Abstract
Chlamydia trachomatis is the causative agent of the most frequently reported bacterial sexually transmitted infection, the total burden of which is underestimated due to the asymptomatic nature of the infection. Untreated C. trachomatis infections can cause significant morbidities, including pelvic inflammatory disease and tubal factor infertility (TFI). The human immune response against C. trachomatis, an obligate intracellular bacterium, is poorly characterized but is thought to rely on cell-mediated immunity, with CD4+ and CD8+ T cells implicated in protection. In this report, we present immune profiling data of subjects enrolled in a multicenter study of C. trachomatis genital infection. CD4+ and CD8+ T cells from subjects grouped into disease-specific cohorts were screened using a C. trachomatis proteomic library to identify the antigen specificities of recall T cell responses after natural exposure by measuring interferon gamma (IFN-γ) levels. We identified specific T cell responses associated with the resolution of infection, including unique antigens identified in subjects who spontaneously cleared infection and different antigens associated with C. trachomatis-related sequelae, such as TFI. These data suggest that novel and unique C. trachomatis T cell antigens identified in individuals with effective immune responses can be considered as targets for vaccine development, and by excluding antigens associated with deleterious sequelae, immune-mediated pathologies may be circumvented.
INTRODUCTION
Chlamydia trachomatis is the leading cause of sexually transmitted bacterial infections around the world; in 2008, the World Health Organization (WHO) estimated that >105 million new cases occurred annually worldwide (1). More than 1.4 million new cases were reported in the United States in 2013 (2). The actual number of C. trachomatis infections is likely to be significantly higher, because many infections are asymptomatic and remain undiagnosed; it has been estimated that there are closer to 2.8 million C. trachomatis infections annually in the United States (3). The diagnosis of C. trachomatis infection is critical, since untreated infections have the potential to cause significant morbidity, often with debilitating consequences: in addition to acute cervicitis, up to 10 to 15% of infected women develop clinical pelvic inflammatory disease (PID), with further long-term sequelae, such as tubal factor infertility (TFI) or ectopic pregnancy, reported in up to 15% of women with PID (4). Despite accurate diagnostic tests and effective therapy, the continued high prevalence of C. trachomatis infections and associated complications underscore the need for a broad prophylactic intervention. A safe and effective C. trachomatis vaccine might have a profound impact on the prevention of disease, a reduction in the rate of C. trachomatis-related complications, and reduce the transmission of C. trachomatis infections.
There have been no human clinical trials for the assessment of vaccines against C. trachomatis genital infection, and vaccination trials directed against trachoma, the ocular form of the disease, have not been successful. The trachoma vaccine trials were conducted using whole inactivated C. trachomatis elementary body (EB) preparations, which induced only partial and short-lived protection against infection (5). Importantly, these studies also reported evidence of disease exacerbation, likely due to immune-mediated inflammation and tissue damage (6). These deleterious responses could not be unequivocally recapitulated in subsequent nonhuman primate studies (7). Recently, a live attenuated plasmid-cured C. trachomatis vaccine strain delivered by the ocular route to macaques showed promising protection against trachoma without exacerbated disease; however, this vaccine was most protective in primates sharing a single major histocompatibility complex class II (MHC II) allele (8, 9). Therefore, the prospect of an inactivated or attenuated EB-based vaccine remains viable; however, it would require substantial additional development prior to evaluation in human studies.
Subunit C. trachomatis vaccines offer a theoretically safer and more well-defined approach to the prevention of genital infection, and several experimental subunit vaccines have been evaluated in animal models, resulting in various levels of protection against experimental challenge (reviewed in reference 10). Collectively, these studies demonstrated that bacterial clearance is achieved by TH1-biased T cells and that a successful vaccine will likely need to induce both interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) responses (11, 12). While murine studies have shown the critical importance of CD4+ T cells in the resolution of experimental C. trachomatis genital infection (13), the relative contributions of CD4+ and CD8+ T cells to protection in humans are currently being defined. Indeed, natural history studies can be ethically conducted only with concomitant treatment with antibiotics, an intervention that has been theorized to arrest the development of a fully functional cell-mediated immune response against C. trachomatis (14).
The limited success of prior vaccination strategies attempted thus far highlights the need for the identification of additional C. trachomatis T cell antigens. This can best be achieved by selecting antigens that stimulate robust IFN-γ responses, are conserved across the major C. trachomatis serogroups, are recognized by individuals with diverse HLA types, and are capable of stimulating both CD4+ and CD8+ T cells. The partial protection induced by C. trachomatis infection suggests that several T cell antigenic responses may be required to properly prime sufficient T cells with an effector phenotype that home to the genital mucosa. Measuring and understanding the breadth of the T cell immune response in subjects who have successfully and naturally cleared their C. trachomatis infection is an advancement toward the identification of novel protective antigens.
Clinical evidence strongly suggests that such a protective immune response can be stimulated in some subjects. Human subjects can resolve acute infection, resist infection upon reexposure, and develop immunity to repeated infections over time (15, 16). However, at the same time, the immune response is also thought to contribute to enhanced pathology in the form of heightened inflammation and tubal scarring in the female reproductive tract, potentially leading to infertility (17). While the nature of the effectors and the specificities associated with protection and disease exacerbation are not known, it is plausible that both are associated with immune priming against distinct sets of C. trachomatis antigens, a hypothesis that readily can be tested by differential immunoprofiling of human subjects.
ATLAS, a high-throughput T cell antigen identification platform, has led to the development of candidate vaccines for the treatment of herpes simplex virus 2 (HSV-2) (18) and prevention of Streptococcus pneumoniae colonization (19), both of which are currently being evaluated in human clinical trials. ATLAS has also been applied to the identification of C. trachomatis antigens in mice; the C. trachomatis screening library was first used to identify the specificity of a protective T cell line generated from infected mice (20) and later used to identify the entire repertoire of immunodominant CD4+ and CD8+ T cell antigens elicited during experimental intraperitoneal C. trachomatis infection. When separately formulated with adjuvant as protein subunit vaccines, two of the antigens identified in mice elicited antigen-specific T cells capable of conferring protection against C. trachomatis genital challenge in a mouse infection model (21). While these data were encouraging, antigen specificities and mechanisms of infection may be very disparate between inbred mice and humans; therefore, we hypothesized that the best antigen candidates would be identified through profiling the specificity and nature of T cell responses in samples from human volunteers that have spontaneously cleared natural infection with C. trachomatis.
Here, we describe the application of the ATLAS technology to profile T cell responses in human subjects with well-characterized outcomes to natural genital C. trachomatis infection. Specifically, the IFN-γ-mediated CD4+ and CD8+ T cell responses to C. trachomatis exposure or infection were evaluated to identify antigens that were associated with either resolution of infection or induction of pathology. While other approaches have been used to identify C. trachomatis-specific T cell antigens, they have not comprehensively analyzed the C. trachomatis proteome (22–24), and, to our knowledge, no studies have identified human T cell antigens that may correlate with the pathologies associated with C. trachomatis infection. By identifying antigens frequently recognized by subjects who can control C. trachomatis infections in addition to antigenic proteins correlating with enhanced disease, the profiling of effective T cell immunity in human subjects may lead to a directed means of selecting the appropriate C. trachomatis antigens for inclusion in a safe and effective subunit vaccine.
MATERIALS AND METHODS
Library composition, construction, and validation.
Library construction and validation were conducted using a methodology similar to one described previously (18). Briefly, 901 unique predicted annotated open reading frames (ORFs) from C. trachomatis serovar D strain UW-3/Cx (25–27) and seven annotated ORFs from the cryptic plasmid (28) were PCR amplified and ligated into a customized expression vector, pDESTSL8, by Gateway recombination. Where present, signal peptides were removed from the gene sequence, and all stop codons were eliminated.
The resulting expression clones contained the inserted ORF flanked by an N-terminal His6 tag and a C-terminal OVA257–264 (SIINFEKL) epitope tag (where OVA stands for ovalbumin) that was used for validation of full-length protein expression. The individual plasmids were transformed into two separate Escherichia coli strains; for the CD8 library, the E. coli strains contained an episome encoding a cytoplasmic variant of listeriolysin O (cLLO) a pore-forming protein derived from Listeria monocytogenes. The addition of cLLO facilitates phagolysosomal escape of the target protein into the cytoplasm, permitting antigen processing and presentation of peptides in the context of MHC I. The CD4+ version of the library was generated by transfection into E. coli that did not contain the cLLO plasmid.
Protein expression was induced by arabinose and isopropyl-β-d-thiogalactopyranoside (IPTG), and full-length protein expression of the individual E. coli transfectants was confirmed by a T cell hybridoma assay, as previously described (18). E. coli clones that did not express to full length were reinduced, tested for expression, and rearrayed together in the library. The final validated libraries contained 960 and 953 clones for the CD4+ and CD8+ libraries, respectively. The induced and validated clones were then fixed with 0.5% paraformaldehyde, washed, and resuspended at 109/ml. Fixed libraries were arrayed into multiple identical copies in 384-well poly-d-lysine-coated plates and frozen at −80°C until ready for use. Similarly, E. coli clones expressing green fluorescent protein (GFP) were induced, fixed, and interspersed throughout the library as negative controls (18). Multiple identical copies of the validated library were aliquoted and frozen for screening.
Study participants.
One hundred forty-one subjects, with 28 (22%) males and 110 (78%) females between the ages of 15 and 60 years, were recruited at five clinics across the United States. The subjects were recruited at Jefferson County Department of Health STD Clinic in Birmingham, AL; Arkansas Children's Hospital Research Institute in Little Rock, AR; San Francisco Department of Public Health STD Prevention and Control Services in San Francisco, CA; University of North Carolina School of Medicine in Chapel Hill, NC; and Women's Health Practice in Champaign, IL. Subjects who were coinfected with gonorrhea or HIV, suffered from immune disorders, had T cell deficiency, used high-dose steroids, or were pregnant were not included in the study. One hundred twenty of the subjects who were exposed to C. trachomatis through an infected partner or had active or previous C. trachomatis infections were classified into one of seven cohorts based on the severity and duration of their infection and evidence of immune control. Twenty-one subjects who had no known previous C. trachomatis exposure or infection were classified as naive. Spontaneously cleared subjects were those who were confirmed C. trachomatis positive by a genital nucleic acid amplification test (NAAT) and who had cleared their infection by the time they returned to the clinic for treatment, based on repeat NAAT. Subjects classified as having persistent infection were those confirmed to be C. trachomatis positive by genital NAAT screening and who remained infected at the time they returned to the clinic for treatment. Exposed subjects were exposed to a C. trachomatis-infected partner but did not become infected, as determined by genital NAAT testing. Subjects considered for this cohort were enrolled when their C. trachomatis-infected partners were evaluated for inclusion in the study by confirmation of active C. trachomatis infection. Unprotected C. trachomatis exposure was determined by medical interview and a questionnaire. Pelvic inflammatory disease (PID) subjects were confirmed to be C. trachomatis positive and were clinically diagnosed with PID based on history, symptoms, and manual examination findings, as defined by the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/std/treatment/2010/pid.htm). Subjects assigned to the tubal factor infertility (TFI) cohort had previous C. trachomatis infections and were clinically diagnosed as having C. trachomatis-associated TFI. The successful pregnancy cohort had experienced one or more C. trachomatis infections, followed by at least one successful pregnancy. Spontaneously cleared and exposed subjects were classified as having an effective immune response. Subjects with sustained persistent or recurring infections or who progressed to clinically diagnosed PID were defined as having an ineffective immune response. Subjects with TFI due to documented previous C. trachomatis infection were separately compared with subjects who had a history of C. trachomatis infection but a subsequent successful pregnancy.
Blood processing.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood by Ficoll gradient sedimentation. The blood was diluted 1:2 with phosphate-buffered saline (PBS), overlaid onto Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ), and centrifuged at 400 × g at 18 to 25°C for 30 min. The mononuclear cell layer was harvested and washed 3 times with PBS. PBMC were resuspended at 107 PBMC/ml in freezing medium composed of T cell medium (TCM) (43% RPMI 1640, 43% Alpha-minimum essential medium [MEM], and 10% heat-inactivated fetal bovine serum [ΔFBS] supplemented with 1% nonessential amino acids [NEAA], 1 mM sodium pyruvate, 10 mM HEPES, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 U penicillin, and 100 μg/ml streptomycin), combined with 50% ΔFBS and 10% dimethyl sulfoxide (DMSO), and frozen at −80°C, followed by storage in the vapor phase of liquid nitrogen (LN2).
T cell and monocyte enrichment and culture.
All medium components and supplements were obtained from Life Technologies (San Diego, CA). Freshly thawed PBMC were added to TCM containing 50 U/ml Benzonase (Millipore, Billerica, MA) and washed twice with prewarmed TCM prior to magnetic bead sorting using autoMACS Pro separator with Miltenyi microbeads and reagents (Miltenyi GmbH, Teterow, Germany). Monocytes were positively selected using CD14+ microbeads, according to the manufacturer's instructions. CD14+ cells were cultured in TCM and supplemented every other day with 300 U/ml recombinant human granulocyte macrophage-cerebrospinal fluid (rhGM-CSF) and 400 U/ml recombinant human interleukin 4 (rhIL-4) (R&D Systems, Minneapolis, MN) for 6 to 7 days to stimulate the maturation of monocyte-derived dendritic cells (MDDC) for use as antigen-presenting cells in the library screen. To enrich for T cells, the CD14+-depleted PBMC fraction was further depleted of all non-T cells using the Miltenyi (San Diego, CA) Pan T isolation kit. The enriched T cell fraction was then subjected to magnetic separation to isolate CD8+ T cells, and the depleted fraction was determined to be enriched for CD4+ T cells. The isolated CD8+ and CD4+ T cells were nonspecifically expanded with anti-CD3/CD28 human T-Activator Dynabeads and 20 U/ml recombinant human IL-2 for 6 to 7 days in T cell expansion medium (AIM-V, 5% ΔFBS with 1% non-NEAA, 1 mM sodium pyruvate, 2 mM l-glutamine, and 50 μM β-mercaptoethanol). Fresh medium containing IL-2 was added 2 and 4 days after stimulation. The cells were rested for 24 h with no cytokine, and the expansion beads were removed ≥4 h before the cell-based assay.
T cell screens.
MDDC were harvested by physical dislodgment in cold PBS, resuspended in TCM, and added to the prearrayed E. coli expression libraries at a target cell ratio of 1:100 MDDC to bacteria. This corresponded to about 3,000 MDDC per well of a 384-well plate. After 2 h of incubation at 37°C, the MDDC-bacterium layer was fixed with 0.1% paraformaldehyde (PFA) and washed with 120 mM lysine buffer, followed by extensive washing with PBS. The expanded T cells were harvested, washed, and resuspended in TCM; 4 × 104 T cells were added to each well of the pulsed MDDC and cocultured for 24 h, after which cell-free supernatants were harvested. Positive-control wells contained T cells stimulated with 1 μg/ml phorbol 12-myristate 13-acetate (PMA) and 20 μM ionomycin. Negative-control wells contained E. coli expressing GFP. The supernatants were assayed in duplicate using the BD OptEIA human IFN-γ enzyme-linked immunosorbent assay (ELISA) kit, modified for use in 384-well format. The IFN-γ concentration in the supernatant was interpolated from a standard curve included on each ELISA plate using a linear regression analysis with SoftMax Pro version 6.1.
Statistical analysis.
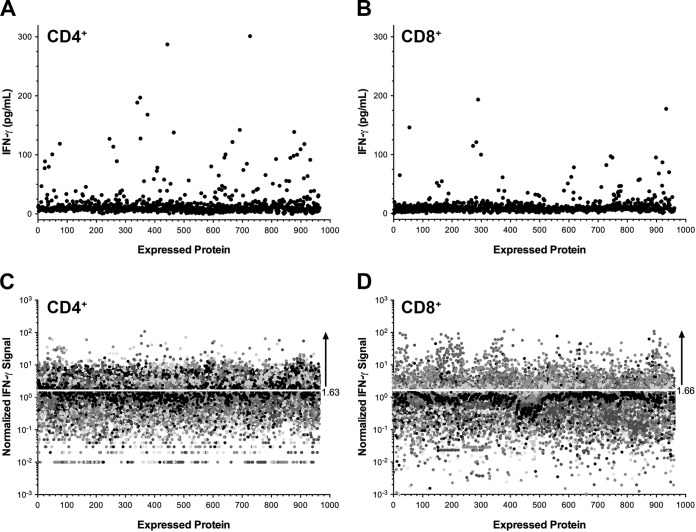
The gamma interferon concentrations calculated by interpolation from the standard curve were examined under the following principles: (i) we imputed values of 0.1 pg/ml to IFN-γ titers that did not reach the limit of detection (LOD); (ii) the data were transformed using a natural logarithm transform to ensure that no heteroscedasticity was apparent in fitting residuals from a linear model, including cohort, subject, and plate as fixed effects; (iii) the negative controls in the assay (wells containing bacteria that expressed GFP) were used to normalize the data by subtracting the average of logarithms of the negative controls for a plate within a subject from each observation. This normalization strategy resulted in a nearly normally distributed data set, with excess positive kurtosis (leptokurtosis). We therefore used a nonparametric estimate of positive signal using cutoffs that resulted, on average, in a ≤10% misassignment error of a negative signal for a positive signal (type I error). These cutoffs were found to be 0.4894 and 0.5086 for the CD4+ and CD8+ data sets, respectively. In their natural logarithm transforms, these cutoffs correspond to 1.63- and 1.66-fold for CD4+ and CD8+, respectively, as shown in Fig. 2. A subject-clone value was classified as a responding signal if the fold ratio of the value over negative control was greater than these thresholds. Percent responders with <10% type I error indicated a higher number of responders than that due to chance alone, with statistical significance (alpha < 0.05) reached when the frequency of responses was >15% (all subjects) or 19% (in comparing only effective and noneffective immune responses).
FIG 2.
T cell response profiling of study subjects against a C. trachomatis-expressed protein library. (A and B) Representative CD4+ (A) and CD8+ (B) T cell responses measured by IFN-γ ELISA against C. trachomatis proteome libraries expressed in E. coli and pulsed onto autologous MDDC. The average IFN-γ value in pg/ml for each clone in the library is represented by a single closed circle. (C and D) Aggregate CD4+ (C) and CD8+ (D) IFN-γ ELISA profiles for all study subjects were generated by log-normal transformation of the IFN-γ value and subsequent normalization of the IFN-γ value to the geometric mean response to an irrelevant antigen (GFP). The threshold used for the determination of positive IFN-γ responses is indicated by a white line at 1.63-fold (for CD4+) and 1.66-fold (for CD8+) signal ratios.
Frequency analysis by cohort.
The antigen-specific T cell response to each expressed protein was quantified by IFN-γ concentration. The interpolated IFN-γ concentrations for each clone were compiled by cohort and analyzed for frequency of response across the cohorts. The distribution of the entire data set, including the GFP (negative) controls, was used to define parameters for the normalization of data across all subjects. To correct for variability between assays and subjects within the data set, the data were log transformed and normalized by subtracting the average of the GFP controls by plate within each subject. In a comparison of cohorts, in contrast to the population evaluation, the cutoff for significance was 19%. Subsequent to identification of the antigens, the frequency of antigen recognition for each protein (responders) was compared between cohorts. Statistically significant differences in the frequency of responses to antigens between protected and unprotected cohorts were determined by Fisher's exact test.
Study approval.
The study protocol, informed consent forms, patient information questionnaires, and advertising materials were reviewed and approved by institutional review boards (IRBs). Studies conducted at Women's Health Practice and San Francisco Department of Public Health were reviewed by Quorum Review, Seattle, WA. Studies conducted at the University of Alabama, the University of North Carolina, and the Arkansas Children's Research Hospital were reviewed by each institution's respective IRB.
RESULTS
Participant demographics and cohort assignments.
For this study, we classified subjects based upon their C. trachomatis infection status at enrollment and their previous exposure history. Specifically, study participants with a diagnosis or history of documented C. trachomatis infection(s) received pelvic examinations and nucleic acid amplification testing (NAAT) for C. trachomatis. Participants also provided blood samples, completed detailed questionnaires regarding their STI history, and received treatment when indicated. An additional cohort of C. trachomatis-naive individuals was also recruited. Clinical evaluation and medical histories collected at the time of enrollment were used to assign 141 subjects to one of seven unique cohorts, as shown in Fig. 1. Ninety-one subjects with acute disease or documented exposure were grouped to reflect the characteristics of their immune response to infection or exposure. Effective or ineffective immune responses were determined by a persistence of infection at follow-up evaluation(s) or by severity of disease. Fifty-four subjects able to either (i) spontaneously clear a documented infection or (ii) remain uninfected despite repeated exposure from an infected partner were classified as having an effective immune response (Fig. 1). In contrast, the 37 subjects who were classified as having an ineffective immune response either (i) sustained persistent infection or experienced recurring infections or (ii) progressed to clinically diagnosed PID. In addition, to evaluate if antigen-specific T cell responses could be associated with infertility, 29 subjects with a history of multiple previous C. trachomatis infections but not Neisseria gonorrhoeae infections were recruited. Of these, 16 participants had developed C. trachomatis-attributed TFI, while 13 subjects did not develop TFI and had a subsequent successful pregnancy(ies).
FIG 1.
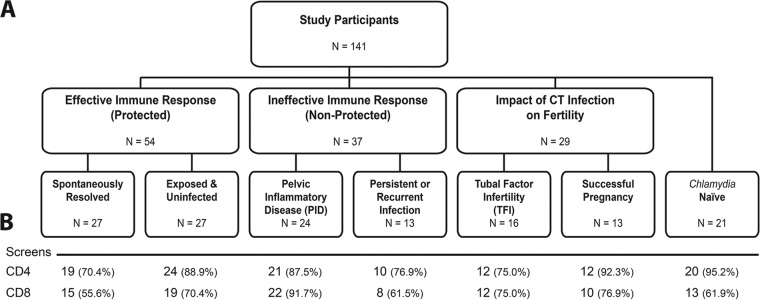
Schematic depiction of the subject cohorts analyzed in this study. (A) The number of subjects in each cohort is indicated in the corresponding box. Multiple cohorts were grouped together for analysis. Subjects who spontaneously cleared their C. trachomatis (CT) infection or were not infected despite repeated exposure were grouped in the effective immune response cohort, subjects who exhibited persistent C. trachomatis infection and/or progressed to PID were grouped in the ineffective immune response, and subjects with documented C. trachomatis exposure that progressed to TFI or were able to complete a pregnancy successfully were grouped in a fertility cohort. A group of subjects, presumably nonexposed to C. trachomatis, were also recruited as naive controls. (B) Numbers and percentages of subject samples screened for T cell responses (CD4+ and CD8+) for each cohort.
Overall, 141 subjects were recruited from five geographically diverse urban areas in the continental United States. The summarized demographics of each cohort are shown in Table 1. The subject ages ranged from 15 to 60 years, and the median age was 25.5 years. The median age of subjects with effective immune responses was greater than that of subjects with ineffective immune responses (30 years versus 25 years, respectively; P = 0.038), consistent with previous findings that increased age is a factor in resistance to C. trachomatis infection (29, 30). The majority of the study subjects (78%) were women, due to the inclusion of cohorts related to PID and the impact on pregnancy, which limits participation to female subjects. Race designation was self-reported and in some instances was not completed on the questionnaires; 51.8% of the enrolled subjects were black and 37% were white. The remaining 11.2% included those with unreported race and one Asian subject.
TABLE 1.
Study participants and demographic information
| Cohort | No. of subjects | No. of sites | Subject information |
|||||
|---|---|---|---|---|---|---|---|---|
| Gender (%) |
Age (median [range]) (yr) | Race (%) |
||||||
| Female | Male | Black | White | Other or unknown | ||||
| Effective immune response | ||||||||
| Spontaneously resolved | 27 | 2 | 96 | 4 | 25 (19–40) | 70 | 19 | 11 |
| Exposed | 27 | 3 | 19 | 81 | 31 (22–60) | 22 | 59 | 19 |
| Total | 54 | 4 | 52 | 48 | 30 (19–60) | 46 | 39 | 15 |
| Ineffective immune response | ||||||||
| PID | 24 | 5 | 100 | 0 | 27 (20–53) | 50 | 42 | 8 |
| Persistent infection | 13 | 1 | 100 | 0 | 23 (17–27) | 77 | 23 | 0 |
| Total | 37 | 5 | 100 | 0 | 25 (17–53) | 59 | 35 | 6 |
| Impact on pregnancy | ||||||||
| Successful pregnancy | 13 | 4 | 100 | 0 | 27 (19–37) | 62 | 23 | 15 |
| TFI | 16 | 3 | 100 | 0 | 24 (21–40) | 69 | 25 | 6 |
| Chlamydia naive | 21 | 2 | 76 | 24 | 19 (15–23) | 33 | 53 | 14 |
T cell library screen.
The two separate E. coli-expressed C. trachomatis proteomic libraries generated for screening CD4+ and CD8+ T cells contained 908 annotated predicted ORFs (25–27). A subset of the ORFs were duplicates, and the final CD4+ and CD8 T cell libraries contained 960 and 953 expressed ORFs, respectively. The CD8+ T cell library clones also coexpressed an episomal nonsecreted variant of listeriolysin-O (cLLO), whose function is to drive MHC I antigen processing. Full-length protein expression was validated by a surrogate T cell activation assay, directed toward the murine ovalbumin-derived SIINFEKL epitope inserted in-frame at the C terminus of each ORF, upstream of the stop codon (18).
CD4+ and CD8+ T cells were isolated from peripheral blood mononuclear cells (PBMC) of each study participant, nonspecifically expanded, and then cocultured with autologous monocyte-derived dendritic cells (MDDC) prepulsed with the E. coli proteomic libraries. The number of MDDC derived from PBMC samples was the limiting factor in the cell-based screens; consequently, in instances in which MDDC numbers were insufficient, only one T cell subset was screened. The numbers of T cell subsets and subjects screened per cohort are summarized in Fig. 1. Antigen-specific T cell recognition was measured by the secretion of IFN-γ into the supernatant during overnight incubation and quantified by ELISA. A representative screen from one subject is shown in Fig. 2A and B, which depicts results from CD4+ and CD8+ library screens, respectively. Each value represents the average IFN-γ value of duplicate wells per expressed protein. The IFN-γ levels induced by the majority of the expressed proteins were near or below the limit of detection (LOD) of the assay, defined by half the concentration of the lowest measurable standard curve point (set at 3 pg/ml). To control for extrinsic experimental factors, such as immune activation status of the donors or cell culture yields, the individual IFN-γ titers were compared to the response measured against an irrelevant antigen (green fluorescent protein [GFP]) distributed at specific positions on each of the assay plates. IFN-γ values were normalized by log-normal (ln) transformation, followed by subtraction of the geometric mean GFP values from each individual assay plate. The normalized IFN-γ distributions for all subjects in the study are depicted in Fig. 2C and D. Considered in total, the majority of the responses to the 960 proteins across all subjects were below the set threshold seen by ≥15% of all subjects positive for T cell antigen responses, as described in Materials and Methods. Across all screened subjects, 270 proteins were identified as CD4+ T cell antigens, and 565 were identified as CD8+ T antigens, representing 30% and 59% of the C. trachomatis proteome, respectively.
Repertoire of T cell-specific antigens differs between protected and unprotected cohorts.
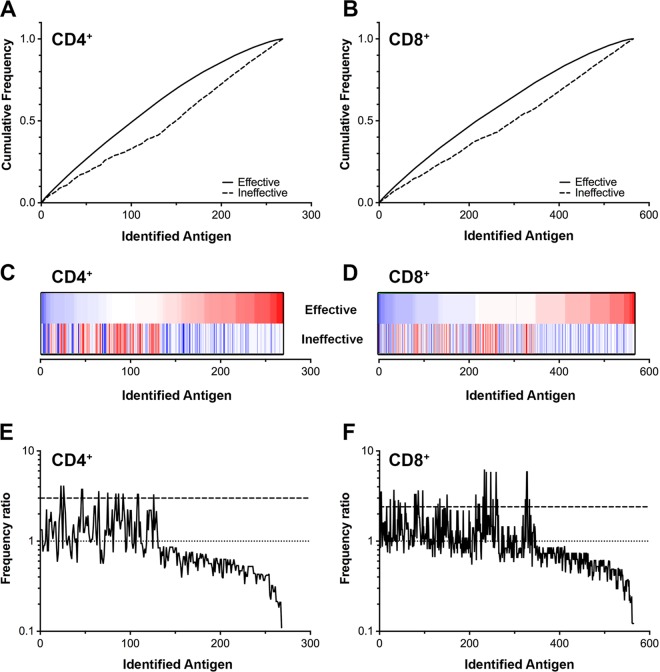
The relative frequency of responses to antigens recognized by CD4+ and CD8+ T cells was compared between the protected and unprotected groups (Fig. 3). The antigens were ordered from highest to lowest frequency in the protected group for the CD4+ (Fig. 3A) and CD8+ (Fig. 3B) T cell subsets. The cumulative frequency plots illustrated a statistically significant difference in the recognition of antigens between protected and unprotected groups (P < 0.005 by Kolmogorov-Smirnov test). The frequency of response to each antigen, in the same order as depicted in Fig. 3A and B, is shown in corresponding heat maps in Fig. 3C and D. The maximum frequency of response to any antigen in the CD4+ T cell screens was 33% (range, 0 to 33%) and was 44% (range, 0 to 44%) for CD8+ T cells (Fig. 3C and D). The disparate patterns of response frequency in the protected and unprotected groups confirm that unique immune recognition repertoires are associated with different clinical outcomes. To identify a set of antigens that were most strongly associated with an effective immune response, a frequency ratio was calculated by dividing the frequency of antigen recognition in the effective group by the recognition frequency in the ineffective group (Fig. 3E and F). Ratios of >3 in favor of the effective response for CD4+ T cells (Fig. 3E) or 2.4 for CD8+ T cells (Fig. 3F) correspond to statistically significant differences between groups (P < 0.05 by Fisher's exact test).
FIG 3.
Response frequency analysis of T cell responses against expressed C. trachomatis proteins. The cumulative frequency of antigens recognized by >15% of CD4+ (A) or CD8+ (B) T cells from all subjects. The frequencies of responses have been arranged in order of increasing frequency for the individuals with effective immune responses (solid line) and the corresponding frequencies for the individuals with ineffective immune responses (dashed line). (C and D) Heat maps of individual response frequencies (ordered by effective immune response) for CD4+ (C) (blue, 35%; red, 0%) and CD8+ (D) (blue, 35%; red, 0%) T cells, respectively. (E and F) Individual frequency ratios calculated by R = f(effective)/f(ineffective) in CD4+ (E) and CD8+ (F), with associated statistical significance thresholds at 3.0 and 2.4, respectively (dashed line). The dotted line represents the baseline ratio, which defines positive versus negative correlation.
A comparison of the frequency of T cell responses to each antigen within each immune cohort yielded a set of antigens associated with protective immunity (listed in Table 2). There were 18 CD8+ and 8 CD4+ T cell antigens that were statistically associated with protection. Different antigens stimulated CD4+ and CD8+ T cells with no overlap, suggesting that the determinants of a successful response are unique to each T cell subset and may include both CD4+ and CD8+ responses. These antigens elicited T cell responses in 17 to 35% of the subjects in the protected cohorts. The names and functions ascribed to the protein antigens are shown in Table 2 and represent a wide range of bacterial proteins.
TABLE 2.
Antigens associated with effective immune responses
| Gene ID by T cell type | Name | Antigen | % response | P value |
|---|---|---|---|---|
| CD4+ | ||||
| CT043 | Slc1 gene | Type III secretion chaperone | 21.7 | 0.05 |
| CT564 | yys | Yop translocation protein T | 17.8 | 0.05 |
| CT572 | gspF | Type II secretion pathway protein | 22.7 | 0.05 |
| CT703 | yphC | GTP-binding protein | 17.8 | 0.05 |
| CT724 | Hypothetical protein | 17.8 | 0.05 | |
| CT725 | birA | Biotin [acetyl-CoA-carboxylase] ligase | 22.7 | 0.05 |
| CT757 | mraY | Phospho-N-acetylmuramoyl-transferase | 25.0 | 0.01 |
| CT856 | ychM | Sulfate transporter | 25.0 | 0.01 |
| CD8+ | ||||
| CT002 | gatC | Glutamyl-tRNA amidotransferase | 25.7 | 0.05 |
| CT142 | ruvB | Holliday junction DNA helicase | 19.5 | 0.05 |
| CT062 | tyrS | Tyrosyl-tRNA synthetase | 19.5 | 0.05 |
| CT064 | lepA | Membrane GTPase | 24.4 | 0.05 |
| CT067 | ytgA | ABC transport protein, binding component | 28.2 | 0.05 |
| CT137 | ywlC | Possible SuA5 family protein, translation | 19.5 | 0.05 |
| CT140 | ypdP | Uncharacterized protein | 24.4 | 0.05 |
| CT037 | Hypothetical protein | 26.8 | 0.05 | |
| CT177 | dsbG | Protein disulfide isomerase | 35.0 | 0.01 |
| CT188 | tdk | Thymidylate kinase | 35.0 | 0.01 |
| CT204 | ybhI | Dicarboxylate translocator | 19.5 | 0.05 |
| CT252 | lgt | Prolipoprotein diacylglyceryl transferase | 25.7 | 0.05 |
| CT366 | aroA | 3-Phosphoshikimate-1-carboxyvinyltransferase | 29.3 | 0.01 |
| CT570 | gspF | General secretion pathway protein F | 28.6 | 0.01 |
| CT578 | copB | Copper-exporting P-type ATPase B | 25.7 | 0.01 |
| CT634 | nqrA | NADH-ubiquinone oxidoreductase | 19.5 | 0.01 |
| CT635 | Hypothetical protein | 19.5 | 0.01 | |
| CTp1 | orf7 | Plasmid virulence factor | 30.0 | 0.01 |
Distinct patterns of antigen-specific CD4+ and CD8+ T cell responses are associated with TFI.
Infertility attributed to C. trachomatis infection is a significant cause of morbidity, and the development of immune-mediated TFI has been associated with antibody responses to specific antigens, such as heat shock protein 60 (HSP60) and major outer membrane protein (MOMP) (31). In this study, we asked if antigen-specific CD4+ or CD8+ T cell responses might also be associated with TFI. While the association would not necessarily be causative, T cell responses to certain antigens might be predictors of persistent infection or the development of C. trachomatis-mediated pathologies. In a comparison with the other cohorts, a small number of T cell antigens might be associated with TFI. Table 3 lists 5 CD4+-specific and 2 CD8+-specific T cell antigens that were most frequently recognized by subjects with C. trachomatis-associated TFI (22.7 to 31.8%) compared with all other cohorts (5 to 12.5%).
TABLE 3.
Antigens associated with tubal factor infertility
| Gene ID by T cell type | Name | Antigen | % response | P value |
|---|---|---|---|---|
| CD4+ | ||||
| CT476 | Hypothetical protein | 31.8 | 0.005 | |
| CT440 | Hypothetical protein | 27.3 | 0.035 | |
| CT203 | hyp | Hypothetical protein | 31.8 | 0.046 |
| CD8+ | ||||
| CT802 | rpsR | 30S ribosomal protein S18 | 31.8 | 0.048 |
| CT788 | Hypothetical protein | 22.7 | 0.017 |
Frequency of CD4+ and CD8+ T cell responses between cohorts.
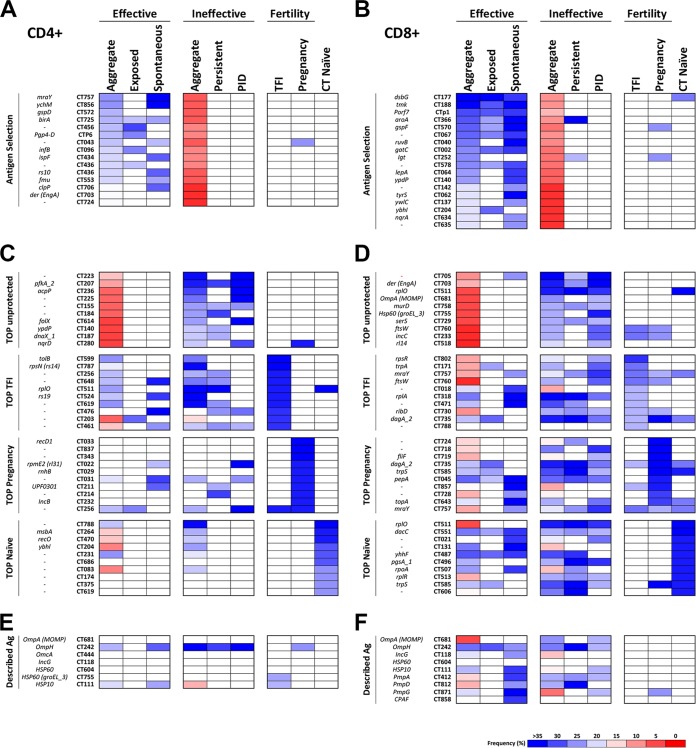
Infection or exposure to C. trachomatis can result in symptomatic or asymptomatic infection that either resolves spontaneously or persists undetected. Without an effective immune response or timely antibiotic treatment, persistent infections may progress to PID. We defined clinical cohorts based upon control or resolution of infection and progression to more advanced disease. Figure 4 illustrates the unique antigens identified in the aggregate effective and ineffective immune groups and the antigen response frequencies in the contributing cohorts.
FIG 4.
Antigen identities and T cell response frequencies for each experimental cohort. The frequencies of subjects responding to selected or described genes are compared to the frequencies of responses in individual cohorts. The frequency ranges are displayed as a blue-to-red heat map. The white squares indicate that the frequency of response against that antigen has failed to meet the selection threshold. The aggregate frequencies of responses to antigens associated with an effective or ineffective immune responses are shown, ordered in rank from high to low occurrence. (A and B) CD4+ (A) and CD8+ (B) T cell responses. (C and D) Same information for TFI, successful pregnancy, and naive cohorts for CD4+ (C) and CD8+ (D). (E and F) Frequency distribution of a select group of antigens previously described in the literature as immunodominant or potentially immunoprotective for all cohorts for CD4+ (E) and CD8+ (F). All standardized C. trachomatis gene identifiers, along with colloquial gene names, when available, are provided.
We first compared the T cell frequencies of antigens that were statistically significantly associated with effective immune responses (spontaneously cleared and exposed uninfected) with those that were associated with ineffective immune responses (Table 2). The 18 CD8+ antigens (Fig. 4B) replicate those in Table 2; however, the 8 statistically significant CD4+ antigens from Table 2 are supplemented with an additional 7 CD4+ antigens that were approaching significance (Fig. 4A). The majority of these antigens associated with the effective immunity cohort were not identified as T cell targets in the ineffective immunity or fertility cohorts. In addition, there was no overlap between antigens identified as CD4+ T cell targets compared with the targets of CD8+ T cell responses (Fig. 4A and B). T cell antigens associated with effective immune responses (protected) were found at a high frequency in spontaneous resolvers and exposed uninfected subjects but not in those who developed PID or sustained persistent infections.
The impact of C. trachomatis infection on fertility and TFI was also investigated in these screens. TFI has been associated with antibodies to MOMP and chlamydial HSP60 (26); however, associations with T cell antigens have not been definitively established. The frequencies of T cell antigens that were most often identified in the fertility cohorts and naive subjects are indicated in Fig. 4C and D. For illustration purposes, the aggregate and cohort-specific frequencies of responses to the top 10 antigens identified in the TFI (TOP TFI), successful pregnancy (TOP Pregnancy), and naive (TOP Naive) cohorts are ranked, rather than limiting the information to those antigens that were statistically overrepresented in the TFI cohort. Several of the top-associated TFI antigens were represented in both the effective and ineffective cohorts and therefore would not be selected as antigens associated with protection. Likewise, there were antigens that were frequently associated with successful pregnancy that were not found to be associated with other clinical outcomes. There were a greater number of CD8+ T cell antigens that overlapped between these cohorts than CD4+ T cell antigens (Fig. 4C and D). There were also antigen-specific T cell responses identified in the presumed naive cohort, which may reflect cross-specificity with other Chlamydia species, such as Chlamydia pneumoniae, or a previously undocumented infection.
Efforts to develop subunit C. trachomatis vaccines have been concentrated on proteins located on the surface of the EB outer membrane or the inclusion body. Other proteins, such as the plasmid protein pgp3, chlamydial protease-like activity factor (CPAF), and HSP, have also been evaluated in animal models (11). The frequency of T cell responses to a group of these described antigens is included in Fig. 4E and F. As was seen in the fertility cohorts, there was a greater frequency of CD8+ T cell responses to this group of antigens (Fig. 4F) than the CD4+ responses (Fig. 4E). In addition, the frequencies of responses to well-defined antibody targets, such as MOMP, were fairly low. Interestingly, when anti-MOMP T cell responses were present, they were associated with ineffective rather than protective immunity, with the highest frequency in the PID cohort (Fig. 4F).
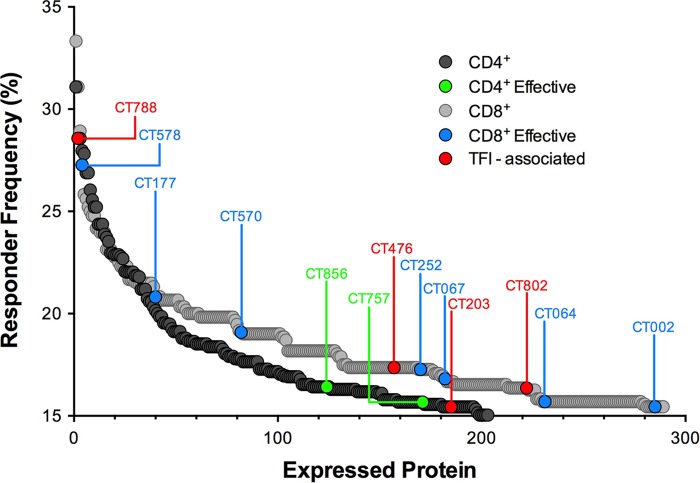
Immunodominant antigen responses do not overlap antigens recognized in effective immune responses.
A comparison of the frequencies of the top antigens recognized by individuals with effective immune responses and antigens recognized by >15% of all study subjects shows that most immunodominant antigens are not associated with protection (Fig. 5). Of interest, CT788, a CD8+ T cell antigen statistically associated with TFI, was close to being the most immunodominant antigen. Therefore, it appears that immunodominance by itself is not a suitable determinant of productive immune responses.
FIG 5.
Immunodominant antigens are not correlated with effective responses against C. trachomatis. Frequency distribution plot of antigens recognized in >15% of all subjects for CD4+ (gray circles) and CD8+ (black circles) T cell responses ordered from high to low frequency. Antigens associated (P < 0.05) with effective immune responses in the CD4+ compartment (green circles), CD8+ compartment (blue circles), and TFI (red circles) are highlighted.
DISCUSSION
The clinical course of human genital C. trachomatis infections encompasses a broad spectrum of asymptomatic, acute, and chronic clinical presentations. Although other factors, such as the genital microbiome (32), coinfection with other pathogens (33, 34), hormone-based contraceptives (35), and timing of antibiotic therapy (14), may impact the burden of disease, clinical presentation is also reflective of the quality of the innate and adaptive immune responses. A variety of outcomes have been defined, ranging from one extreme of protective immunity to subsequent infections to the other extreme of sequelae, such as TFI. Individuals who are incapable of mounting protective immune responses may have misdirected responses to decoy immunodominant antigens. An ineffective immune response may also predispose an individual to repeated infections, a risk factor for more serious sequelae, such as PID or TFI (36). In this study, we examined the entire C. trachomatis proteome to identify antigen specificities of human CD4+ and CD8+ T cell responses, as defined by recall IFN-γ secretion, which are either immunodominant, unique to the clinical resolution of C. trachomatis infection, or associated with immune-mediated inflammation and tubal scarring. Disparate immune signatures were identified based upon clinical evidence of disease severity and recovery.
In this study, a comprehensive frequency analysis of IFN-γ responses yielded antigenic signatures that were unique to the immune and fertility cohorts and disparate from immunodominant antigens. Interestingly, antigens that were correlated with immune protection did not exclusively fall into predicted antigen categories, such as outer membrane, secreted, or surface-expressed proteins, or virulence factors. The identified T cell antigens included hypothetical proteins and membrane proteins that are expressed throughout all stages of the developmental cycle and do not represent proteins localized to any unique subcellular compartment, which is consistent with previously reported findings (37).
It was unexpected that many of the previously described antigens were not prioritized in this study. T cell responses to these antigens were indeed identified in the ATLAS screens; in fact, CD4+ T cell responses were measured to OmpH and HSP10, and CD8+ T cell responses were measured to MOMP, OmpH, IncG, HSP10, PmpA, PmpD, PmpG, and CPAF but not HSP60. However, none of the responses to these antigens could be correlated with protective immunity, suggesting that they may be immunodominant but not protective. This outcome may not be surprising, given that many of the previously defined T cell antigens were identified in mouse models (38–40) or upon searches for T cell responses to defined antibody targets based upon the principle of linked recognition (41). Even more unexpected was the association of CD8+ T cell responses with antigens, such as OmpA (MOMP) and HSP60 (groEL), with ineffective immune responses. There were no substantial CD4+ T cell responses measured to these previously defined antigens, with the exception of CD4+ T cell responses to groEL in the TFI cohort, supporting the hypothesis that responses to this antigen are associated with negative sequelae related to C. trachomatis infection.
Of the antigens that were prioritized by the ATLAS screens, only one, CT043 (Slc1), which was prioritized as a protective CD4+ T cell target, had been identified as a human T cell target and subsequently studied as a vaccine antigen. It was originally described as a C. trachomatis T cell antigen in screens of human PBMC (37, 42, 43) and later elicited protective responses in mice when administered as a subunit vaccine (44). In those studies, mice immunized with CT043 protein plus CAF01 adjuvant generated antibodies that bound to the surface of C. trachomatis but did not neutralize the bacteria, and CD4+ T cell responses that reduced the bacterial burden in the lower reproductive tract by approximately 1 log after challenge with the human C. trachomatis serovar D, compared with the control group. These data are promising but also suggest that more than one antigen will be required in a subunit vaccine for optimal vaccine efficacy.
A separate examination of the fertility cohorts revealed that a group of T cell antigens was statistically associated with C. trachomatis-attributable TFI (Table 3). However, this group did not include expected targets, such as HSP60 (CD4+ T cell responses were measured to this antigen in subjects comprising the TFI cohort, but they did not segregate with the infertility cohort compared to the other cohorts). It is possible that this is because the majority of the human studies characterizing HSP60 responses were focused on antibodies (45–48), although previous studies have reported T cell responses based on proliferation as well (49). One of the T cell targets that was prioritized as a protective antigen in this study from the CD8+ T cell screens, CT634 (nqrA), has been implicated as an antigen associated with reactive arthritis in HLA-B27-expressing subjects (50), but it was not identified as a T cell target in the infertility cohort. Interestingly, all of the statistically prioritized CD4+ TFI antigens are hypothetical proteins, and only one of the CD8+ TFI antigens is a known protein. The small number of significant antigens identified in this cohort may be a reflection of multiple C. trachomatis exposures leading to the huge diversity of T cell responses in subjects with advanced disease.
The general consensus in the field, based predominantly on mouse studies, is that CD4+ T cells secreting IFN-γ are responsible for the resolution of infections (30). In this study, we identified a group of antigens that elicited CD4+ T cell responses that segregated with protective immune responses, as expected. However, we also identified a group of antigens that were uniquely recognized in the CD8+ T cell screens and that also elicited greater frequency responses overall than those identified with the CD4+ T cell subset. These data suggest that in humans, IFN-γ-secreting CD8+ T cells may also play a critical role in protection from disease. It is curious that (i) there was a greater number of CD8+ T cell antigens that elicited responses that were statistically more frequent in the protected cohorts than in unprotected cohorts, and (ii) there was no overlap between the top CD8+ and CD4+ T cell antigens. One hypothesis to explain the observation is that the different T cell subsets play unique roles in protection that are temporally or spatially associated with the unique life cycle of this bacterium. T cells producing other cytokines, such as IL-10, IL-17, IL-22, and IL-23, might also play a role in either protective or deleterious immune responses to C. trachomatis infection; however, the evidence in human subjects is sparse and inconclusive. In mice, the presence or deletion of IL-17, IL-22, and IL-23 in experimental genital Chlamydia muridarum infection does not significantly impact the resolution of infection or the development of oviduct pathology (51). On the other hand, the preponderance of existing literature clearly points to IFN-γ as the major immune effector. The secretion of increased levels of IL-17 and IL-22 has been reported in cervical washes of C. trachomatis-infected women (52); however, PBMC samples from C. trachomatis-infected women produce C. trachomatis-specific IFN-γ but not IL-17 (53). The mucosal localization of cytokine responses other than IFN-γ further validates the use of IFN-γ as the readout for antigen-specific responses in the ATLAS screening. As described above, other groups have conducted human studies designed to identify C. trachomatis-specific antigens, resulting in different outcomes. Comparisons of T cell identification platforms and their strengths and weaknesses were discussed in in a recent review (54). The reasons for the identification of novel and unpredicted antigens may lie in the uniqueness of the ATLAS approach. First, the ATLAS screens are comprehensive, such that every antigen predicted to be expressed by the pathogen is interrogated individually using autologous antigen-presenting cells and T cells from each subject. This eliminates guesswork and skewing of data due to predictions based on bioinformatics (37) or epitope algorithms (55). Second, these screens were based on T cell reactivity after natural exposure rather than determining if previously identified antibody targets are also T cell targets (56, 57). Third, CD4+ and CD8+ T cell antigen recognition profiles were determined separately and independently from one another. The contribution of IFN-γ-producing CD4+ T cells to the clearance of C. trachomatis infection and induction of memory responses is generally accepted; however, the roles of CD8+ T cells are less well understood (30). Future work to evaluate the function and nature of the antigens identified from each subset may provide insight into the resolution of infection or pathologies associated with C. trachomatis infection. Fourth, by delivering full-length protein antigens to autologous antigen-presenting cells, each subject's own antigen-processing machinery will naturally process and present epitopes in the context of their own MHC I or II molecules. This represents a more natural means of antigen presentation than exogenous loading of preprocessed overlapping peptides. Finally, the depth in discriminating antigen-specific responses based on the clinical outcome of the donors is unique to this proteomic screen for C. trachomatis-specific responses. This is the first study in which genetically diverse human subjects with well-characterized outcomes to natural genital C. trachomatis infection or exposure (any serovar) were used to identify T cell antigens associated with protective and, conversely, pathological T cell responses.
In conclusion, we have presented a comprehensive T cell immune signature of human clinical genital C. trachomatis infection that reflects immune competence. The unique T cell recognition patterns stratified by resolution of infection highlight the importance of effective immune priming of T cells in the genital mucosa to specific antigens likely to lead to a favorable clinical outcome. In addition, we have shown that the most frequently recognized antigens across all subjects exposed to or infected with C. trachomatis may fall into any of the disease cohorts, suggesting that immunodominance may not be predictive of resolution of infection. The practical application of these antigen signatures to vaccine development may yield vaccine formulations that could provide immune protection to C. trachomatis infection and significantly aid in the prevention and control of the C. trachomatis infection epidemic.
ACKNOWLEDGMENTS
We thank E. W. Hook III for thoughtful discussions on the project and for critical review of the manuscript. We also thank Amy Schurlock (University of Arkansas Children's Hospital, Little Rock, AR), Suzanne Trupin (Women's Health Practice, Champaign, IL), Peter Leone (University of North Carolina, Chapel Hill, NC), and Jeffrey Klausner (San Francisco Department of Health, San Francisco, CA) for enrollment, sample collection, and coordination. We also thank Yana Ostrovsky and Judith Jacques for their assistance with the molecular biology work associated with this project. Finally, we thank Frank Gohs for help with the statistical analysis of the profiling data.
M.D.P., J.-L.B., A.L., J.P., V.C., D.E.H., G.R.S., and J.B.F. disclose a financial interest in Genocea Biosciences, Inc.
REFERENCES
- 1.WHO. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2014. Sexually transmitted disease surveillance 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/stats13/surv2013-print.pdf. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2011. CDC Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep 60:370–373. [PubMed] [Google Scholar]
- 4.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. 2010. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 201(Suppl 2):S134–S155. [DOI] [PubMed] [Google Scholar]
- 5.Sowa S, Sowa J, Collier LH, Blyth WA. 1969. Trachoma vaccine field trials in The Gambia. J Hyg (Lond) 67:699–717. doi: 10.1017/S0022172400042157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabey DC, Hu V, Bailey RL, Burton MJ, Holland MJ. 2014. Towards a safe and effective chlamydial vaccine: lessons from the eye. Vaccine 32:1572–1578. doi: 10.1016/j.vaccine.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol 182:8063–8070. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kari L, Goheen MM, Randall LB, Taylor LD, Carlson JH, Whitmire WM, Virok D, Rajaram K, Endresz V, McClarty G, Nelson DE, Caldwell HD. 2011. Generation of targeted Chlamydia trachomatis null mutants. Proc Natl Acad Sci U S A 108:7189–7193. doi: 10.1073/pnas.1102229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivares-Zavaleta N, Whitmire WM, Kari L, Sturdevant GL, Caldwell HD. 2014. CD8+ T cells define an unexpected role in live-attenuated vaccine protective immunity against Chlamydia trachomatis infection in macaques. J Immunol 192:4648–4654. doi: 10.4049/jimmunol.1400120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farris CM, Morrison RP. 2011. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun 79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison SG, Farris CM, Sturdevant GL, Whitmire WM, Morrison RP. 2011. Murine Chlamydia trachomatis genital infection is unaltered by depletion of CD4+ T cells and diminished adaptive immunity. J Infect Dis 203:1120–1128. doi: 10.1093/infdis/jiq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SG, Su H, Caldwell HD, Morrison RP. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun 68:6979–6987. doi: 10.1128/IAI.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison SG, Morrison RP. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect Immun 69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunham RC, Rekart ML. 2008. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 35:53–54. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 15.Geisler WM, Wang C, Morrison SG, Black CM, Bandea CI, Hook EW III. 2008. The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis 35:119–123. doi: 10.1097/OLQ.0b013e318151497d. [DOI] [PubMed] [Google Scholar]
- 16.Geisler WM, Lensing SY, Press CG, Hook EW III. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 207:1850–1856. doi: 10.1093/infdis/jit094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Hartog JE, Morré SA, Land JA. 2006. Chlamydia trachomatis-associated tubal factor subfertility: immunogenetic aspects and serological screening. Hum Reprod Update 12:719–730. doi: 10.1093/humupd/dml030. [DOI] [PubMed] [Google Scholar]
- 18.Long D, Skoberne M, Gierahn TM, Larson S, Price JA, Clemens V, Baccari AE, Cohane KP, Garvie D, Siber GR, Flechtner JB. 2014. Identification of novel virus-specific antigens by CD4(+) and CD8(+) T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology 464–465:296–311. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Gierahn T, Thompson CM, Trzcinski K, Ford CB, Croucher N, Gouveia P, Flechtner JB, Malley R, Lipsitch M. 2012. Distinct effects on diversifying selection by two mechanisms of immunity against Streptococcus pneumoniae. PLoS Pathog 8:e1002989. doi: 10.1371/journal.ppat.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roan NR, Gierahn TM, Higgins DE, Starnbach MN. 2006. Monitoring the T cell response to genital tract infection. Proc Natl Acad Sci U S A 103:12069–12074. doi: 10.1073/pnas.0603866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard MD, Cohane KP, Gierahn TM, Higgins DE, Flechtner JB. 2012. High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine 30:4387–4393. doi: 10.1016/j.vaccine.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, Meoni E, Bonci A, Agnusdei M, Nardelli F, Bartolini E, Scarselli M, Caproni E, Laera D, Zedda L, Skibinski D, Giovinazzi S, Bastone R, Ianni E, Cevenini R, Grandi G, Grifantini R. 2011. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc Natl Acad Sci U S A 108:9969–9974. doi: 10.1073/pnas.1101756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen AW, Follmann F, Højrup P, Leah R, Sand C, Andersen P, Theisen M. 2007. Identification of human T cell targets recognized during Chlamydia trachomatis genital infection. J Infect Dis 196:1546–1552. doi: 10.1086/522524. [DOI] [PubMed] [Google Scholar]
- 24.Karunakaran KP, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, Gabel BR, Yu H, Foster LJ, Brunham RC. 2008. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol 180:2459–2465. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- 25.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 26.Carlson JH, Porcella SF, McClarty G, Caldwell HD. 2005. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun 73:6407–6418. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen KT, Petersen L, Falk S, Iversen P, Andersen P, Theisen M, Krogh A. 2006. Novel overlapping coding sequences in Chlamydia trachomatis. FEMS Microbiol Lett 265:106–117. doi: 10.1111/j.1574-6968.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 28.Comanducci M, Ricci S, Cevenini R, Ratti G. 1990. Diversity of the Chlamydia trachomatis common plasmid in biovars with different pathogenicity. Plasmid 23:149–154. doi: 10.1016/0147-619X(90)90034-A. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb SL, Brunham R, Byrne GI, Martin DH. 2010. Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J Infect Dis 201(Suppl 2):S190–S204. [DOI] [PubMed] [Google Scholar]
- 30.Darville T, Hiltke TJ. 2010. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201(Suppl 2):S114–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hjelholt A, Christiansen G, Johannesson TG, Ingerslev HJ, Birkelund S. 2011. Tubal factor infertility is associated with antibodies against Chlamydia trachomatis heat shock protein 60 (HSP60) but not human HSP60. Hum Reprod 26:2069–2076. doi: 10.1093/humrep/der167. [DOI] [PubMed] [Google Scholar]
- 32.Brotman RM. 2011. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberts CJ, Schim van der Loeff MF, Papenfuss MR, da Silva RJ, Villa LL, Lazcano-Ponce E, Nyitray AG, Giuliano AR. 2013. Association of Chlamydia trachomatis infection and herpes simplex virus type 2 serostatus with genital human papillomavirus infection in men: the HPV in men study. Sex Transm Dis 40:508–515. doi: 10.1097/OLQ.0b013e318289c186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanover J, Sun J, Deka S, Kintner J, Duffourc MM, Schoborg RV. 2008. Herpes simplex virus co-infection-induced Chlamydia trachomatis persistence is not mediated by any known persistence inducer or anti-chlamydial pathway. Microbiology 154:971–978. doi: 10.1099/mic.0.2007/012161-0. [DOI] [PubMed] [Google Scholar]
- 35.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. 2006. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol 26:222–232. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 36.Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. 1997. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol 176:103–107. doi: 10.1016/S0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 37.Follmann F, Olsen AW, Jensen KT, Hansen PR, Andersen P, Theisen M. 2008. Antigenic profiling of a Chlamydia trachomatis gene-expression library. J Infect Dis 197:897–905. doi: 10.1086/528378. [DOI] [PubMed] [Google Scholar]
- 38.Coler RN, Bhatia A, Maisonneuve JF, Probst P, Barth B, Ovendale P, Fang H, Alderson M, Lobet Y, Cohen J, Mettens P, Reed SG. 2009. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS Immunol Med Microbiol 55:258–270. doi: 10.1111/j.1574-695X.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina DM, Pal S, Kayala MA, Teng A, Kim PJ, Baldi P, Felgner PL, Liang X, de la Maza LM. 2010. Identification of immunodominant antigens of Chlamydia trachomatis using proteome microarrays. Vaccine 28:3014–3024. doi: 10.1016/j.vaccine.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz-Fisher MI, Cheng C, Sun G, Pal S, Teng A, Molina DM, Kayala MA, Vigil A, Baldi P, Felgner PL, Liang X, de la Maza LM. 2011. Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infect Immun 79:246–257. doi: 10.1128/IAI.00626-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker DC. 1993. T cell-dependent B cell activation. Annu Rev Immunol 11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 42.Olsen AW, Follmann F, Jensen K, Højrup P, Leah R, Sørensen H, Hoffmann S, Andersen P, Theisen M. 2006. Identification of CT521 as a frequent target of Th1 cells in patients with urogenital Chlamydia trachomatis infection. J Infect Dis 194:1258–1266. doi: 10.1086/508203. [DOI] [PubMed] [Google Scholar]
- 43.Meoni E, Faenzi E, Frigimelica E, Zedda L, Skibinski D, Giovinazzi S, Bonci A, Petracca R, Bartolini E, Galli G, Agnusdei M, Nardelli F, Buricchi F, Norais N, Ferlenghi I, Donati M, Cevenini R, Finco O, Grandi G, Grifantini R. 2009. CT043, a protective antigen that induces a CD4+ Th1 response during Chlamydia trachomatis infection in mice and humans. Infect Immun 77:4168–4176. doi: 10.1128/IAI.00344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen AW, Andersen P, Follmann F. 2014. Characterization of protective immune responses promoted by human antigen targets in a urogenital Chlamydia trachomatis mouse model. Vaccine 32:685–692. doi: 10.1016/j.vaccine.2013.11.100. [DOI] [PubMed] [Google Scholar]
- 45.Wagar EA, Schachter J, Bavoil P, Stephens RS. 1990. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis 162:922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- 46.Brunham RC, Peeling R, Maclean I, Kosseim ML, Paraskevas M. 1992. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis 165:1076–1081. doi: 10.1093/infdis/165.6.1076. [DOI] [PubMed] [Google Scholar]
- 47.Toye B, Laferrière C, Claman P, Jessamine P, Peeling R. 1993. Association between antibody to the chlamydial heat-shock protein and tubal infertility. J Infect Dis 168:1236–1240. doi: 10.1093/infdis/168.5.1236. [DOI] [PubMed] [Google Scholar]
- 48.Arno JN, Yuan Y, Cleary RE, Morrison RP. 1995. Serologic responses of infertile women to the 60-kd chlamydial heat shock protein (hsp60). Fertil Steril 64:730–735. [DOI] [PubMed] [Google Scholar]
- 49.Witkin SS, Jeremias J, Toth M, Ledger WJ. 1994. Proliferative response to conserved epitopes of the Chlamydia trachomatis and human 60-kilodalton heat-shock proteins by lymphocytes from women with salpingitis. Am J Obstet Gynecol 171:455–460. doi: 10.1016/0002-9378(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 50.Cragnolini JJ, Garcia-Medel N, de Castro JA. 2009. Endogenous processing and presentation of T-cell epitopes from Chlamydia trachomatis with relevance in HLA-B27-associated reactive arthritis. Mol Cell Proteomics 8:1850–1859. doi: 10.1074/mcp.M900107-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frazer LC, Scurlock AM, Zurenski MA, Riley MM, Mintus M, Pociask DA, Sullivan JE, Andrews CW Jr, Darville T. 2013. IL-23 induces IL-22 and IL-17 production in response to Chlamydia muridarum genital tract infection, but the absence of these cytokines does not influence disease pathogenesis. Am J Reprod Immunol 70:472–484. doi: 10.1111/aji.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jha R, Srivastava P, Salhan S, Finckh A, Gabay C, Mittal A, Bas S. 2011. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect 13:167–178. doi: 10.1016/j.micinf.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Barral R, Desai R, Zheng X, Frazer LC, Sucato GS, Haggerty CL, O'Connell CM, Zurenski MA, Darville T. 2014. Frequency of Chlamydia trachomatis-specific T cell interferon-gamma and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females. J Reprod Immunol 103:29–37. doi: 10.1016/j.jri.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grubaugh D, Flechtner JB, Higgins DE. 2013. Proteins as T cell antigens: methods for high-throughput identification. Vaccine 31:3805–3810. doi: 10.1016/j.vaccine.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 55.Capo S, Nuti S, Scarselli M, Tavarini S, Montigiani S, Mori E, Finco O, Abrignani S, Grandi G, Bensi G. 2005. Chlamydia pneumoniae genome sequence analysis and identification of HLA-A2-restricted CD8+ T cell epitopes recognized by infection-primed T cells. Vaccine 23:5028–5037. doi: 10.1016/j.vaccine.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 56.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. 2010. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol 185:1670–1680. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]