Abstract
Purpose
Hot flashes are a common and debilitating symptom among survivors of breast cancer. This study aimed at evaluating the effects of electroacupuncture (EA) versus gabapentin (GP) for hot flashes among survivors of breast cancer, with a specific focus on the placebo and nocebo effects.
Patients and Methods
We conducted a randomized controlled trial involving 120 survivors of breast cancer experiencing bothersome hot flashes twice per day or greater. Participants were randomly assigned to receive 8 weeks of EA or GP once per day with validated placebo controls (sham acupuncture [SA] or placebo pills [PPs]). The primary end point was change in the hot flash composite score (HFCS) between SA and PP at week 8, with secondary end points including group comparisons and additional evaluation at week 24 for durability of treatment effects.
Results
By week 8, SA produced significantly greater reduction in HFCS than did PP (−2.39; 95% CI, −4.60 to −0.17). Among all treatment groups, the mean reduction in HFCS was greatest in the EA group, followed by SA, GP, and PP (−7.4 v −5.9 v −5.2 v −3.4; P = < .001). The pill groups had more treatment-related adverse events than did the acupuncture groups: GP (39.3%), PP (20.0%), EA (16.7%), and SA (3.1%), with P = .005. By week 24, HFCS reduction was greatest in the EA group, followed by SA, PP, and GP (−8.5 v −6.1 v −4.6 v −2.8; P = .002).
Conclusion
Acupuncture produced larger placebo and smaller nocebo effects than did pills for the treatment of hot flashes. EA may be more effective than GP, with fewer adverse effects for managing hot flashes among breast cancer survivors; however, these preliminary findings need to be confirmed in larger randomized controlled trials with long-term follow-up.
INTRODUCTION
Vasomotor symptoms, such as hot flashes, affect millions of menopausal women world wide.1 Survivors of breast cancer have more severe and persistent hot flashes because of premature menopause resulting from chemotherapy and surgery, as well as from the use of hormonal treatments, such as tamoxifen and aromatase inhibitors.2,3
The management of hot flashes in survivors of breast cancer is challenging because estrogen-based hormonal treatment is contraindicated.4 Nonhormonal-based treatments, such as selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and gabapentin (GP), provide partial relief for women.4,5 Also, many women prefer nonpharmacologic therapies to avoid adverse effects or adding to their already complex medication regimens.6
Acupuncture, a nonpharmacologic therapy, may be helpful in the management of hot flashes. Electroacupuncture (EA), in particular, has been shown to affect endorphins and other central neuropeptides, offering biologic plausibility for addressing hot flashes.7 Because acupuncture is a complex intervention that involves specific needling techniques and patient-provider interaction in the therapeutic context,8 it is challenging to evaluate its efficacy. Small and short-term trials have often found that real acupuncture has effects equivalent to sham acupuncture (SA),9–12 yet the magnitude of these effects is comparable to other active treatments.13 Previous research in pain, but not hot flashes, has found that the placebo effect in acupuncture seems to be greater than in pills.14,15 Equally intriguing is the nocebo effect, defined as the negative effects on treatment efficacy and tolerability induced or driven by psychological factors.16 We conducted this randomized controlled trial to evaluate the effects of EA versus GP on hot flashes in survivors of breast cancer, with a specific focus on placebo/nocebo responses. The predefined primary end point for the trial was change in hot flashes between SA and placebo pill (PP) at week 8 from random assignment. As a secondary objective, we evaluated the long-term durability of the effects after the period of therapy.
PATIENTS AND METHODS
Study Participants
We conducted a four-arm randomized controlled trial (EA, SA, GP, and PP) from November 2009 through June 2013 at the Abramson Cancer Center of the Hospital of the University of Pennsylvania in Philadelphia. The University of Pennsylvania Institutional Review Board approved the study protocol. Eligible patients were women with a history of early-stage breast cancer (stages I to III), free of cancer as determined by an oncologist or primary care physician, with at least two hot flashes each day during the 7-day screening period. Hot flashes had to have been present for at least a month before study enrollment and premenopausal women had to be willing to use nonhormonal contraceptives during the duration of the study. Exclusions were as follows: metastatic breast cancer, active breast cancer treatment, initiation or change in hormonal adjuvant therapy within the past 4 weeks, plans to initiate or change hormonal treatment in the coming 14 weeks, pregnancy or breast feeding, bleeding disorders or use of warfarin/heparin, an allergy to or previous use of GP for hot flashes, current use of an anticonvulsant, or documented renal failure in the past 12 months. All participants provided informed consent before random assignment.
Study Design
Participants were randomly assigned to treatment groups using computer-generated numbers sealed in opaque envelopes. Random assignment was stratified by hormonal therapy status and included random block sizes of four or eight participants. Participants were first randomly assigned to the acupuncture or pill group, followed by random assignment to active and placebo interventions.
EA
Two licensed nonphysician acupuncturists with 8 and 20 years of experience, respectively, administered interventions twice per week for 2 weeks, then once per week for 6 more weeks, for a total of 10 treatments during 8 weeks. We developed a semistandardized treatment manual on the basis of existing literature,17 with input from acupuncture experts (Data Supplement). For EA, the acupuncturist chose standard points depending on subjects' preferred positions. In addition, up to four acupuncture points were chosen on the basis of subjects' other presenting symptoms (eg, fatigue and insomnia). The needles (30 or 40 mm and 0.25 mm gauge; Seirin-America, Weymouth, MA) were inserted and manipulated until De Qi (sensation of soreness and tingling) was reported by patients.18 A bilateral 2-Hz current was connected between two acupuncture points using a transcutaneous electrical nerve stimulation unit. The needles were left in place for 30 minutes with brief manipulation at the beginning, middle, and end of therapy.
SA
Treatment was the same for SA, except for the following: We used Streitberger needles, which act like a stage dagger with the shaft of the needle retracting into the handle, creating a shortened appearance to lead patients to believe that needles were inserted into their skin.19 The acupuncturist selected the same number of nonacupuncture, nontrigger points. Instead of eliciting De Qi, the needles were minimally manipulated to avoid eliciting sensations other than the initial contact with skin. Instead of adding a small electric current to the needles, the dial of the transcutaneous electrical nerve stimulation unit was turned on to a different channel so that the subject could observe the light blinking without receiving the electricity.
GP and PPs
A total dose of 900 mg once per day was chosen for GP on the basis of its effectiveness in survivors of breast cancer.20 There was a 6-day titration phase during which participants took one pill (300 mg) at bedtime for 3 days, then twice per day for 3 days, and then three times per day for the remaining 50 days (a total of 8 weeks). At the beginning of week 9, the participants tapered the medication by taking one 300 mg pill twice per day for 3 days and then once per day for 3 more days before discontinuing. The University of Pennsylvania Investigational Drug Services purchased GP 300-mg pills and masked the medication by placing the whole intact dose into a larger opaque gelatin capsule shell. Placebo capsules were prepared by back-filling identical empty gelatin capsule shells with lactose monohydrate.
Outcome Measures and Follow-Up
The primary outcome was the once per week average hot flash composite score (HFCS) as measured by the Daily Hot Flash Diary, which is known to be reliable, valid, and responsive to treatment effects.21 The participants documented their hot flash frequency and severity each day starting from baseline until end of the intervention and then for 1 week at weeks 12 and 24. Each participant recorded the number and severity of hot flashes each day. The composite score for each day was calculated by multiplying the number of mild, moderate, severe, or very severe hot flashes by 1, 2, 3, or 4, respectively, and adding the values.21 Research staff blinded to treatment assignment monitored adverse events (AEs) using a standard AE case report form each week during the intervention phase.
Masking
The principal investigator, study investigators, patients, study staff, and the statistician were all blinded to the treatment assignments between active and placebo interventions, but not blinded to whether the patient received acupuncture or pill interventions. The acupuncturists were not blinded to EA or SA.
Statistical Analysis
The predefined primary end point for the trial was the two-group comparison of mean change in HFCS from baseline to end of intervention (week 8) between SA and PP. Because placebo response to a pill has been previously reported to be approximately 20%,20 assuming baseline HFCS had a mean of 16 with a standard deviation of 4, we needed 26 participants in each placebo group to detect a 20% difference (3.2 HFCS/day) with 80% power using a two-sided significance level of .05. Assuming a 15% dropout rate, we needed to recruit 30 subjects per arm, for a total of 120 subjects. Our trial was not designed to evaluate the efficacy of EA or GP because sample size requirements would be much larger on the basis of previous literature. Evaluations of the short- and long-term effects between EA and other groups were secondary aims.
An analysis of variance or the χ2 test was used to compare baseline variables among groups. Because primary and secondary outcome measures were repeated over time, differences of changes were assessed from baseline to weeks 8 and 24 using mixed-effects models.22 Time and group were treated as categorical variables, and a random intercept term was included in the mixed-effects model.23 Tests of intention-to-treat differences between intervention arms with respect to the change were based on time-intervention interactions in the mixed-effects models. The goodness of fit of the mixed-effects models was checked using residual plots. We did not find evidence of violations for any model assumptions and lack of fit. The impact of missing data on the results was evaluated through sensitivity analyses, including last observation carried forward and analyses of completers only. We did not find that any results differed from our intent-to-treat analyses. All analyses were two sided, with P < .05 indicating significance. Statistical analyses were conducted using STATA (version 12.0; STATA Corporation, College Station, TX) and SAS (version 9.2; SAS Institute, Cary, NC).
RESULTS
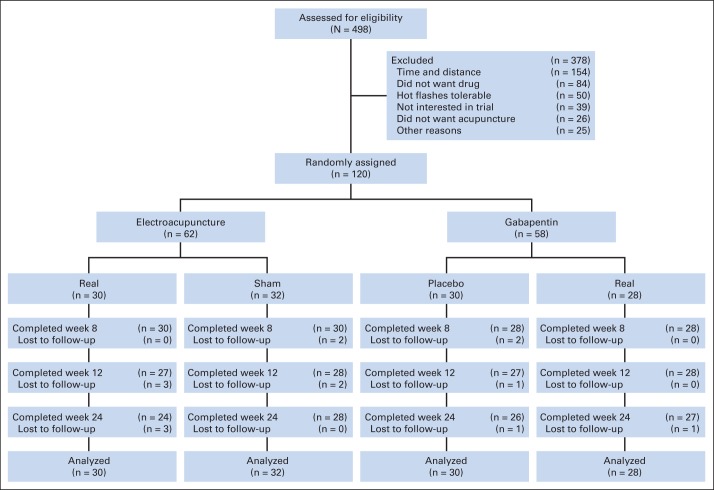
Between November 2009 and June 2013, we screened 498 patients (Fig 1); 154 (30.9%) declined enrollment because of distance or time commitment, and 84 (16.9%) and 26 (5.2%) did not want to take a drug or receive acupuncture for their hot flashes, respectively. Of 120 patients who were randomly assigned, 116 (96.7%) provided evaluable data at week 8 (primary end point) and 105 (87.5%) at week 24 from random assignment. Among participants, 27 (90.0%) in the EA group and 26 (81.2%) in the SA group received all 10 acupuncture treatments; and 21 (75.0%) in the GP group and 24 (80.0%) in the PP group were adherent to medications on the basis of pill count.
Fig 1.
CONSORT diagram.
Baseline Patient Characteristics
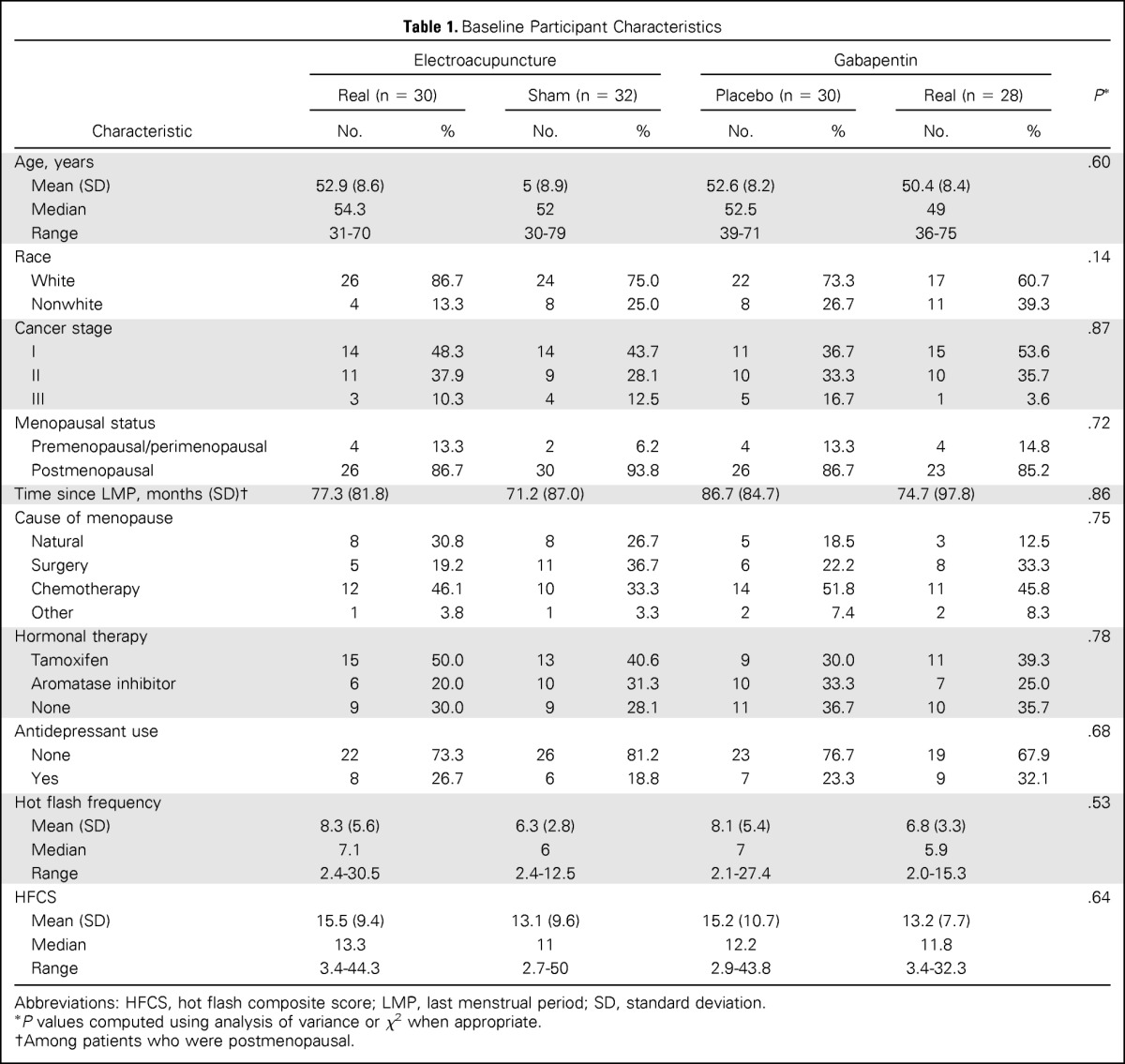
Table 1 shows baseline data for the 120 participants. The mean age of the women enrolled was 52.3 ± 8.5 years (range, 30 to 79 years); 89 (74.2) were white, and 27 (22.5%) were African American. Of the patients, 87.5% were postmenopausal, 25.0% had surgically induced menopause, 39.2% had chemotherapy-induced menopause, 67.5% were receiving a hormonal therapy, and 25.0% were receiving an antidepressant (either a selective serotonin reuptake inhibitor or a serotonin norepinephrine reuptake inhibitor). The mean HFCS was 14.3 (standard deviation, 9.4). Baseline characteristics were well balanced and not significantly different among the four groups.
Table 1.
Baseline Participant Characteristics
| Characteristic | Electroacupuncture |
Gabapentin |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Real (n = 30) |
Sham (n = 32) |
Placebo (n = 30) |
Real (n = 28) |
P* | |||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age, years | .60 | ||||||||
| Mean (SD) | 52.9 (8.6) | 5 (8.9) | 52.6 (8.2) | 50.4 (8.4) | |||||
| Median | 54.3 | 52 | 52.5 | 49 | |||||
| Range | 31-70 | 30-79 | 39-71 | 36-75 | |||||
| Race | .14 | ||||||||
| White | 26 | 86.7 | 24 | 75.0 | 22 | 73.3 | 17 | 60.7 | |
| Nonwhite | 4 | 13.3 | 8 | 25.0 | 8 | 26.7 | 11 | 39.3 | |
| Cancer stage | .87 | ||||||||
| I | 14 | 48.3 | 14 | 43.7 | 11 | 36.7 | 15 | 53.6 | |
| II | 11 | 37.9 | 9 | 28.1 | 10 | 33.3 | 10 | 35.7 | |
| III | 3 | 10.3 | 4 | 12.5 | 5 | 16.7 | 1 | 3.6 | |
| Menopausal status | .72 | ||||||||
| Premenopausal/perimenopausal | 4 | 13.3 | 2 | 6.2 | 4 | 13.3 | 4 | 14.8 | |
| Postmenopausal | 26 | 86.7 | 30 | 93.8 | 26 | 86.7 | 23 | 85.2 | |
| Time since LMP, months (SD)† | 77.3 (81.8) | 71.2 (87.0) | 86.7 (84.7) | 74.7 (97.8) | .86 | ||||
| Cause of menopause | .75 | ||||||||
| Natural | 8 | 30.8 | 8 | 26.7 | 5 | 18.5 | 3 | 12.5 | |
| Surgery | 5 | 19.2 | 11 | 36.7 | 6 | 22.2 | 8 | 33.3 | |
| Chemotherapy | 12 | 46.1 | 10 | 33.3 | 14 | 51.8 | 11 | 45.8 | |
| Other | 1 | 3.8 | 1 | 3.3 | 2 | 7.4 | 2 | 8.3 | |
| Hormonal therapy | .78 | ||||||||
| Tamoxifen | 15 | 50.0 | 13 | 40.6 | 9 | 30.0 | 11 | 39.3 | |
| Aromatase inhibitor | 6 | 20.0 | 10 | 31.3 | 10 | 33.3 | 7 | 25.0 | |
| None | 9 | 30.0 | 9 | 28.1 | 11 | 36.7 | 10 | 35.7 | |
| Antidepressant use | .68 | ||||||||
| None | 22 | 73.3 | 26 | 81.2 | 23 | 76.7 | 19 | 67.9 | |
| Yes | 8 | 26.7 | 6 | 18.8 | 7 | 23.3 | 9 | 32.1 | |
| Hot flash frequency | .53 | ||||||||
| Mean (SD) | 8.3 (5.6) | 6.3 (2.8) | 8.1 (5.4) | 6.8 (3.3) | |||||
| Median | 7.1 | 6 | 7 | 5.9 | |||||
| Range | 2.4-30.5 | 2.4-12.5 | 2.1-27.4 | 2.0-15.3 | |||||
| HFCS | .64 | ||||||||
| Mean (SD) | 15.5 (9.4) | 13.1 (9.6) | 15.2 (10.7) | 13.2 (7.7) | |||||
| Median | 13.3 | 11 | 12.2 | 11.8 | |||||
| Range | 3.4-44.3 | 2.7-50 | 2.9-43.8 | 3.4-32.3 | |||||
Abbreviations: HFCS, hot flash composite score; LMP, last menstrual period; SD, standard deviation.
P values computed using analysis of variance or χ2 when appropriate.
Among patients who were postmenopausal.
Hot Flashes Change During Active Treatment
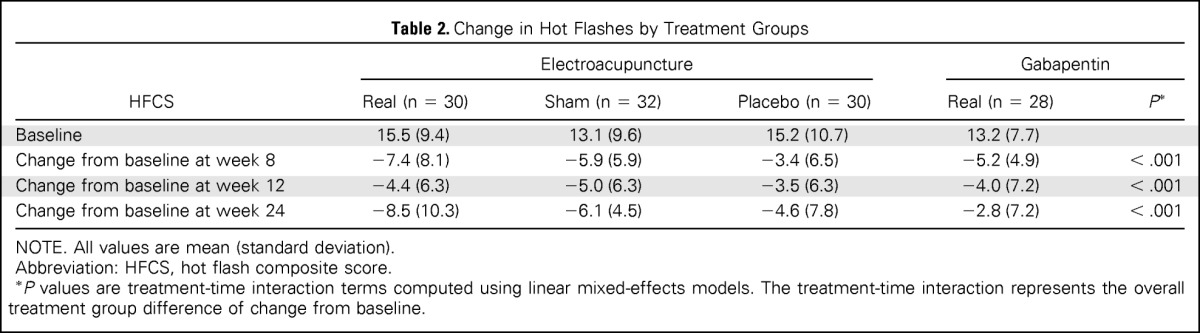
By week 8 (end of the intervention), acupuncture produced a significantly greater placebo effect than did pills, with the SA group having a significantly lower HFCS than the PP group (−2.39; 95% CI, −4.60 to −0.17; P = .035). Compared with PP, EA also had improvement in hot flashes (−4.1; 95% CI, −7.0 to −1.3; P = .005), whereas GP showed nonsignificant improvement (−1.8; 95% CI, −3.9 to 0.2; P = .085). We observed differences among treatment groups in the HFCS score (P < .001 for time and treatment interaction), with −7.4 for EA, −5.9 for SA, −5.2 for GP, and −3.4 for PP (Table 2 and Fig 2). Participants in the active treatment groups (EA and GP) experienced 47.8% and 39.4% improvement in hot flashes, respectively, compared with baseline. Placebo participants in the SA and PP groups experienced 45.0% and 22.3% improvement, respectively.
Table 2.
Change in Hot Flashes by Treatment Groups
| HFCS | Electroacupuncture |
Gabapentin |
|||
|---|---|---|---|---|---|
| Real (n = 30) | Sham (n = 32) | Placebo (n = 30) | Real (n = 28) | P* | |
| Baseline | 15.5 (9.4) | 13.1 (9.6) | 15.2 (10.7) | 13.2 (7.7) | |
| Change from baseline at week 8 | −7.4 (8.1) | −5.9 (5.9) | −3.4 (6.5) | −5.2 (4.9) | < .001 |
| Change from baseline at week 12 | −4.4 (6.3) | −5.0 (6.3) | −3.5 (6.3) | −4.0 (7.2) | < .001 |
| Change from baseline at week 24 | −8.5 (10.3) | −6.1 (4.5) | −4.6 (7.8) | −2.8 (7.2) | < .001 |
NOTE. All values are mean (standard deviation).
Abbreviation: HFCS, hot flash composite score.
P values are treatment-time interaction terms computed using linear mixed-effects models. The treatment-time interaction represents the overall treatment group difference of change from baseline.
Fig 2.
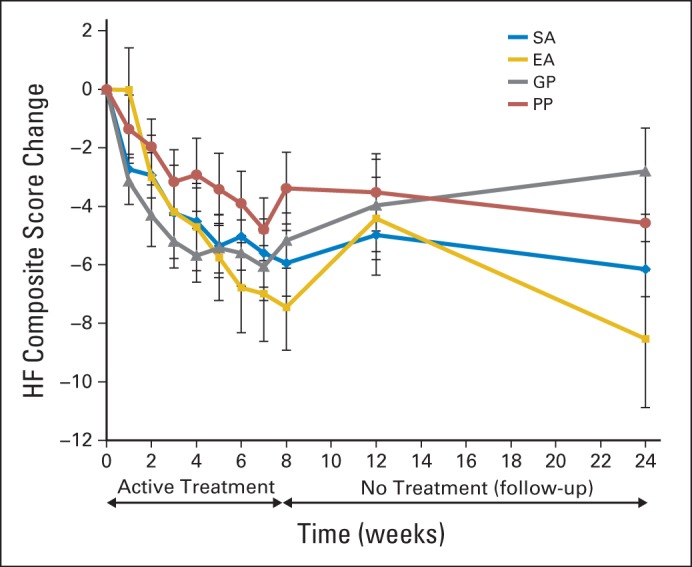
Mean change in hot flashes (HFs) by treatment groups over time. EA, electroacupuncture; GP, gabapentin; PP, placebo pill; SA, sham acupuncture.
Durability of Treatment Effects
By week 24 from random assignment, or 4 months off therapy, group differences were seen, with the EA group having the best long-term effect (−8.5), followed by SA (−6.1), PP (−4.6), and GP (−2.8), with P < .001 (Table 2). Participants in active treatment groups (EA and GP) experienced 54.8% and 21.2% improvement in hot flashes at 24 weeks, respectively. Placebo participants in SA and PP groups experienced 46.6% and 30.3% improvement at 24 weeks, respectively.
AEs
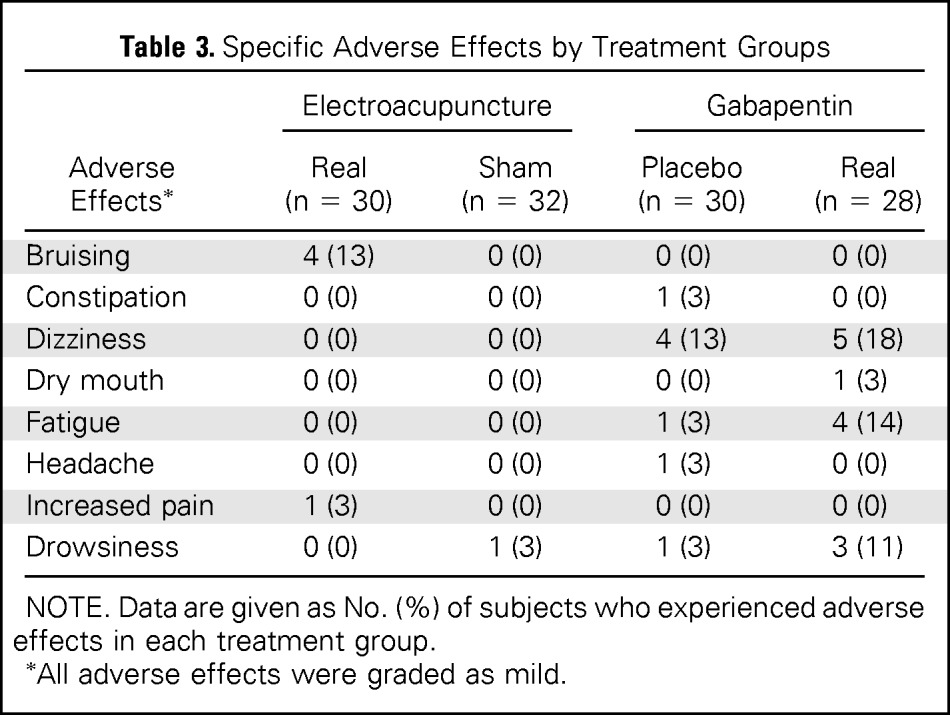
No case of infection or reports of development or worsening of lymphedema occurred in either acupuncture group. No serious AEs were observed in any treatment groups. More treatment-related AEs (Table 3) were reported in the pill groups than in the acupuncture groups (39.3% for GP, 20.0% for PP, 16.7% for EA, and 3.1% for SA), with P = .005. More AEs were reported in the PP group compared with SA (P = .05).
Table 3.
Specific Adverse Effects by Treatment Groups
| Adverse Effects* | Electroacupuncture |
Gabapentin |
||
|---|---|---|---|---|
| Real (n = 30) | Sham (n = 32) | Placebo (n = 30) | Real (n = 28) | |
| Bruising | 4 (13) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| Dizziness | 0 (0) | 0 (0) | 4 (13) | 5 (18) |
| Dry mouth | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Fatigue | 0 (0) | 0 (0) | 1 (3) | 4 (14) |
| Headache | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| Increased pain | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Drowsiness | 0 (0) | 1 (3) | 1 (3) | 3 (11) |
NOTE. Data are given as No. (%) of subjects who experienced adverse effects in each treatment group.
All adverse effects were graded as mild.
Assessment of Blinding
The proportion of individuals who guessed that they received EA versus not sure versus SA was 51.7%, 34.5%, and 13.8% for the EA group and 36.7%, 40.0%, and 23.3% for the SA group (P = .45). The proportion of individuals who guessed that they received GP versus not sure versus PP was 53.8%, 26.9%, and 19.2% for the GP group and 33.3%, 29.2%, and 37.5% for the PP group (P = .26). The percentage of participants in the SA and PP groups who believed they received true interventions was almost identical (36.7% v 33.3%; P = .50).
DISCUSSION
In this randomized, placebo-controlled trial, we found that acupuncture elicited greater placebo and smaller nocebo effects than did pills for management of hot flashes among survivors of breast cancer. EA resulted in the greatest reduction in hot flashes both at the end of treatment and 4 months off treatment among treatment groups. GP had a similar effect while receiving treatment, but not off treatment.
Placebo response to acupuncture or pills has not previously been investigated in hot flashes. A hot flash is a psychophysiologic response that occurs in menopause and can be exacerbated by premature ovarian failure or estrogen deprivation resulting from cancer therapies.1 The magnitude of placebo response in our pill group is similar to that in other drug trials.20,21 We found that acupuncture elicited a greater placebo response than did pills, consistent with observations in studies of pain.14 Although not completely understood, the enhanced placebo effect seen in acupuncture may be a combination of positive expectancy, patient-provider interaction, active patient engagement, relaxation, and light sensory stimulation by the sham needling.8,24
Nocebo effects are unwanted AEs attributed to nonspecific effects.16 Although little is known about the mechanism of the nocebo effect, research suggests that it involves different regions of brain activation and neuropeptides than does the placebo effect.25,26 We found that more AEs were reported for the PP group than for the SA group. The informed consent process itself may contribute to this degree of nocebo effect.14 The process of taking a pill once per day may have led some participants to associate normal fluctuations of daily experience (eg, fatigue) with the pill they were taking. In contrast, participants may only associate proximal events to acupuncture needling. A better understanding of what contributes to nocebo effects and how to accurately communicate risk while decreasing these effects in clinical research and practice could improve adherence to interventions.27
The durability of the therapeutic effects off treatment in acupuncture (EA or SA) has not been previously evaluated, yet offers substantial therapeutic potential. By using positron emission tomography with 11C-carfentanil, researchers found that real acupuncture produced long-term increases in μ-opioid receptor binding potential in brain structures, including the cingulate (dorsal and perigenual), caudate, and amygdale; however, these effects were not obverted for SA.28 The enhanced and durable effect observed in the SA group challenges how we should interpret the clinical relevance of acupuncture. The additional benefit of EA compared with SA was small (Cohen's d of 0.21) at week 8, but became moderate (Cohen's d of 0.31) at week 24 (16 weeks after the acupuncture treatments). This raises the question of whether EA may produce further symptom improvement beyond the treatment period; however, these preliminary findings will need to be confirmed in a follow-up study that is powered to detect such a difference.
Few trials have compared active interventions for hot flashes in survivors of breast cancer. Venlafaxine was compared with GP in a short-term cross-over trial (each for 4 weeks). The drugs produced similar effects for hot flashes, with venlafaxine preferred because of AE profiles.29 Venlafaxine was also compared with acupuncture for hot flashes in a 12-week trial among 50 participants. Both treatments had similar benefits, but acupuncture had fewer adverse effects.13 Our trial results offer a more definitive comparison in the long-term outcomes between acupuncture and GP. The Cohen's d between EA and GP groups was 0.33 at week 8 and 0.67 at week 24, indicating a moderate increased benefit of EA compared with GP. Albeit preliminary, these clinically important differences should be confirmed in future adequately powered trials.
Several limitations need acknowledgment. Without a no-treatment group, the responses cannot be separated from that of natural history or regression to the mean. Our trial was not powered to examine the efficacy of acupuncture or GP. Also, because we did not evaluate the long-term use of acupuncture versus GP, the results should not be interpreted to conclude that continued drug therapy would not be beneficial longer term. Furthermore, part of the response to the SA may be attributed to sensory stimulation of the skin30; however, SA without any skin sensation could be less credible. Last, this is a single-center trial, limiting generalizability.
In conclusion, acupuncture is associated with enhanced placebo effects and lower nocebo effects when compared with taking pills for hot flashes among survivors of breast cancer. Eight weeks of EA produced promising short- and long-term treatment outcomes for hot flashes compared with other comparators and had fewer adverse effects than GP. These preliminary findings need to be confirmed in larger studies with long-term follow-up. For survivors of breast cancer experiencing hot flashes, acupuncture could be preferable to GP because of sustained benefit after treatment and fewer adverse effects, whereas patients who dislike needles or do not have the time required for acupuncture treatments may prefer GP.
Acknowledgment
We thank our acupuncturists, Lorna Lee and Adam Schreiber, for their help in designing and delivering the interventions. We thank Christina Seluzicki, Rana Leed, Shawn Fernandes, Qing S. Li, and our student workers for their dedication to clinical trial coordination, data collection and management, and statistical programming. We also thank Kenneth Rockwell, PharmD, and the staff at the University of Pennsylvania Investigational Drug Service for their contribution. Sincere thanks also go to the patients, oncologists, nurse practitioners, and clinical staff for their support of this study.
Footnotes
Supported by National Center for Complementary and Integrative Health Grant No. K23-AT004112.
The funding agencies had no role in the design or conduct of the study. J.J.M. has full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01005108.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Jun J. Mao, Marjorie A. Bowman, Deborah Bruner, Angela DeMichele, John T. Farrar
Financial support: Jun J. Mao
Administrative support: Jun J. Mao, John T. Farrar
Collection and assembly of data: Jun J. Mao
Data analysis and interpretation: Jun J. Mao, Marjorie A. Bowman, Sharon X. Xie, John T. Farrar
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Electroacupuncture Versus Gabapentin for Hot Flashes Among Breast Cancer Survivors: A Randomized Placebo-Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jun J. Mao
No relationship to disclose
Marjorie A. Bowman
No relationship to disclose
Sharon X. Xie
No relationship to disclose
Deborah Bruner
No relationship to disclose
Angela DeMichele
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer (Inst), Genentech (Inst), Incyte (Inst), Millennium Pharmaceuticals (Inst), Bayer AG (Inst), Veridex (Inst), Calithera Biosciences (Inst), GlaxoSmithKline (Inst), Wyeth (Inst)
Travel, Accommodations, Expenses: Pfizer
John T. Farrar
Consulting or Advisory Role: Bayer Schering Pharma, Biogen Idec, Mallinckrodt Pharmaceuticals, Campbell Collaboration, Janssen Pharmaceuticals, Daiichi Sankyo, Cara Therapuetics, Novartis
Research Funding: Pfizer, Depomed
REFERENCES
- 1.Stearns V, Ullmer L, Lopez JF, et al. Hot flashes. Lancet. 2002;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter JS, Johnson D, Wagner L, et al. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 3.Harris PF, Remington PL, Trentham-Dietz A, et al. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage. 2002;23:501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 4.Stearns V. Clinical update: New treatments for hot flashes. Lancet. 2007;369:2062–2064. doi: 10.1016/S0140-6736(07)60959-3. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao JJ, Leed R, Bowman MA, et al. Acupuncture for hot flashes: Decision making by breast cancer survivors. J Am Board Fam Med. 2012;25:323–332. doi: 10.3122/jabfm.2012.03.110165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ. 2005;330:1202–1205. doi: 10.1136/bmj.330.7501.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter JS, Neal JG. Other complementary and alternative medicine modalities: Acupuncture, magnets, reflexology, and homeopathy. Am J Med. 2005;118:109–117. doi: 10.1016/j.amjmed.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 10.Deng G, Vickers A, Yeung S, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25:5584–5590. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 11.Vincent A, Barton DL, Mandrekar JN, et al. Acupuncture for hot flashes: A randomized, sham-controlled clinical study. Menopause. 2007;14:45–52. doi: 10.1097/01.gme.0000227854.27603.7d. [DOI] [PubMed] [Google Scholar]
- 12.Bao T, Cai L, Snyder C, et al. Patient-reported outcomes in women with breast cancer enrolled in a dual-center, double-blind, randomized controlled trial assessing the effect of acupuncture in reducing aromatase inhibitor-induced musculoskeletal symptoms. Cancer. 2014;120:381–389. doi: 10.1002/cncr.28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: A randomized controlled trial. J Clin Oncol. 2010;28:634–640. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 14.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: Randomised controlled trial of two placebo treatments. BMJ. 2006;332:391–397. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner K, Fassler M, Rucker G, et al. Differential effectiveness of placebo treatments: A systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941–1951. doi: 10.1001/jamainternmed.2013.10391. [DOI] [PubMed] [Google Scholar]
- 16.Bingel U. Avoiding nocebo effects to optimize treatment outcome. JAMA. 2014;312:693–694. doi: 10.1001/jama.2014.8342. [DOI] [PubMed] [Google Scholar]
- 17.Huang MI, Nir Y, Chen B, et al. A randomized controlled pilot study of acupuncture for postmenopausal hot flashes: Effect on nocturnal hot flashes and sleep quality. Fertil Steril. 2006;86:700–710. doi: 10.1016/j.fertnstert.2006.02.100. [DOI] [PubMed] [Google Scholar]
- 18.Mao JJ, Farrar JT, Armstrong K, et al. De qi: Chinese acupuncture patients' experiences and beliefs regarding acupuncture needling sensation—An exploratory survey. Acupunct Med. 2007;25:158–165. doi: 10.1136/aim.25.4.158. [DOI] [PubMed] [Google Scholar]
- 19.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364–365. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 20.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 23.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 24.Bauml J, Xie SX, Farrar JT, et al. Expectancy in real and sham electroacupuncture: Does believing make it so? J Natl Cancer Inst Monogr. 2014;2014:302–307. doi: 10.1093/jncimonographs/lgu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetti F, Amanzio M, Vighetti S, et al. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colloca L, Benedetti F. Nocebo hyperalgesia: How anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–439. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 27.Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA. 2012;307:567–568. doi: 10.1001/jama.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RE, Zubieta JK, Scott DJ, et al. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009;47:1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147–5152. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]
- 30.Langevin HM, Wayne PM, Macpherson H, et al. Paradoxes in acupuncture research: Strategies for moving forward. Evid Based Complement Alternat Med. 2011;2011:180805. doi: 10.1155/2011/180805. [DOI] [PMC free article] [PubMed] [Google Scholar]