinical counseling. Although RB survivors were coping well, survivors of bilateral disease endorsed greater fears of recurrence or second malignant neoplasms as well as fears of passing RB to their children, perhaps highlighting a greater need for long-term psychosocial and genetic counseling. In addition, this study should provide reassurance to RB patients, families, and their health care providers that survivors will likely be functioning psychosocially well. In fact, RB survivors did not have symptoms of anxiety, depression, or somatization. Some of our findings indicated that RB survivors were doing better psychosocially, compared with CCSS siblings, perhaps indicating a high level of resilience in RB survivors.
This study has some limitations, including those with cognitive disabilities were excluded from participation, we did not have a measure of learning disabilities/special education for CCSS siblings, and there were some survivors lost to follow-up, which may introduce bias. In addition, contemporary treatment for RB includes abandonment of external-beam irradiation, marked diminution in enucleation rates, and using more systemic, intra-arterial, and intravitreal chemotherapy. Examination of psychosocial differences in more contemporarily treated RB survivors would be an area worthy of future research.
However, our data, among a large cohort of RB survivors, indicate that overall this is a group who are functioning fairly well and that bilateral RB survivors are not faring worse psychosocially than unilateral RB survivors. In addition, our findings suggest that adult RB survivors do not have poorer psychosocial functioning compared with noncancer comparators.
Survivorship programs should recognize the overall positive functioning for most RB survivors. However, attention to symptoms of post-traumatic stress and worries about future health and childbearing should be given to this cohort of survivors. Fertility and genetic counseling may be warranted and indicated for survivors of childhood RB. Future research should focus on interventions targeted toward post-traumatic stress symptoms and health-related fears raised by RB survivors, especially those that affect having children.
Appendix
Fig A1.
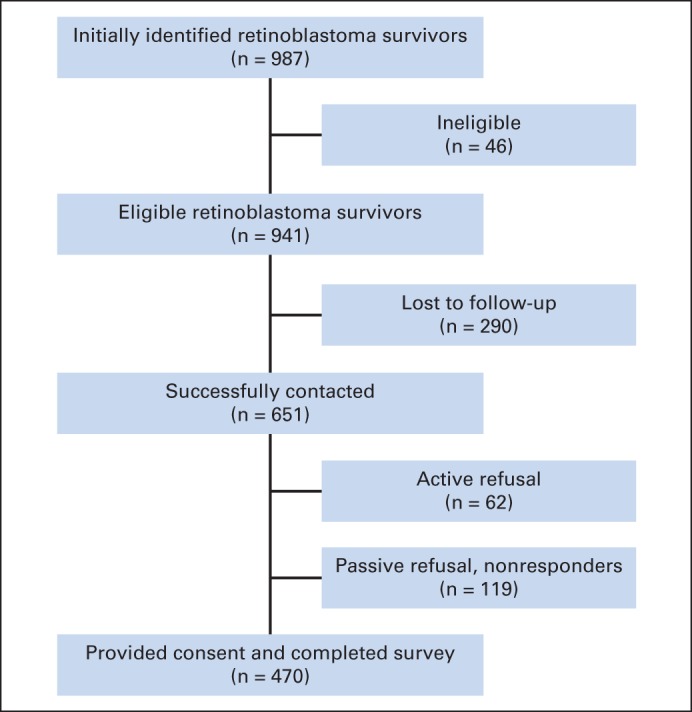
Recruitment and participation for retinoblastoma survivors.
Footnotes
Supported by the New York Community Trust, Pearl Vision, Frueauff Foundation, Perry's Promise Fund, St Jude Grant No. U24CA55727 (G.T. Armstrong, principal investigator), Memorial Sloan Kettering Cancer Grant No. P30CA008748 (C.B. Thompson, principal investigator), and Memorial Sloan Kettering Cancer Center Grant No. K05CA160724 (K.C.O., principal investigator); D.N.F. is supported in part by National Cancer Institute/National Institutes of Health Grant No. KL2TR000458 of the Clinical Trial and Translational Science Center at Weill Cornell Medical College, the intramural program of the National Institutes of Health, National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer S. Ford, Charles A. Sklar, Kevin C. Oeffinger, Mary McCabe, Ira J. Dunkel
Financial support: David H. Abramson, Ira J. Dunkel
Provision of study materials or patients: Jennifer S. Ford, Leslie L. Robison, Ruth A. Kleinerman, Ira J. Dunkel
Collection and assembly of data: Jennifer S. Ford, Joanne F. Chou, Charles A. Sklar, Kevin C. Oeffinger, Danielle Novetsky Friedman, Mary McCabe, Leslie L. Robison, Yuelin Li, Brian P. Marr, Ira J. Dunkel
Data analysis and interpretation: Jennifer S. Ford, Joanne F. Chou, Charles A. Sklar, Kevin C. Oeffinger, Danielle Novetsky Friedman, Ruth A. Kleinerman, Yuelin Li, Brian P. Marr, David H. Abramson, Ira J. Dunkel
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Psychosocial Outcomes in Adult Survivors of Retinoblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jennifer S. Ford
No relationship to disclose
Joanne F. Chou
No relationship to disclose
Charles A. Sklar
Honoraria: Sandoz
Travel, Accommodations, Expenses: Sandoz
Kevin C. Oeffinger
No relationship to disclose
Danielle Novetsky Friedman
No relationship to disclose
Mary McCabe
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Ruth A. Kleinerman
No relationship to disclose
Yuelin Li
No relationship to disclose
Brian P. Marr
No relationship to disclose
David H. Abramson
No relationship to disclose
Ira J. Dunkel
Consulting or Advisory Role: Bristol-Myers Squibb, Ipsen
Research Funding: Genentech (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Ipsen
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2009 (Vintage 2009 Populations) http://seer.cancer.gov/csr/1975_2009_pops09/
- 2.Wong FL, Boice JD, Jr, Abramson DH, et al. Cancer incidence after retinoblastoma: Radiation dose and sarcoma risk. JAMA. 1997;278:1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 3.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 4.Kleinerman RA, Tucker MA, Abramson DH, et al. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99:24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- 5.Kleinerman RA, Schonfeld SJ, Tucker MA. Sarcomas in hereditary retinoblastoma. Clin Sarcoma Res. 2012;2:15. doi: 10.1186/2045-3329-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis JH, Kleinerman RA, Seddon JM, et al. Increased risk of secondary uterine leiomyosarcoma in hereditary retinoblastoma. Gynecol Oncol. 2012;124:254–259. doi: 10.1016/j.ygyno.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dommering CJ, Garvelink MM, Moll AC, et al. Reproductive behavior of individuals with increased risk of having a child with retinoblastoma. Clin Genet. 2012;81:216–223. doi: 10.1111/j.1399-0004.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk J, Grootenhuis MA, Imhof SM, et al. Coping strategies of retinoblastoma survivors in relation to behavioural problems. Psychooncology. 2009;18:1281–1289. doi: 10.1002/pon.1507. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk J, Huisman J, Moll AC, et al. Health-related quality of life of child and adolescent retinoblastoma survivors in the Netherlands. Health Qual Life Outcomes. 2007;5:65. doi: 10.1186/1477-7525-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk J, Imhof SM, Moll AC, et al. Quality of life of adult retinoblastoma survivors in the Netherlands. Health Qual Life Outcomes. 2007;5:30. doi: 10.1186/1477-7525-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk J, Oostrom KJ, Huisman J, et al. Restrictions in daily life after retinoblastoma from the perspective of the survivors. Pediatr Blood Cancer. 2010;54:110–115. doi: 10.1002/pbc.22230. [DOI] [PubMed] [Google Scholar]
- 12.van Dijk J, Oostrom KJ, Imhof SM, et al. Behavioural functioning of retinoblastoma survivors. Psychooncology. 2009;18:87–95. doi: 10.1002/pon.1381. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub N, Rot I, Shoshani N, et al. Participation in daily activities and quality of life in survivors of retinoblastoma. Pediatr Blood Cancer. 2011;56:590–594. doi: 10.1002/pbc.22790. [DOI] [PubMed] [Google Scholar]
- 14.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 15.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derogatis L. Minneapolis, MN: NCS Pearson; 2000. BSI 18 Brief Symptom Inventory 18, Administration, Scoring and Procedures Manual. [Google Scholar]
- 18.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Joseph S. Psychometric evaluation of Horowitz's Impact of Event Scale: A review. J Trauma Stress. 2000;13:101–113. doi: 10.1023/A:1007777032063. [DOI] [PubMed] [Google Scholar]
- 20.Lesko LM, Ostroff JS, Mumma GH, et al. Long-term psychological adjustment of acute leukemia survivors: Impact of bone marrow transplantation versus conventional chemotherapy. Psychosom Med. 1992;54:30–47. doi: 10.1097/00006842-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Sundin EC, Horowitz MJ. Impact of Event Scale: Psychometric properties. Br J Psychiatry. 2002;180:205–209. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Sundin EC, Horowitz MJ. Horowitz's Impact of Event Scale evaluation of 20 years of use. Psychosom Med. 2003;65:870–876. doi: 10.1097/01.psy.0000084835.46074.f0. [DOI] [PubMed] [Google Scholar]
- 23.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 24.Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4:213–220. [PubMed] [Google Scholar]
- 25.Hilton BA. The relationship of uncertainty, control, commitment, and threat of recurrence to coping strategies used by women diagnosed with breast cancer. J Behav Med. 1989;12:39–54. doi: 10.1007/BF00844748. [DOI] [PubMed] [Google Scholar]
- 26.Program CTE. Common terminology criteria for adverse events, version 4.03. National Cancer Institute. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE4.032010-06-14QuickReference8.5x11.pdf.
- 27.Oeffinger KC, Mertens AC, Sklar CS, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuber ML, Meeske KA, Krull KR, et al. Prevalence and predictors of posttraumatic stress disorder in adult survivors of childhood cancer. Pediatrics. 2010;125:e1124–e1134. doi: 10.1542/peds.2009-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zebrack BJ, Zeltzer LK, Whitton J, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin's disease, and non-Hodgkin's lymphoma: A report from the Childhood Cancer Survivor Study. Pediatrics. 2002;110:42–52. doi: 10.1542/peds.110.1.42. [DOI] [PubMed] [Google Scholar]
- 31.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: A report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49:47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]


