Abstract
Laboratory testing for the diagnosis of Lyme disease is performed primarily by serologic assays and is accurate for detection beyond the acute stage of the infection. Serodiagnostic assays to detect the early stages of infection, however, are limited in their sensitivity, and improvement is warranted. We analyzed a series of Borrelia burgdorferi proteins known to be induced within feeding ticks and/or during mammalian infection for their utility as serodiagnostic markers against a comprehensive panel of Lyme disease patient serum samples. The antigens were assayed for IgM and IgG reactivity in line immunoblots and separately by enzyme-linked immunosorbent assay (ELISA), with a focus on reactivity against early Lyme disease with erythema migrans (EM), early disseminated Lyme neuroborreliosis, and early Lyme carditis patient serum samples. By IgM immunoblotting, we found that recombinant proteins BBA65, BBA70, and BBA73 reacted with early Lyme EM samples at levels comparable to those of the OspC antigen used in the current IgM blotting criteria. Additionally, these proteins reacted with serum samples from patients with early neuroborreliosis and early carditis, suggesting value in detecting early stages of this disease progression. We also found serological reactivity against recombinant proteins BBA69 and BBA73 with early-Lyme-disease samples using IgG immunoblotting and ELISA. Significantly, some samples that had been scored negative by the Centers for Disease Control and Prevention-recommended 2-tiered testing algorithm demonstrated positive reactivity to one or more of the antigens by IgM/IgG immunoblot and ELISA. These results suggest that incorporating additional in vivo-expressed antigens into the current IgM/IgG immunoblotting tier in a recombinant protein platform assay may improve the performance of early-Lyme-disease serologic testing.
INTRODUCTION
Accurate diagnoses are essential to treat patients with Lyme disease, a tick-borne illness caused by the bacterial agent Borrelia burgdorferi. Diagnosis in the initial stages of Lyme disease can be made by clinical signs such as the onset of flu-like symptoms with the presence of a rash termed erythema migrans (EM) at the site of the tick bite (1, 2). Aiding the diagnostic evaluation, Lyme disease in the United States is endemic and transmitted by the tick vectors Ixodes scapularis in the Northeast and upper Midwest, and Ixodes pacificus in parts of the Pacific Northwest (http://www.cdc.gov/lyme/stats/index.html). However, it is not always apparent that a patient was bitten by an infected tick, and the EM may not appear or may go unnoticed, leading to a disseminated infection with more severe clinical symptoms, including arthritis, carditis, and neuropathy (2). In these instances, diagnosis is performed by serological testing to determine if the patient has been exposed to B. burgdorferi.
The standard for serologic Lyme disease testing is a 2-tiered test recommended by the Centers for Disease Control and Prevention whereby the first tier is commonly an enzyme immunoassay (such as enzyme-linked immunosorbent assay [ELISA]), usually performed against a whole-cell sonicate of cultured B. burgdorferi cells (http://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html) (3). Additionally, the Food and Drug Administration has approved the use of the antigen VlsE or a subunit peptide of VlsE termed C6 in first-tier ELISA (4). When the ELISA result is positive or equivocal, immunoblotting (also known as Western blotting) is performed as the second-tier test for the final determination. The 2-tiered testing can be for detection of IgM or IgG antibodies against B. burgdorferi antigens, depending on the time following onset of illness at which the serum sample was collected. IgM and/or IgG antibody testing is utilized for samples taken ≤30 days following onset of illness (4). For suspected Lyme disease cases that have progressed past 4 weeks, 2-tiered IgG testing is used and IgM antibody testing is not recommended (5). Despite limitations in the Western blot assays, 2-tiered IgG testing for late-Lyme-arthritis cases performs well and detects 97 to 100% of infections (6). However, the current 2-tiered testing is not sensitive (40% or less) for detection of disease in the early acute stages of infection (7, 8), and there are drawbacks to this system of testing. The second-tier Western blot requires more technical expertise, due to multiple steps in the procedure, than does ELISA. Moreover, the results of a Western blot are not quantitative, thereby requiring a subjective decision by an individual scorer based on band intensity compared to controls (although some labs can read blots by densitometry). Additionally, some manufacturers' blots are composed of fractionated B. burgdorferi proteins from whole-cell lysates originating from in vitro culture, which can be difficult to standardize. The criteria for a positive IgG immunoblot calls for the observation of at least 5 defined reactive antigen bands out of a total of 10 antigens identified as immunodominant in B. burgdorferi infections. The IgM criterion is the presence of 2 out of 3 defined immunoreactive bands (for a review, see reference 5). Because of subtle variances in blot strips made from cultured borrelial lysates, reactive bands appearing on blots may be erroneously scored as positive. A major flaw with utilizing whole-cell borrelial lysates in immunoblots is that the profile of proteins synthesized from in vitro culture is not wholly representative of the gene products expressed by B. burgdorferi during host infection. Many proteins are expressed during in vivo infection that are either not expressed in culture or expressed at very low levels (9, 10). Therefore, antibodies produced by Lyme disease patients against B. burgdorferi in vivo-expressed antigens that are not expressed by cultured organisms will be undetected by Western blots. To improve serologic testing for early acute-phase Lyme disease, we hypothesized that additional alternative antigens expressed early following in vivo infection could be incorporated as recombinant proteins into the Western blot testing criteria.
Previous studies from our laboratory have focused on a series of B. burgdorferi gene products expressed during in vivo infection, i.e., BBA64, -65, -66, -68, -69, -70, and -73 (9–11). The genes encoding these proteins are located on the B. burgdorferi 54-kb linear plasmid (lp54) that is essential for borrelial growth and survival (12). The genes are expressed in either ticks and/or mammalian hosts and are regulated by the RpoS/RpoN regulatory cascade (9, 11, 13–16). The genes encode surface-localized membrane lipoproteins that elicit antibody responses in humans and experimental animals following B. burgdorferi infection (11, 17–19). Functional aspects of the genes and their encoded products have been investigated, with the findings that BBA64 and BBA66 are involved in B. burgdorferi transmission from ticks (13, 20); BBA68 is designated a complement regulator-acquiring surface protein (CRASP) that plays a role in the inactivation of complement (21, 22), and BBA70 has recently been described as a plasminogen-binding protein (23). Historically, these genes have been characterized as paralogs and were originally annotated as belonging to one of several paralogous gene families (pgf54 or PFam 54) in the B. burgdorferi genome (10, 24, 25). However, nucleotide alignments show that these genes share little homology, i.e., from 26 to 33% nucleotide identity; amino acid identity ranges from 11.5 to 21% (10; our unpublished results). The exception is BBA68 and BBA69, which share extensive homology, i.e., 67% nucleotide identity and 55% amino acid identity (10).
In this study, we investigated the immunoreactivities of serum samples from Lyme disease patients and controls against recombinant proteins encoded by these in vivo expressed genes. Using a comprehensive collection of serum samples representing several stages of disease, we found that BBA65, BBA70, and BBA73 were reactive by IgM serology in early Lyme EM, neuroborreliosis, and carditis patient serum samples. Additionally, BBA69 and BBA73 were reactive by IgG immunoblotting against serum samples from early stage Lyme disease patients. The findings also show that some patients' samples that had been categorized as negative by 2-tiered testing were reactive against one or more of the in vivo-expressed proteins. We discuss the implications of including recombinant in vivo-synthesized proteins into improved early serologic testing for Lyme disease.
MATERIALS AND METHODS
Serum samples.
The Lyme Serum Repository was the source of human serum panels used in this study, and samples were obtained from the Division of Vector Borne Diseases, Bacterial Diseases Branch, Centers for Disease Control and Prevention. A detailed description of the Lyme Serum Repository, which is composed of serum obtained from well-characterized Lyme disease patients, control serum from healthy individuals, and serum from patients with other diseases, has been published (26). Lyme disease patient samples were subdivided into groups as follows: early Lyme disease with erythema migrans (EM), which consisted of paired patient serum samples taken at the acute and convalescent stages of disease (n = 23 pairs [46 total]); early Lyme neuroborreliosis (n = 10); early Lyme carditis (n = 6); and late Lyme arthritis (n = 8). Inclusion criteria for all Lyme disease patients included appropriate epidemiological risk for this infection, and all patient exposure had to occur within a state where Lyme disease is endemic. Overall, the majority of these patients reported exposure in the northeastern United States or adjacent states. Serum samples from patients with other diseases numbered 8 each: fibromyalgia, rheumatoid arthritis, multiple sclerosis, mononucleosis, syphilis, and severe periodontitis. These serum samples were collected from patients living in areas where Lyme disease is and is not endemic, including Maryland, Virginia, New Jersey, Florida, Washington, California, Montana, Colorado, and Arizona. Serum samples from healthy patients in areas where Lyme disease is and is not endemic (donors residing in New York and Texas, respectively) numbered 16 each.
Recombinant protein expression and purification.
Truncated (i.e., lacking signal sequence and lipidation motif) genes encoding BBA64, BBA65, BBA66, BBA68, BBA69, BBA70, BBA73, OspA, OspC, and DbpA were amplified by PCR from B. burgdorferi strain B31 genomic DNA using primers designed for cloning into the pETite N-His vector in accordance with the T7 Expresso system instructions (Lucigen, Middleton, WI). Primers for specific gene amplification into this plasmid are listed in Table 1. The resultant expression plasmids were transformed into Escherichia coli 10G (Lucigen) and selected for growth on Luria-Bertani (LB) medium plates supplemented with 50 μg/ml kanamycin. Plasmid DNA from transformant colonies was purified by miniprep (Qiagen, Valencia, CA) and was sequenced for insert confirmation. Recombinant plasmids with the correct gene inserts were transformed into E. coli BL21(DE3) (Lucigen). Following transformant screening for the appropriate clones, colonies were grown in LB-kanamycin (50 μg/ml) broth, and recombinant-protein expression was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM). Cells were harvested at late-log-phase growth, and recombinant protein was purified under nondenaturing conditions using a nickel-nitrilotriacetic acid (Ni-NTA) Fast Start His tag affinity purification kit (Qiagen). Proteins were dialyzed into 250 mM Tris (pH 8.0) and quantified by bicinchoninic acid (BCA) assay (Thermo-Fisher Scientific, Rockford, IL) before use.
TABLE 1.
Primers used for generation of N-his tagged recombinant proteins (5′-3′)
| Gene | Primer sequence |
|
|---|---|---|
| Forward | Reverse | |
| BBA64 | CATCATCACCACCATCACTCTCTGGAAGTCAAAGACAGC | GTGGCGGCCGCTCTATTACTGAATTGGAGCAAGAATATT |
| BBA65 | CATCATCACCACCATCACGATCTAAACAACAAAGACAAC | GTGGCGGCCGCTCTATTAATTATTATTAAATTTAAATAA |
| BBA66 | CATCATCACCACCATCACACGATTGATGCCAATCTAAAC | GTGGCGGCCGCTCTATTACATTATACTAATGTATGCTTC |
| BBA68 | CATCATCACCACCATCACGCACCTTTTAGCAAAATCGATCCT | GTGGCGGCCGCTCTATTAGTAAAAGGCAGGTTTTAAAGT |
| BBA69 | CATCATCACCACCATCACGCACCTTTTAACAAAATCAATCCC | GTGGCGGCCGCTCTATTAATAAAAGGCAGATTGTTAAGA |
| BBA70 | CATCATCACCACCATCACGCTCCAGAAGTAAACAGCTAC | GTGGCGGCCGCTCTATTACTTTTTCTCTGATTATATTTT |
| BBA73 | CATCATCACCACCATCACTCTTTTTATTCTAAATCAAAC | GTGGCGGCCGCTCTATTAGTAGTGTATGTGGTCACAACA |
| DbpA | CATCATCACCACCATCACGGACTAACAGGAGCAACA | GTGGCGGCCGCTCTATTATTAGTTATTTTTGCATTTTTC |
| OspA | CATCATCACCACCATCACAAGCAAAATGTTAGCAGCCTT | GTGGCGGCCGCTCTATTATTTTAAAGCGTTTTTAATTTC |
| OspC | CATCATCACCACCATCACATTAATTCAGGGAAAGATGGG | GTGGCGGCCGCTCTATTAAGGTTTTTTTGGACTTTCTGC |
Line immunoblotting.
Nitrocellulose membranes were hydrated in Tris-buffered saline–Tween 20 (TBS-T; 20 mM Tris, 140 mM NaCl, 2.7 mM KCl, 0.05% Tween 20 [pH 7.4]) and placed in a Miniblotter45 (Immunetics, Boston, MA). Protein (10 μg) in TBS-T was loaded in each lane and incubated for 10 min with rocking at room temperature. The protein solution was aspirated by vacuum from the lanes followed by three washes in TBS-T. The membrane was removed from the manifold and blocked in T20 blocking buffer (Thermo-Fisher Scientific) at room temperature for 30 min. The membrane was rotated 90° and placed back in the manifold with serum samples diluted in T20 blocking buffer (1:100) added to the appropriate lane, followed by incubation at room temperature for 1 h on a rocking platform. After three washes in TBS-T, alkaline phosphatase-conjugated goat anti-human IgG or IgM antibodies (KPL, Gaithersburg, MD) diluted in blocking buffer (1:10,000) were added to each lane and incubated with rocking at room temperature for 1 h. Following aspiration of the secondary antibody solution, the membrane was removed from the apparatus, washed three times with TBS-T, and developed with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) (Thermo-Fisher Scientific). Optimal conditions for protein concentration and for primary and secondary antibody dilutions were determined in a series of preliminary assays prior to utilization of the serum panels. Seroreactivity for each antigen was scored as either positive or negative independently by five lab members, with final blot interpretation according to the majority score.
ELISA.
Recombinant antigens were diluted with carbonate buffer (90 mM NaHCO3, 60 mM Na2CO3; pH 9.6) and bound to 96-well Immulon 2HB format plates overnight at 4°C (Thermo Scientific) at a final concentration of 200 ng/well. The plate wells were subjected to five washes with TBS-T using a BioTek 405 Select plate washer (BioTek, Winooski, VT), followed by addition of blocking buffer (TBS-T with 5% fetal bovine serum) for 45 min at room temperature. Serum samples diluted at either 1:100 (IgM) or 1:200 (IgG) in blocking buffer (200 μl) were added to the wells, and the plates were incubated for 45 min with moderate agitation at room temperature followed by five washes with TBS-T. Alkaline phosphatase-conjugated goat anti-human antibodies (KPL, Gaithersburg, MD) were added at 1:2,000 (IgM) or 1:5,000 (IgG) in blocking buffer, and plates were incubated for 45 min with agitation at room temperature followed by the wash step. For development, 100 μl of para-nitrophenyl phosphate (PNPP) substrate (Thermo-Fisher Scientific) was added to each well, followed by incubation with agitation at room temperature for 20 min. The reaction was stopped by adding 50 μl of 2 N NaOH to wells. Plates were read at an optical density at 405 nm (OD405) using an ELx808IU Ultra microplate reader (BioTek). Samples were determined to be positive if the absorbance at 405 nm was three standard deviations above the mean for serum samples from healthy patients in areas where Lyme disease is not endemic (n = 16).
RESULTS
Line immunoblotting and ELISA screening of serum panels.
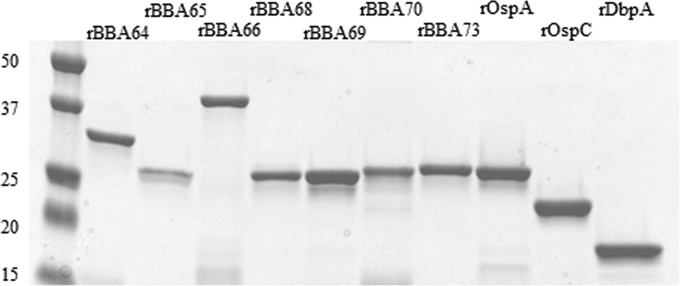
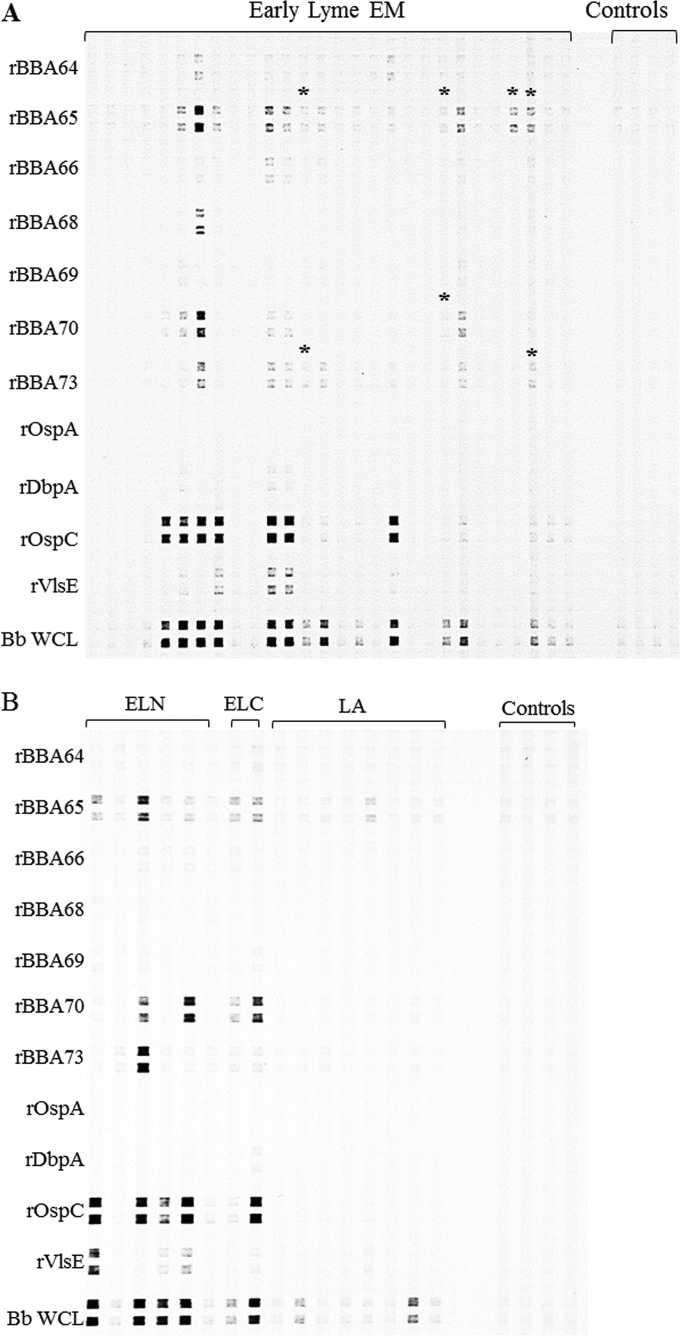
Recombinant proteins used in the immunoassays were purified from the soluble fraction of lysed E. coli cells by His tag affinity column chromatography. Figure 1 shows each protein as a major single band with minimal degradation or E. coli contamination. Initially, each recombinant protein was screened in duplicate against a blinded panel of 124 serum samples from Lyme disease patients, other disease controls, and healthy controls by line immunoblotting. Included in the line immunoblot were recombinant proteins currently employed in 2-tiered serodiagnosis, i.e., outer surface protein C (OspC), decorin-binding protein A (DbpA), and VMP (variable membrane protein)-like sequence expressed (VlsE), and also included were OspA and B. burgdorferi strain B31 whole-cell lysate. Representative line immunoblots with antigen reactivities against early Lyme EM serum samples by IgM immunoblotting are shown in Fig. 2A, and reactivities against early Lyme neuroborreliosis, early Lyme carditis, and Lyme arthritis serum samples are shown in Fig. 2B. A number of serum samples demonstrated positive banding patterns with various signal intensities, as is commonly seen with Western blots. In this initial analysis to screen all antigens, IgM immunoblotting identified rBBA65, rBBA70, and rBBA73 as candidates for further screening because of their high percentage of seropositivity, which was comparable to that of VlsE, OspC, and B31 lysate (Table 2).
FIG 1.
Recombinant proteins used in this study. Proteins were fractionated by SDS-PAGE and stained with GelCode Blue (Thermo Scientific, Rockford, IL). A 0.5- to 1-μg portion of protein was loaded in each lane. The first lane contains size markers, with the molecular masses (in kilodaltons) on the left.
FIG 2.
Representative IgM line blot with serum samples from patients with early Lyme erythema migrans (EM) (A) and with early disseminated Lyme neuroborreliosis (ELN), early disseminated Lyme carditis (ELC), and Lyme arthritis (LA) (B) tested for reactivity against the B. burgdorferi recombinant antigens on the left (Bb WCL, B. burgdorferi strain B31 whole-cell lysate). Results for selected negative controls from healthy individuals are presented on the right for comparative purposes, and these were tested on the same membrane. *, positive call for the antigens BBA65, BBA70, and BBA73 for patients scored as serologically negative by the 2-tiered testing algorithm.
TABLE 2.
Initial IgM line blot screening of all antigens
| Categorya | No. of samples | No. of IgM-reactive serum samples (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rBBA64 | rBBA65 | rBBA66 | rBBA68 | rBBA69 | rBBA70 | rBBA73 | rOspA | rDbpA | rOspC | rVlsE | B31 | ||
| Lyme disease | |||||||||||||
| Early Lyme disease with EM | 28 | 2 (7) | 12 (43) | 2 (7) | 1 (4) | 1 (4) | 7 (25) | 9 (32) | 0 (0) | 3 (11) | 9 (32) | 3 (11) | 13 (46) |
| Early neurologic Lyme disease | 6 | 0 (0) | 5 (83) | 2 (33) | 0 (0) | 0 (0) | 3 (50) | 3 (50) | 0 (0) | 0 (0) | 4 (67) | 3 (50) | 5 (83) |
| Early cardiac Lyme disease | 2 | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 1 (50) | 0 (0) | 1 (50) | 2 (100) | 0 (0) | 2 (100) |
| Late Lyme disease (Lyme arthritis) | 8 | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (43) |
| All early Lyme disease | 36 | 2 (6) | 19 (53) | 4 (11) | 1 (3) | 1 (3) | 12 (33) | 13 (36) | 0 (0) | 4 (11) | 15 (42) | 6 (17) | 20 (56) |
| Total Lyme disease | 44 | 2 (5) | 20 (45) | 4 (9) | 1 (2) | 1 (2) | 13 (30) | 13 (30) | 0 (0) | 4 (9) | 15 (34) | 6 (14) | 23 (52) |
| Other diseases | |||||||||||||
| Fibromyalgia | 8 | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rheumatoid arthritis | 8 | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Multiple sclerosis | 8 | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Mononucleosis | 8 | 0 (0) | 6 (75) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 5 (63) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Syphilis | 8 | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Severe periodontitis | 8 | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Healthy controls | |||||||||||||
| Healthy; endemic | 16 | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (19) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Healthy; nonendemic | 16 | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Total non-Lyme disease | 80 | 0 (0) | 16 (20) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 8 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (5) |
“Endemic” and “nonendemic” refer to samples from patients in areas where Lyme disease is and is not endemic, respectively.
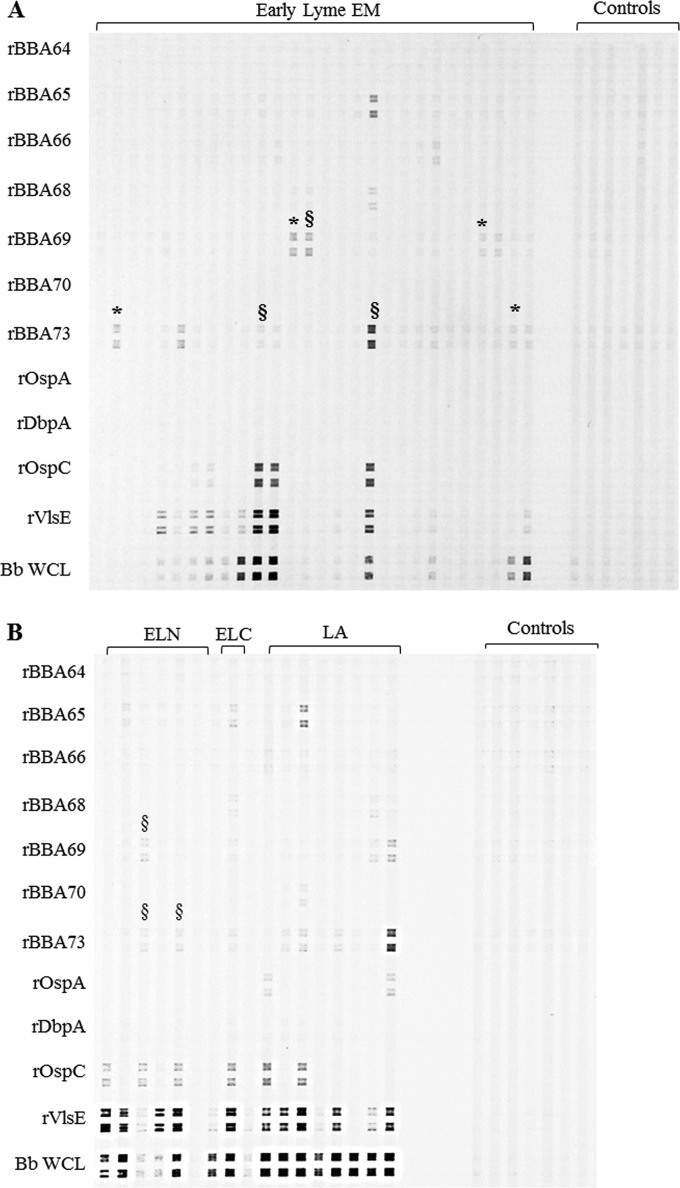
IgG immunoblotting, performed in the same manner as for IgM, identified rBBA69 and rBBA73 as candidates for further analysis (Table 3). Representative IgG line blots are shown in Fig. 3; Fig. 3A shows antigen reactivities against early Lyme EM serum samples, and Fig. 3B shows reactivities against early Lyme neuroborreliosis, early Lyme carditis, and Lyme arthritis serum samples. rBBA64, rBBA66, rBBA68, and rBBA69 demonstrated little to no reactivity in the IgM blots (Table 2), and rBBA64, rBBA65, rBBA66, rBBA68, and rBBA70 likewise demonstrated little to no reactivity in the IgG blots (Table 3); therefore, these antigens were not tested further.
TABLE 3.
Initial IgG line blot screening of all antigens
| Categorya | No. of samples | No. of reactive sera IgG (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rBBA64 | rBBA65 | rBBA66 | rBBA68 | rBBA69 | rBBA70 | rBBA73 | rOspA | rDbpA | rOspC | rVlsE | B31 | ||
| Lyme disease | |||||||||||||
| Early Lyme disease with EM | 28 | 0 (0) | 1 (4) | 2 (7) | 3 (11) | 4 (14) | 0 (0) | 8 (29) | 0 (0) | 0 (0) | 5 (18) | 9 (32) | 12 (43) |
| Early neurologic Lyme disease | 6 | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 2 (33) | 0 (0) | 0 (0) | 3 (50) | 5 (83) | 5 (83) |
| Early cardiac Lyme disease | 2 | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 2 (100) |
| Late Lyme disease-Lyme arthritis | 8 | 0 (0) | 1 (13) | 2 (25) | 0 (0) | 2 (25) | 1 (13) | 4 (14) | 2 (25) | 0 (0) | 2 (25) | 6 (86) | 8 (100) |
| Early Lyme disease | 36 | 0 (0) | 3 (8) | 2 (6) | 3 (8) | 6 (17) | 0 (0) | 11 (31) | 0 (0) | 0 (0) | 9 (25) | 14 (39) | 19 (53) |
| Total Lyme disease | 44 | 0 (0) | 4 (9) | 4 (9) | 3 (8) | 8 (18) | 1 (2) | 15 (34) | 2 (5) | 0 (0) | 11 (25) | 20 (45) | 27 (61) |
| Other diseases | |||||||||||||
| Fibromyalgia | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rheumatoid arthritis | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Multiple sclerosis | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Mononucleosis | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Syphilis | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 3 (38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Severe periodontitis | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Healthy controls | |||||||||||||
| Healthy; endemic | 16 | 0 (0) | 2 (13) | 0 (0) | 2 (13) | 1 (6) | 1 (6) | 3 (19) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (6) |
| Healthy; nonendemic | 16 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total non-Lyme disease | 80 | 0 (0) | 2 (3) | 0 (0) | 2 (3) | 5 (6) | 1 (1) | 9 (11) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 2 (3) |
“Endemic” and “nonendemic” refer to samples from patients in areas where Lyme disease is and is not endemic, respectively.
FIG 3.
Representative IgG line blot with serum samples from patients with early Lyme erythema migrans (EM) (A) and with early disseminated Lyme neuroborreliosis (ELN), early disseminated Lyme carditis (ELC), and Lyme arthritis (LA) (B) tested for reactivity against the B. burgdorferi recombinant antigens on the left (Bb WCL, B. burgdorferi strain B31 whole-cell lysate). Results for selected negative controls from healthy individuals are presented on the right for comparative purposes, and these were tested on the same membrane. *, positive calls for antigens for patients scored as serologically negative by the 2-tiered testing algorithm; §, positive calls for antigens for patients that were IgG Western blot negative by the 2-tiered algorithm.
Following the initial assessment, we tested 27 additional samples from Lyme disease patients with early Lyme disease with EM, early disseminated carditis, and early disseminated neuroborreliosis from the CDC Lyme Serum Repository. These samples were screened by IgM blotting against rBBA65, rBBA70, and rBBA73 and by IgG blotting against rBBA69 and rBBA73. While blots are useful for diagnostic purposes, they are subjective, especially in the cases of bands with borderline intensities. Therefore, we also tested the recombinant antigens against all serum samples by the quantitative ELISA format. Cumulative results for the individual antigens are presented separately below and are summarized in Table 4 (immunoblotting) and Table 5 (ELISA).
TABLE 4.
Line immunoblotting of serum samples
| Category (na) | No. of positive reactions (%) |
||||
|---|---|---|---|---|---|
| IgM |
IgG |
||||
| rBBA65 | rBBA70 | rBBA73 | rBBA69 | rBBA73 | |
| Lyme disease | |||||
| Early Lyme disease with EM | |||||
| Acute phase (23) | 7 (30) | 5 (22) | 6 (26) | 2 (9) | 4 (17) |
| Convalescent phase (23) | 9 (39) | 8 (35) | 11 (48) | 2 (9) | 6 (26) |
| Lyme neuroborreliosis (10) | 7 (70) | 3 (30) | 5 (50) | 2 (20) | 3 (30) |
| Lyme carditis (6) | 2 (33) | 2 (33) | 1 (17) | 2 (33) | 1 (17) |
| Late Lyme diseases (Lyme arthritis) (8) | 1 (13) | 1 (13) | 0 (0) | 2 (25) | 4 (50) |
| Total early Lyme disease (62) | 25 (40) | 18 (29) | 23 (37) | 8 (13) | 14 (23) |
| Total Lyme disease (70) | 26 (37) | 19 (27) | 23 (33) | 10 (14) | 18 (26) |
| Other diseases | |||||
| Fibromyalgia (8) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rheumatoid arthritis (8) | 2 (25) | 0 (0) | 0 (0) | 1 (13) | 1 (13) |
| Multiple sclerosis (8) | 2 (25) | 0 (0) | 0 (0) | 1 (13) | 1 (13) |
| Mononucleosis (8) | 6 (75) | 0 (0) | 5 (63) | 0 (0) | 1 (13) |
| Syphilis (8) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 3 (38) |
| Severe periodontitis (8) | 1 (13) | 0 (0) | 0 (0) | 1 (13) | 0 (0) |
| Healthy controls | |||||
| Healthy; endemic (16) | 1 (6) | 0 (0) | 3 (19) | 1 (6) | 3 (19) |
| Healthy; nonendemic (16) | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total non-Lyme disease (80) | 18 (23) | 0 (0) | 8 (10) | 5 (6) | 9 (11) |
| Lyme patient samples 2-tier-test negative (n = 23) but reactive with test antigens | 4 (17) | 1 (4) | 2 (9) | 3 (13) | 2 (9) |
n, number of samples. “Endemic” and “nonendemic” refer to samples from patients in areas where Lyme disease is and is not endemic, respectively.
TABLE 5.
ELISA of sera
| Category (na) | No. of positive reactions (%) |
||||
|---|---|---|---|---|---|
| IgM |
IgG |
||||
| rBBA65 | rBBA70 | rBBA73 | rBBA69 | rBBA73 | |
| Early Lyme disease with EM | |||||
| Acute phase (23) | 5 (22) | 6 (26) | 5 (22) | 1 (4) | 5 (22) |
| Convalescent phase (23) | 10 (43) | 15 (65) | 8 (35) | 1 (4) | 6 (26) |
| Lyme neuroborreliosis (10) | 5 (50) | 7 (70) | 6 (60) | 1 (10) | 2 (20) |
| Lyme carditis (6) | 2 (33) | 3 (50) | 2 (33) | 0 (0) | 1 (17) |
| Late Lyme disease (Lyme arthritis) (8) | 0 (0) | 2 (25) | 0 (0) | 2 (25) | 2 (25) |
| Total Early Lyme disease (62) | 22 (35) | 33 (53) | 21 (34) | 3 (5) | 14 (23) |
| Total Lyme disease (70) | 22 (31) | 35 (50) | 21 (30) | 5 (7) | 16 (23) |
| Other diseases | |||||
| Fibromyalgia (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rheumatoid arthritis (8) | 1 (13) | 1 (13) | 0 (0) | 0 (0) | 0 (0) |
| Multiple sclerosis (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mononucleosis (8) | 1 (13) | 2 (25) | 0 (0) | 0 (0) | 0 (0) |
| Syphilis (8) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) |
| Severe periodontitis (8) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) |
| Healthy controls | |||||
| Healthy; endemic (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (13) |
| Healthy; nonendemic (16) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (6) |
| Total non-Lyme (80) | 2 (3) | 4 (5) | 0 (0) | 2 (3) | 3 (4) |
| Lyme patient samples 2-tier-test negative (n = 23) but reactive with test antigens | 1 (4) | 3 (13) | 1 (4) | 1 (4) | 3 (13) |
n, number of samples. “Endemic” and “nonendemic” refer to samples from patients in areas where Lyme disease is and is not endemic, respectively.
rBBA65 IgM line blotting and ELISA.
By IgM line blotting, we found seropositivity against rBBA65 for 7/23 acute-phase early-EM samples and 9/23 paired convalescent-phase samples. By ELISA, 5/23 acute-phase early-EM and 10/23 convalescent-phase early-EM samples were positive. Numbers of serum samples that were positive by both line blotting and ELISA were 4/23 acute-phase samples and 5/23 convalescent-phase samples (Table 6). rBBA65 was reactive against 7/10 samples from neuroborreliosis patients and 2/6 from carditis patients by immunoblotting, with 5/10 and 2/6 positive by ELISA, respectively. Numbers of samples positive by both line blotting and ELISA were 4/10 and 1/6 for neuroborreliosis and carditis samples, respectively (Table 6). Overall, rBBA65 reacted against 25/62 (40%) of the total early-Lyme-disease patient serum samples by immunoblotting (Table 4) and 22/62 (35%) by ELISA (Table 5).
TABLE 6.
Numbers of samples both immunoblot and ELISA positive, by antigen
| Category (n) | No. positive (blotting/ELISA/both) |
||||
|---|---|---|---|---|---|
| IgM |
IgG |
||||
| rBBA65 | rBBA70 | rBBA73 | rBBA69 | rBBA73 | |
| Acute EM Lyme disease (23) | 7/5/4 | 5/6/4 | 6/5/5 | 2/1/1 | 4/5/3 |
| Convalescent EM Lyme disease (23) | 9/10/5 | 8/15/8 | 11/8/6 | 2/1/1 | 6/6/4 |
| Early neurologic Lyme disease (10) | 7/5/4 | 3/7/3 | 5/6/4 | 2/1/1 | 3/2/2 |
| Early cardiac Lyme disease (6) | 2/2/1 | 2/3/2 | 1/2/1 | 2/0/0 | 1/1/0 |
| Lyme arthritis (8) | 1/0/0 | 1/2/1 | 0/0/0 | 2/2/2 | 4/2/2 |
| Total Early Lyme disease (62) | 25/22/14 | 18/32/17 | 23/22/16 | 8/3/3 | 14/14/9 |
| Total Lyme disease (70) | 26/22/14 | 19/34/18 | 23/22/16 | 10/5/5 | 18/16/11 |
The specificity of immunoblotting was low, as 18/80 (77% specificity) control serum samples were scored as positive, indicating that background reactivity was somewhat problematic with this recombinant antigen in the line blot format. Much of the nonspecificity was due to rBBA65 cross-reactivity with serum samples from mononucleosis patients (6/8). However, ELISA demonstrated significantly higher specificity, with only 2/80 (97% specificity) control samples testing positive.
rBBA70 IgM line blotting and ELISA.
By immunoblotting, rBBA70 reacted against 5/23 acute-phase early-EM samples, with 8/35 positives among the paired convalescent-phase samples. By ELISA, 6/23 acute-phase samples were positive, with 15/23 of the convalescent-phase samples showing positive reactivity. By both line blotting and ELISA, 4/23 (acute phase) and 8/23 (convalescent phase) samples were positive (Table 6). rBBA70 was reactive by immunoblotting against 3/10 and 2/6 of the neuroborreliosis and carditis patient serum samples, respectively. ELISA identified 7/10 and 3/6 neuroborreliosis and carditis patient samples, respectively, as positive. For neuroborreliosis patients and cardiac patients, 3/10 and 2/6 samples were positive by both immunoblotting and ELISA (Table 6). Overall, rBBA70 reacted against 18/62 (29%) of the total samples from patients with early Lyme disease in line blotting (Table 4), and 33/62 (53%) were reactive in ELISA (Table 5).
In contrast to rBBA65, rBBA70 demonstrated excellent specificity (100%), with 0/80 of the control samples exhibiting reactivity by immunoblotting. The specificity of ELISA was also high (95%), with 4/80 of the control samples being reactive against rBBA70.
rBBA73 IgM line blotting and ELISA.
rBBA73 was reactive by line blotting against 6/23 acute-phase early Lyme EM patient samples and 11/23 of the convalescent-phase samples. ELISA showed seropositivity for 5/23 and 8/23 acute-phase and convalescent-phase samples, respectively. There were 5/23 samples that were both immunoblotting and ELISA positives in the acute-phase EM group and 6/23 in the convalescent group (Table 6). Of the neuroborreliosis patient samples, 5/10 were immunoblotting positive and 6/10 were ELISA positive, with 4/10 of the samples being positive by both assays. In the carditis patient samples, 1/6 were line blotting positive and 2/6 were ELISA positive, with 1/6 samples being positive by both assays (Table 6). Overall, 23/62 (37%) total early-Lyme-disease patient samples were immunoblotting positive for rBBA73, and 21/62 (34%) were positive by ELISA.
rBBA73 was scored positive by line blotting in 8/80 (90% specificity) of the control samples. These 8 positives among the control groups were split between the mononucleosis samples (5/8) and the healthy samples from patients in areas of endemicity (3/16), with all other control groups demonstrating no false-positive cross-reactivity against rBBA73.
rBBA69 and rBBA73 IgG line blot and ELISA.
rBBA69 was reactive by line blotting against 2/23 early Lyme EM acute-phase patient samples and 2/23 convalescent-phase samples. By ELISA, 1/23 and 1/23 acute- and convalescent-phase samples, respectively, were positive. Of the neuroborreliosis patient samples, 2/10 were positive by line blotting and 1/10 was positive by ELISA. Of the carditis patient samples, 2/6 were positive by immunoblotting and 0/6 were positive by ELISA. Overall, 8/62 (13%) of early-Lyme-disease patient samples were rBBA69 reactive by line blotting and only 3/62 (5%) were positive by ELISA.
rBBA73 exhibited higher reactivity than rBBA69, with 4/23 early Lyme EM acute-phase patient samples being positive and 6/23 convalescent-phase samples being positive. By rBBA73 ELISA, 5/23 acute-phase and 6/23 convalescent-phase samples were positive. There were 3/10 reactive positives for the neuroborreliosis group by line blotting and 2/10 by ELISA. There were 1/6 reactive positives from the carditis patient samples by line blotting and ELISA. Overall, 14/62 (23%) total early-Lyme-disease patient samples were positive for rBBA73 by immunoblotting and ELISA, with 9/14 being positive by both assays (Table 6).
Of the late-Lyme-arthritis patient samples, 2/8 were reactive against rBBA69 and 4/8 were reactive against rBBA73 by line blotting. By ELISA, 2/8 were rBBA69 positive and 2/8 were rBBA73 positive. By line blotting, rBBA69 was reactive against 5/80 (94% specificity) of the control serum samples and rBBA73 was positive against 9/80 (89% specificity) (Table 4). rBBA73 showed the most cross-reactivity against the syphilis patient group, with 3/8 scoring positive. ELISA testing resulted in better specificity (97%), with rBBA69 being reactive against 2/80 and rBBA73 being reactive against 3/80 (96% specificity) control patient samples (Table 5).
rBBA65, rBBA70, and rBBA73 IgM serology compared to CDC-recommended 2-tiered testing.
The results presented above clearly demonstrate that the antigens react with serum samples from Lyme disease patients. In order to assess if inclusion of these antigens in existing diagnostics could improve detection of early-Lyme-disease cases, it was important to determine whether the antigens demonstrated seropositivity against Lyme disease patient serum that tested negative by the CDC's recommended 2-tiered algorithm. Twenty-three of the 62 early Lyme EM (acute- and convalescent-phase), neuroborreliosis, and cardiac patient samples were scored as Lyme disease negative by the 2-tiered testing algorithm. rBBA65 was reactive with 4 of these samples by immunoblotting and with 1 sample by ELISA. rBBA70 detected 1 sample that was negative in the two-tiered test by immunoblotting and 3 samples by ELISA. rBBA73 was reactive with 2 two-tier-test-negative samples by immunoblotting and 1 sample by ELISA. The line blots for these samples are presented in Fig. 2A. ELISA testing identified additional samples as positive that differed from the line blots (Table 5).
The complete IgM serological data for rBBA65, rBBA70, and rBBA73 are broken down categorically for comparison against results obtained by 2-tiered testing in Table 7.
TABLE 7.
Comparison to 2-tiered testing as reported in reference 26
| Category (na) | No. of positive reactions (%) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-tiered testing of sera (26) |
Two-tiered testing |
Line blot testing of sera |
ELISA of sera |
|||||||||||||
| WCS EIS (Vidas)b | WBc |
EIA WB IgMd | Standarde | EIA WB IgG | IgM |
IgG |
IgM |
IgG |
||||||||
| IgM | IgG | rBBA65 | rBBA70 | rBBA73 | rBBA69 | rBBA73 | rBBA65 | rBBA70 | rBBA73 | rBBA69 | rBBA73 | |||||
| Lyme disease | ||||||||||||||||
| Early Lyme disease with EM | ||||||||||||||||
| Acute phase (23) | 14 (61) | 9 (40) | 2 (9) | 7 (30) | 7 (30) | 1 (4) | 7 (30) | 5 (22) | 6 (26) | 2 (9) | 4 (17) | 5 (22) | 6 (26) | 5 (22) | 1 (4) | 5 (22) |
| Convalescent phase (23) | 22 (96) | 16 (70) | 6 (26) | 16 (70) | 18 (78) | 7 (30) | 9 (39) | 8 (35) | 11 (48) | 2 (9) | 6 (26) | 10 (43) | 15 (65) | 8 (35) | 1 (4) | 6 (26) |
| Lyme neuroborreliosis (10) | 9 (90) | 10 (100) | 3 (30) | 9 (90) | 9 (90) | 4 (40) | 7 (70) | 3 (30) | 5 (50) | 2 (20) | 3 (30) | 5 (50) | 7 (70) | 6 (60) | 1 (10) | 2 (20) |
| Lyme carditis (6) | 6 (100) | 3 (50) | 5 (83) | 4 (67) | 5 (83) | 3 (50) | 2 (33) | 2 (33) | 1 (17) | 2 (33) | 1 (17) | 2 (33) | 3 (50) | 2 (33) | 0 (0) | 1 (17) |
| Late Lyme disease (Lyme arthritis) (8) | 8 (100) | 1 (13) | 8 (100) | 1 (13) | 8 (100) | 8 (100) | 1 (13) | 1 (13) | 0 (0) | 2 (25) | 4 (50) | 0 (0) | 2 (25) | 0 (0) | 2 (25) | 2 (25) |
| Total Early Lyme (62) | 51 (82) | 38 (61) | 16 (26) | 36 (58) | 39 (63) | 15 (24) | 25 (40) | 18 (29) | 23 (37) | 8 (13) | 14 (23) | 22 (35) | 33 (53) | 21 (34) | 3 (5) | 14 (23) |
| Total Lyme disease (70) | 59 (84) | 39 (55) | 24 (34) | 37 (53) | 47 (67) | 23 (33) | 26 (37) | 19 (27) | 23 (33) | 10 (14) | 18 (26) | 22 (31) | 35 (50) | 21 (30) | 5 (7) | 16 (23) |
| Look-alike diseases | ||||||||||||||||
| Fibromyalgia (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rheumatoid arthritis (8) | 2 (25) | 1 (13) | 0 (0) | 1 (13) | 1 (13) | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 1 (13) | 1 (13) | 1 (13) | 1 (13) | 0 (0) | 0 (0) | 0 (0) |
| Multiple sclerosis (8) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (38) | 0 (0) | 0 (0) | 1 (13) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mononucleosis (8) | 4 (50) | 1 (13) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 7 (88) | 0 (0) | 5 (63) | 0 (0) | 1 (13) | 1 (13) | 2 (25) | 0 (0) | 0 (0) | 0 (0) |
| Syphilis (8) | 6 (75) | 1 (13) | 0 (0) | 1 (13) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (38) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) |
| Severe periodontitis (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (13) | 0 (0) |
| Healthy controls | ||||||||||||||||
| Healthy; endemic (16) | 5 (31) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 3 (19) | 1 (6) | 3 (19) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (13) |
| Healthy; nonendemic (16) | 3 (19) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (6) |
| Total non-Lyme disease (80) | 21 (26) | 3 (4) | 0 (0) | 3 (4) | 2 (3) | 0 (0) | 18 (23) | 0 (0) | 8 (10) | 4 (5) | 9 (11) | 2 (3) | 4 (5) | 0 (0) | 2 (3) | 3 (4) |
n, number of samples. “Endemic” and “nonendemic” refer to samples from patients in areas where Lyme disease is and is not endemic, respectively.
WCS, whole-cell sonicate; EIA, enzyme immunoassay; Vidas, Lyme disease IgM and IgG polyvalent assay (bioMérieux, Inc., Durham, NC).
WB, Western blotting; IgM and IgG immunoblotting assays (MarDx Diagnostics, Inc., Carlsbad, CA).
Positive IgM WB results are recorded regardless of the duration of illness prior to specimen collection.
IgM WB results are recorded only when the duration of illness was <30 days.
rBBA69 and rBBA73 IgG serology compared to CDC-recommended 2-tiered testing.
In IgG immunoblotting, 3/23 and 2/23 samples previously scored as Lyme disease negative by the 2-tiered test were reactive against rBBA69 and rBBA73, respectively. The line blots for these samples are presented in Fig. 3A. ELISA results found 1/23 and 3/23 of the 2-tiered negative samples positive for rBBA69 and rBBA73, respectively. Additionally, some patient samples were scored as negative using the IgG immunoblot criterion of 5/10 positive bands but were considered reactive against rBBA69 and/or rBBA73 by immunoblotting; however, these samples were deemed positive by 2-tiered testing based on IgM criteria. Results for these samples are presented in Fig. 3.
The complete IgG serological data for rBBA69 and rBBA73 are broken down categorically for comparison against results obtained by 2-tiered testing in Table 7.
DISCUSSION
We performed this study to determine whether B. burgdorferi proteins synthesized during human in vivo infection elicit antibodies that could prove useful for improved serological testing. The data presented here demonstrate that the in vivo-expressed proteins BBA65, BBA69, BBA70, and BBA73 are reactive with Lyme disease patient serum during the early EM, neuroborreliosis, and carditis stages of infection, thereby indicating that these proteins are produced by B. burgdorferi early following infection and are immunogenic.
We utilized a line immunoblotting procedure to enable an initial screening of patient serum samples against several purified soluble recombinant proteins. By this method, we were able to determine the antigens that were most reactive and thus warranted further study. As expected, there was no reactivity of the Lyme disease patient sera against recombinant OspA, which served as an internal control. Interestingly, we found very little seroreactivity against the antigens BBA64 and BBA66, which had been postulated from previous studies to be a potential serodiagnostic. We also observed few seropositive results with rDbpA, which may indicate that the 18-kDa band recognized as DbpA in Western blots generated from cultured borrelial lysates may be a distinct antigen. The immunodominant antigens rVlsE and rOspC, and also whole-cell lysate from B. burgdorferi strain B31, were observed in many of the Lyme disease patient samples and served as internal positive controls for line blotting.
The data from our IgM serological screenings indicated that the antigens BBA65, BBA70, and BBA73 elicit an antibody response in humans early following the onset of infection with B. burgdorferi. This finding suggests that these proteins are functional in tick-to-skin transition and/or play a role in the establishment of infection and dissemination in the human host similar to that known for OspC, an immunodominant antigen that is one of the first serologically detectable in Lyme disease.
Individually, these 3 antigens demonstrated a range of IgM/IgG seropositivity from 29% to 53% against the total serum samples from early-Lyme-disease patients (i.e., EM, neuroborreliosis, and carditis) by either line blotting or ELISA. These numbers compared favorably with those for the immunodominant antigens, VlsE and OspC, that are currently employed in 2-tiered serodiagnostic testing. A significant finding was the high percentage of rBBA65-, rBBA70-, and rBBA73-seropositive samples from patients with early disseminated Lyme neuroborreliosis, with up to 70% of the samples being detected by either line blotting or ELISA. This result suggests that these gene products are differentially expressed depending on the stage of infection or the target organs during dissemination and may have potential as biomarkers for disease manifestations.
Certainly our test antigens were seroreactive with samples that were positive by the 2-tiered test based on the current antigen algorithm and therefore would not have affected the final interpretation. However, we found several instances of Lyme disease patient serum samples that were interpreted as negative according to the 2-tiered algorithm but were reactive to one or more of our test antigens. Although we observed a few non-Lyme disease patient sera that were reactive against individual test antigens, utilizing these proteins as part of a multiantigen algorithm should mitigate reporting of false positives. Our data suggest that the identification of additional antigens that elicit early IgM responses, such as ones described here, could be employed in IgM Western blots to significantly improve test sensitivity and specificity.
IgG immunoblotting was performed using the early-Lyme-disease EM patient samples. Although IgG serology is occasionally utilized for early-Lyme-disease samples, it is generally thought that sensitivity is low for detection because the IgG humoral response typically appears after the first 2 to 3 weeks postinfection. Therefore, IgM testing is more routinely employed for serum samples collected less than 30 days after onset of illness. Nevertheless, we observed IgG seroreactivity against rBBA69 and rBBA73. rBBA69 and rBBA73 were detectable bands in immunoblots that either were scored as negative in the 2-tiered test or were IgG immunoblotting negative but were 2-tier-test positive based solely on the IgM results. Therefore, the possibility of identifying antigens that elicit an early IgG response <2 weeks postexposure exists. Current serological testing of late-Lyme-disease patient samples (e.g., Lyme arthritis) by IgG serology is highly sensitive and specific; therefore, we did not extensively test our antigens against a large number of late-Lyme-arthritis patient samples. Although we observed some seroreactivity in the late-Lyme-arthritis patient serum samples, it was not suggestive of improvement for the current test.
The results indicated that subjective interpretation of immunoblots can be further refined to aid in borderline calls by utilizing ELISA as a quantitative tool; however, this assay also had its limitations. We set an ELISA absorbance cutoff for positive seroreactivity at 3 standard deviations above the mean for the samples from healthy patients in areas where Lyme disease is endemic. Although a majority of these samples gave a low baseline, there were outliers reactive with individual antigens that may have set the cutoff higher than expected. However, these cutoffs resulted in higher specificity at the possible expense of sensitivity. We also found that although there were several examples of agreement between line blotting and ELISA calls, there was not always consensus. This effect may be attributed to nonspecific background in line blots observed with some antigen preparations and some serum samples. However, in some instances, nonspecific background was not an issue; e.g., IgM line blots with rBBA70 resulted in 100% specificity. Future studies should focus on epitope mapping of the antigens with patient serum to produce immunoreactive peptides specific for Lyme disease serology to reduce background and cross-reactivity. We expect that further refinement with reagents and assay conditions could feasibly decrease the disparity between blots and ELISA, which will lead to an improved signal-to-noise ratio.
One approach to improve IgM serological testing (and IgG testing) is to use recombinant antigens in the first-tier ELISA and/or the second-tier Western blotting. This alleviates several problems associated with use of whole-cell borrelial lysates: (i) the genes encoding the antigens have been cloned, thereby ensuring the identity of the protein; (ii) a standard amount of recombinant protein can be applied to the membrane strip or wells; (iii) the recombinant protein can be applied to the strip in a specific location and is not subject to the impreciseness of lot-to-lot gel fractionation; (iv) any number of recombinant antigens can be incorporated in a blot strip or ELISA well test; and (v) band reactivities in immunoblots can be densitometrically scanned, resulting in less-subjective scoring. In addition to using recombinant antigens, synthetic peptides can be used in combination with the same advantages. Serodiagnostic testing in Europe could be assessed with the addition of the homologous antigens from Borrelia garinii and Borrelia afzelii (27). Importantly, antigens that are immunogenic in humans with Lyme borreliosis that may not be produced by in vitro culture can be added to the membrane test strip, allowing greater sensitivity and specificity.
The CDC recommendation of a 2-tiered algorithm for the serodiagnosis of Lyme disease calls for IgM and IgG antibody testing from serum samples collected <30 days after onset of disease. Test sensitivity at this stage is predictably lower, because antibodies against antigens of the infectious agent may not have had time to be synthesized. IgM class antibodies are present; however, IgM testing can lend itself to nonspecific reactivities because of the inherently “sticky” nature of the pentameric IgM molecule, and false positives are a concern (28). Even with these limitations, IgM testing is currently part of the recommended testing scheme for serum samples taken early after onset of illness; therefore, it is imperative to improve the sensitivity and specificity of these tests. An advantage of IgM serology is that these antibodies arise within a few days postinfection; therefore, an improved IgM test can be exploited to improve the low sensitivity and specificity in the current second-tier test or evolving serology-based assays. One of the limitations of the current IgM Western blot assay is that only 3 antigens are used for scoring, with a requirement for two being visible for a positive test. One of these 3 antigens is FlaB, the flagellin protein, which elicits antibodies that are cross-reactive against a variety of nonborrelial proteins, exacerbating the problem of low serological specificity due to false positives.
Recombinant-based immunoblot tests using the 5/10 and 2/3 antigen algorithm for IgG and IgM testing, respectively, are currently commercially available (4). Additional improvements to the current testing algorithm using the VlsE antigen have been proposed (29–31), as has using peptide subunits from the antigens OspC, OppA, BBK07, and DbpAB to reduce cross-reactive background (32–36). Different platforms, including luciferase immunoprecipitation systems (LIPS) and immuno-PCR, also demonstrate the potential to upgrade current serological testing, with the latter employing recombinant antigens (37–39). Line immunoblotting was also successfully used in a study designed to improve serodiagnosis of Lyme borreliosis with recombinant antigens from three European borrelial species (27). Barbour et al. have taken a global approach to identify proteins reactive with serum from infected humans that are candidates for serodiagnostic markers (40).
In conclusion, acknowledged limitations in the performance and implementation of the current 2-tier serological assays can be addressed. The findings presented here suggest that IgM Western blotting may be improved by the addition of the BBA65, BBA70, and BBA73 antigens to the second tier of the assay and reassessing the algorithm as to the number of reactive bands necessary for a positive test; e.g., if 6 antigens are used, perhaps a positive test would be defined as 3 to 5 visible bands out of the 6, instead of the current requirement for 2 positive bands out of 3. Likewise, for IgG immunoblotting, the inclusion of additional recombinant antigens could improve the sensitivity of 2-tiered testing. We will continue to evaluate the performance of these antigens concerning sensitivity and specificity, with comparisons to the existing two-tier assays. The data from our study provide evidence that certain borrelial antigens elicit antibody responses that can be detected within a few days postinfection. The results from this study should spur investigations into additional in vivo-expressed gene products that are produced during mammalian infection and that may be immunodominant in human Lyme borreliosis.
ACKNOWLEDGMENTS
We acknowledge the contributions of Christopher Sexton, John W. Young, Laura V. Ashton, and Ryan Pappert in establishing the CDC serum repository used in this study.
Z.P.W. was supported as a fellow through the American Society for Microbiology/CDC Program in Infectious Disease and Public Health Microbiology. R.M.C. was supported as an Emerging Infectious Disease Training Fellow through the Association for Public Health Laboratories (APHL) and CDC.
REFERENCES
- 1.Shapiro ED. 2014. Lyme disease. N Engl J Med 371:684. doi: 10.1056/NEJMc1407264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BJB. 2011. Laboratory diagnostic testing for Borrelia burgdorferi infection. CAB International, Oxfordshire, United Kingdom. [Google Scholar]
- 5.Marques AR. 2015. Laboratory Diagnosis of Lyme Disease: Advances and Challenges. Infect Dis Clin North Am 29:295–307. doi: 10.1016/j.idc.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD Jr, Philipp MT, Steere AC, Wormser GP, Marques AR, Johnson BJ. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 187:1187–1199. doi: 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguero-Rosenfeld ME, Nowakowski J, Bittker S, Cooper D, Nadelman RB, Wormser GP. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol 34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguero-Rosenfeld ME. 2008. Lyme disease: laboratory issues. Infect Dis Clin North Am 22:301–313, vii. doi: 10.1016/j.idc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore RD Jr, Howison RR, Schmit VL, Carroll JA. 2008. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice. Microb Pathog 45:355–360. doi: 10.1016/j.micpath.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JL, Nolder CL, Nowalk AJ, Clifton DR, Howison RR, Schmit VL, Gilmore RD Jr, Carroll JA. 2008. Borrelia burgdorferi surface-localized proteins expressed during persistent murine infection are conserved among diverse Borrelia spp. Infect Immun 76:2498–2511. doi: 10.1128/IAI.01583-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore RD Jr, Howison RR, Schmit VL, Nowalk AJ, Clifton DR, Nolder C, Hughes JL, Carroll JA. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect Immun 75:2753–2764. doi: 10.1128/IAI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. 2012. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore RD Jr, Howison RR, Dietrich G, Patton TG, Clifton DR, Carroll JA. 2010. The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc Natl Acad Sci U S A 107:7515–7520. doi: 10.1073/pnas.1000268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patton TG, Dietrich G, Dolan MC, Piesman J, Carroll JA, Gilmore RD Jr. 2011. Functional analysis of the Borrelia burgdorferi bba64 gene product in murine infection via tick infestation. PLoS One 6:e19536. doi: 10.1371/journal.pone.0019536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol 65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. 2011. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun 79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks CS, Vuppala SR, Jett AM, Akins DR. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun 74:296–304. doi: 10.1128/IAI.74.1.296-304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clifton DR, Nolder CL, Hughes JL, Nowalk AJ, Carroll JA. 2006. Regulation and expression of bba66 encoding an immunogenic infection-associated lipoprotein in Borrelia burgdorferi. Mol Microbiol 61:243–258. doi: 10.1111/j.1365-2958.2006.05224.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilmore RD Jr, Kappel KJ, Johnson BJ. 1997. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol 35:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton TG, Brandt KS, Nolder C, Clifton DR, Carroll JA, Gilmore RD. 2013. Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse Infection by tick transmission. Infect Immun 81:2488–2498. doi: 10.1128/IAI.00140-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol 175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- 22.Kraiczy P, Stevenson B. 2013. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis 4:26–34. doi: 10.1016/j.ttbdis.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenigs A, Hammerschmidt C, Jutras BL, Pogoryelov D, Barthel D, Skerka C, Kugelstadt D, Wallich R, Stevenson B, Zipfel PF, Kraiczy P. 2013. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J Biol Chem 288:25229–25243. doi: 10.1074/jbc.M112.413872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35:490–516. [DOI] [PubMed] [Google Scholar]
- 25.Wywial E, Haven J, Casjens SR, Hernandez YA, Singh S, Mongodin EF, Fraser-Liggett CM, Luft BJ, Schutzer SE, Qiu WG. 2009. Fast, adaptive evolution at a bacterial host-resistance locus: the PFam54 gene array in Borrelia burgdorferi. Gene 445:26–37. doi: 10.1016/j.gene.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molins CR, Sexton C, Young JW, Ashton LV, Pappert R, Beard CB, Schriefer ME. 2014. Collection and characterization of samples for establishment of a serum repository for Lyme disease diagnostic test development and evaluation. J Clin Microbiol 52:3755–3762. doi: 10.1128/JCM.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goettner G, Schulte-Spechtel U, Hillermann R, Liegl G, Wilske B, Fingerle V. 2005. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J Clin Microbiol 43:3602–3609. doi: 10.1128/JCM.43.8.3602-3609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. 2012. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 18:1236–1240. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 29.Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2010. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis 50:20–26. doi: 10.1086/648674. [DOI] [PubMed] [Google Scholar]
- 30.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. 2011. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 31.Ledue TB, Collins MF, Young J, Schriefer ME. 2008. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaboldi PM, Sambir M, Dattwyler RJ. 2014. Decorin binding proteins A and B in the serodiagnosis of Lyme disease in North America. Clin Vaccine Immunol 21:1426–1436. doi: 10.1128/CVI.00383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signorino G, Arnaboldi PM, Petzke MM, Dattwyler RJ. 2014. Identification of OppA2 linear epitopes as serodiagnostic markers for Lyme disease. Clin Vaccine Immunol 21:704–711. doi: 10.1128/CVI.00792-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnaboldi PM, Seedarnee R, Sambir M, Callister SM, Imparato JA, Dattwyler RJ. 2013. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early lyme disease. Clin Vaccine Immunol 20:474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman AS, Rossmann E, Yang X, Song H, Lamichhane CM, Iyer R, Schwartz I, Pal U. 2011. BBK07 immunodominant peptides as serodiagnostic markers of Lyme disease. Clin Vaccine Immunol 18:406–413. doi: 10.1128/CVI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jobe DA, Lovrich SD, Asp KE, Mathiason MA, Albrecht SE, Schell RF, Callister SM. 2008. Significantly improved accuracy of diagnosis of early Lyme disease by peptide enzyme-linked immunosorbent assay based on the borreliacidal antibody epitope of Borrelia burgdorferi OspC. Clin Vaccine Immunol 15:981–985. doi: 10.1128/CVI.00079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burbelo PD, Issa AT, Ching KH, Cohen JI, Iadarola MJ, Marques A. 2010. Rapid, simple, quantitative, and highly sensitive antibody detection for Lyme disease. Clin Vaccine Immunol 17:904–909. doi: 10.1128/CVI.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halpern MD, Jain S, Jewett MW. 2013. Enhanced detection of host response antibodies to Borrelia burgdorferi using immuno-PCR. Clin Vaccine Immunol 20:350–357. doi: 10.1128/CVI.00630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halpern MD, Molins CR, Schriefer M, Jewett MW. 2014. Simple objective detection of human Lyme disease infection using immuno-PCR and a single recombinant hybrid antigen. Clin Vaccine Immunol 21:1094–1105. doi: 10.1128/CVI.00245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun 76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]