Abstract
The serodiagnosis of human tegumentary leishmaniasis (TL) presents some problems, such as the low level of antileishmanial antibodies found in most of the patients, as well as the cross-reactivity in subjects infected by other trypanosomatids. In the present study, an immunoproteomic approach was performed aimed at identification of antigens in total extracts of stationary-phase promastigote and amastigote-like forms of Leishmania (Viannia) braziliensis using sera from TL patients. With the purpose of reducing the cross-reactivity of the identified proteins, spots recognized by sera from TL patients, as well as those recognized by antibodies present in sera from noninfected patients living in areas where TL is endemic and sera from Chagas disease patients, were discarded. Two Leishmania hypothetical proteins and 18 proteins with known functions were identified as antigenic. The study was extended with some of them to validate the results of the immunoscreening. The coding regions of five of the characterized antigens (enolase, tryparedoxin peroxidase, eukaryotic initiation factor 5a, β-tubulin, and one of the hypothetical proteins) were cloned in a prokaryotic expression vector, and the corresponding recombinant proteins were purified and evaluated for the serodiagnosis of TL. The antigens presented sensitivity and specificity values ranging from 95.4 to 100% and 82.5 to 100%, respectively. As a comparative antigen, a preparation of Leishmania extract showed sensitivity and specificity values of 65.1 and 57.5%, respectively. The present study has enabled the identification of proteins able to be employed for the serodiagnosis of TL.
INTRODUCTION
Leishmaniasis consists of a spectrum of diseases caused by protozoan parasites of the genus Leishmania, which present high morbidity and mortality throughout the world (1). Approximately 350 million people in 98 countries are at risk of contracting the infection, while nearly 1.0 to 1.5 million cases of tegumentary leishmaniasis (TL) and 0.2 to 0.5 million cases of visceral leishmaniasis (VL) are registered annually (2). Although TL is not a fatal disease, it is endemic in more than 70 countries, and 90% of the cases have occurred in Afghanistan, Algeria, Brazil, Pakistan, Peru, Saudi Arabia, and Syria (3). The disease exhibits distinct clinical manifestations, such as cutaneous leishmaniasis (CL), diffuse cutaneous leishmaniasis (DCL), and mucosal leishmaniasis (ML) (4, 5). In Brazil, TL is caused mainly by infection with the species Leishmania (Viannia) braziliensis, Leishmania (V.) guyanensis, and Leishmania (Leishmania) amazonensis, although parasitological and molecular evidence has shown that L. braziliensis is the most important etiological agent of the disease (6, 7).
At present, there is no gold standard test for TL diagnosis, and a combination of diagnostic methods is frequently needed to obtain more precise results (8). Parasitological diagnosis is definitive and consists of the microscopic examination of Giemsa-stained biopsy specimen smears, of histopathological examination or immunohistochemical approaches in lesion fragment triturates, and by parasite in vitro cultures, as well as PCR. However, these tests' sensitivity varies according to when the lesions occurred, and a low number of parasites can be present in the late lesions, hampering the sensitivity of the tests (8–13). Additionally, these tests require invasive procedures of sample collection, which limit their use.
The Montenegro skin test (MST) has been employed for the immunological diagnosis of TL, although it does not make it possible to distinguish if the patients present either the acute or chronic disease or if they have already been cured or are noninfected subjects living in areas where the disease is endemic (14). In this context, tests based on antileishmanial serology could be used to contribute to the laboratory diagnosis of the disease (15). However, the antigens usually employed can indicate false-positive results in serum samples from noninfected subjects living in areas of endemicity where leishmaniasis is commonly found or in those infected by other trypanosomatids, such as Trypanosoma cruzi. On the other hand, these preparations can also indicate false-negative results for patients presenting low titers of antileishmanial serology (8, 13, 16–20).
It is believed that the serum concentration of antileishmanial antibodies can be directly related to the parasite load and to the time of exposure to Leishmania antigens (21–23). Although the role of antibodies in TL is not completely understood, evidence has shown that the humoral response determined by specific IgG reactivity to Leishmania antigens could well be used as an indicator of the development of the disease, since some authors have positively correlated the clinical cure of disease with a decrease in the antileishmanial antibody titers, as well as to assist the clinical follow-up during and after healing by the treatment of the patients (23–27).
In this context, the search for more refined antigens of Leishmania spp. is necessary to identify molecules able to improve the sensitivity and specificity values for the serodiagnosis of TL. In addition, the identification of proteins able to predict the success of a given treatment will help the follow-up of patients after treatment. Only a few reports that applied proteomic methods for the identification of L. braziliensis-excreted proteins or those related to the development of different clinical forms of TL have been published so far (28, 29). In this context, the present study applied for the first time an immunoproteomic approach to the stationary-phase promastigote and amastigote-like stages of L. braziliensis using sera from TL patients. The objective was the identification of the most antigenic proteins to be applied for the improvement of serodiagnosis of TL. With the purpose of refining the selection of the candidate antigens, sera from noninfected subjects living in regions of TL endemicity, as well as sera from Chagas disease (CD) patients, were also used in the immunoblots to exclude the cross-reactive proteins.
MATERIALS AND METHODS
Ethics statement.
This study was conducted according to the Declaration of Helsinki principles, and it was approved by the Ethics Committee from Federal University of Minas Gerais (protocol no. CAAE–323431 14.9.0000.5149), Belo Horizonte, Minas Gerais, Brazil. All patients received an individual copy of the study policy, which was reviewed by an independent person, and all participants gave their consent form in Portuguese before the collection of their blood sample.
Parasite.
Leishmania (Viannia) braziliensis strain MHOM/BR/1975/M2904 was used. Parasites were used until the 5th in vitro culture passage. The promastigotes were grown at 24°C in Schneider's medium (Sigma, St. Louis, MO), which was supplemented with 10% inactivated fetal bovine serum (FBS) (Sigma), 20 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (pH 7.4) after reaching the stationary phase of the culture. The soluble Leishmania antigenic extract (SLA) was prepared as described previously (30). For the preparation of the amastigote-like forms of L. braziliensis, 1 × 109 stationary promastigotes were washed in 5 ml of sterile phosphate-buffered saline (PBS). The parasites then were incubated in 5 ml of FBS for 48 h at 37°C. After that, parasites were washed two times in sterile PBS and visualized in an optical light microscopy (31). The cellular density was estimated by counting in a Neubauer chamber. Also, the amastigote-like forms were used in in vitro experiments to infect macrophages as a functional viability parameter.
Preparation of total extracts to 2DE gels.
The total extracts of stationary-phase promastigotes and amastigotes-like forms of L. braziliensis, as well as two-dimensional electrophoresis (2DE), were performed following a modified technical protocol (32). Briefly, parasites in each stage (1 × 108) were resuspended in a DeStreak rehydration solution (GE Healthcare, Uppsala, Sweden) containing a protease inhibitor cocktail (GE Healthcare) plus 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich). After homogenization, samples were disrupted by sonication in ice bath for 15 min by applying a continuous pulse (with pulses of 30 s, with a 15-s interval between them, at 38 MHz) and centrifuged at 20,000 × g for 7 min at 4°C. The supernatant was collected, and the protein concentration was estimated by the Bradford method (33).
IEF and SDS-PAGE.
For the first-dimension electrophoresis, 150 μg of total extracts of the stationary promastigote and amastigote-like organisms were resuspended in a volume of 125 μl of rehydration solution containing a DeStreak rehydration solution in 1% immobilized pH gradient (IPG) buffer at pH 4 to 7. Next, samples were applied to IPG strips (7 cm, pH 4 to 7 [GE Healthcare]) for passive rehydration for 18 h at room temperature. After gel rehydration for 18 h, isoelectric focusing (IEF) was performed using the Ettan IPGphor3 system at 1,000 V for 300 V/h, 5,000 V for 4,800V/h, and holding at 5,000 V for 3,000 V/h. After IEF, each strip was incubated for 15 min in a solution composed of 50 mM Tris-HCl buffer (pH 8.8), 6 M urea, 30% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.002% bromophenol blue, and 1% (wt/vol) dithiothreitol (DTT), followed by a second incubation step in the same buffer solution, excluding DTT, which was replaced by 125 mM iodoacetamide. IPG strips were transferred to 12% polyacrylamide and sealed with agarose solution (agarose and bromophenol blue in a Tris-glycine cathode buffer). A protein standard was used (Invitrogen BenchMark protein ladder). Electrophoresis was performed in a Mini-Protean II system (Bio-Rad) connected to a MultiTemp II cooling bath (Amersham Biosciences), in a Tris-glycine-SDS buffer. Proteins were separated at 30 mA/gel until the dye front had reached the bottom of the 2DE gel.
Human sera.
Blood samples from patients with a confirmed diagnosis of TL (n = 43), representing those developing either CL (n = 23; including 17 males and 6 females, with ages ranging from 30 to 65 years) or ML (n = 20; including 15 males and 5 females, with ages ranging from 25 to 60 years), all of them living in an area of leishmaniasis endemicity (Belo Horizonte, Minas Gerais, Brazil), were used in this study. Diagnosis of TL was confirmed when, in addition to clinical evaluation of lesions compatible with either CL or ML, parasites were observed in Giemsa-stained smears of biopsy specimens of skin (CL) and/or mucosal (ML) fragments. As well, all patients presented positive results for the Montenegro skin test. For this, the Leishmania antigen used was obtained from the L. amazonensis IFLA/BR/1967/PH-8 strain containing 40 mg of protein nitrogen per ml, with 0.005 g per ml of phenol, which was provided by CPPI (Instituto de Saúde do Paraná, Centro de Produção e Pesquisa de Imunobiológicos, Curitiba, Paraná, Brazil). The magnitude of the skin response was assessed 48 to 72 h after intradermal injection of 0.1 ml of the antigen into the anterior right forearm. The diameter of the induration was measured in millimeters by outlining the indurated border, with MST considered positive if it measured ≥5 mm (34). In addition, two biopsy samples collected from each patient were submitted to a conventional PCR technique, and they presented positive results for L. braziliensis DNA. None of the TL patients had been previously treated with antileishmanial drugs before the sample collection. All patients in this study were integrated into the clinical assistance program of the Clinical Hospital from UFMG and received continuous medical assistance. Concomitantly, blood samples were taken from a control group consisting of noninfected individuals (n = 30, including 10 males and 20 females, with ages ranging from 17 to 30 years). Subjects were selected from healthy people living in area of disease endemicity (Belo Horizonte, Minas Gerais, Brazil). These subjects were also clinically evaluated through anamnesis and physical examination, and they did not present any clinical signs or suspicion of leishmaniasis. To exclude the cross-reactive spots in the immunoblot assays, blood samples from Chagas disease (CD) patients (n = 10, including 7 males and 3 females, with ages ranging from 24 to 58 years) were also collected and employed. Infection was confirmed by hemoculture or by both the Chagatest recombinant enzyme-linked immunosorbent assay (ELISA) v.3.0 kit (Wiener Lab, Argentina) and the Chagatest hemagglutination inhibition (HAI) assay (Wiener Lab). All blood samples were collected by venipuncture of the medial vein in tubes without anticoagulant and were kept at 37°C for 15 min, when they were centrifuged at 3,000 × g for 15 min, and serum samples were separated and kept at −80°C until use.
Cloning, expression, and purification of the recombinant proteins.
The gene regions codifying the enolase (LbrM.14.1330), tryparedoxin peroxidase (LbrM.15.1100), hypothetical protein (LbrM.30.3350), eukaryotic initiation factor 5a (LbrM.25.0580), and β-tubulin (LbrM.33.0920) were obtained and cloned in an expression vector. Afterward, the recombinant proteins were expressed and purified to be evaluated in ELISAs. For this, the L. braziliensis genomic DNA was used to amplify the specific sequences using the following primers: for enolase, forward primer 5′GCTAGCATGCCGATCCAGAAGGTGTA and reverse primer 5′AAGCTTTTACGCCCAGCCGGAGTA; for tryparedoxin peroxidase, forward primer 5′GCTAGCATGTCCTGCGGTGACGCC and reverse primer 5′AAGCTTTTACGCCCAGCCGGAGTA; for the hypothetical protein, forward primer 5′GCTAGCATGATGTACACGGGCGAA and reverse primer 5′AAGCTTCTAGTAGTCAGAATTGCTGA; for β-tubulin, forward primer GCTAGCATGCGTGAGATCGTTTCCTG and reverse primer 5′GAGCTCCTAGTAGGCCTCCTCCTC; and for eukaryotic initiation factor 5a, forward primer 5′GCTAGCATGTCTGACGAGGACCAC and reverse primer 5′AAGCTTCTACTTCTCCGCGGCATT. The restriction sites for the NheI (GCTAGC), HindIII (AAGCTT), and SacI (GAGCTC) restriction enzymes are underlined in the sequences. The sequences were added to the oligonucleotides for cloning purposes. The PCR-amplified products were analyzed on agarose gels. They were excised from the gels, purified, digested with the respective restriction enzymes, and ligated to a similarly digested pET28a-TEV vector. The recombinant plasmids were introduced to electrocompetent Escherichia coli BL21 Arctic Express (DE3) cells (Agilent Technologies) by electroporation using a MicroPulser electroporation apparatus (Bio-Rad Laboratories). The correct sequences' insertion was confirmed by their sequencing using the T7 primer (Macrogen, South Korea). The recombinant proteins were expressed by adding 1.0 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (Promega, Canada) for 24 h at 12°C with shaking at 200 × g per min. Then the cells were lysed by sonication (applying continuous pulses of 30 s, with 15-s intervals between them, at 38 MHz) and centrifuged at 13,000 × g for 20 min at 4°C. The recombinant proteins were purified under nondenaturing conditions, using 5 ml His-Trap columns (GE Healthcare Life Science) attached to a fast protein liquid chromatography (FPLC) system (GE Healthcare Life Science). After purification, the recombinant proteins were passed through a polymyxin-agarose column (Sigma) to remove residual endotoxin content. Also, to verify their purity, the purified products were submitted to 10% SDS-PAGE.
ELISA for the serodiagnosis of TL.
Previous titration curves were performed to determine the most appropriate antigen concentration and antibody dilution to be used. In order to evaluate the diagnostic performance of the selected proteins for the TL serodiagnosis, the enolase, tryparedoxin peroxidase, hypothetical protein, eukaryotic initiation factor 5a, and β-tubulin proteins (employed at concentrations of 1.0, 0.5, 0.5, 0.05, and 0.01 μg per well, respectively) were employed as antigens. L. braziliensis SLA (2.0 μg per well) was used as a comparative antigen. For the assays, Falcon flexible microtiter immunoassay plates (Becton Dickinson) were coated with the antigens diluted in 100 μl of coating buffer (50 mM carbonate buffer) at pH 9.6 for 18 h at 4°C. Afterward, free binding sites were blocked using 200 μl of PBS-T (PBS plus 0.05% Tween 20 at pH 7.0) containing 5% casein for 1 h at 37°C. After the plates had been washed five times with PBS-T, they were incubated with 100 μl of individual sera (diluted 1:100 in PBS-T containing 0.5% casein), for 1 h at 37°C. The plates were washed five times using PBS-T and incubated with a 1:10,000 dilution of horseradish peroxidase-conjugated anti-human IgG antibody (catalog no. SAB3701282; Sigma-Aldrich) for 1 h at 37°C. After the plates had been washed seven times with PBS-T, the reaction was developed by adding 100 μl of a solution composed of 2 mg ortho-phenylenediamine, 2 μl H2O2 (30 vol), and 10 ml citrate-phosphate buffer (pH 5.0) for 30 min and in the dark. The reaction was stopped by adding 100 μl of a solution composed of 25 μl 2 N H2SO4, and the optical density at 492 nm (OD492) was read in an ELISA microplate spectrophotometer (Molecular Devices, Spectra Max Plus, Canada). The ELISAs were performed twice, and the data shown in this study are representative of one of them. In addition, each serum sample was evaluated in triplicate, and positive and negative controls were used in each plate in the different experiments.
2DE immunoblot assays and protein identification.
The immunoblots were performed to select the protein spots that were recognized only by antibodies in sera from CL and/or ML patients, excluding those also reactive with sera from Chagas disease patients or from noninfected subjects. For this, total extracts of stationary promastigotes and amastigote-like parasites were separated electrophoretically and transferred onto nitrocellulose membranes (Schleicher & Schull, Dassel, Germany) by semidry blotting for 2 h at 400 mA. Membranes were blocked in 5% (wt/vol) low-fat dried milk and diluted in 10 ml of PBS-T for 2 h and at room temperature. Next, membranes were washed 5 times (10 min each) with PBS-T and incubated with the individual pools of sera. For this, serum samples from CL (n = 23) or ML (n = 20) patients, as well as sera from noninfected subjects (n = 30) and those with Chagas′ disease (n = 10), were pooled in equal volumes, diluted in PBS-T (1:100 dilution), and individually incubated with the membranes for 2 h at room temperature. After this, the blots were washed 5 times with PBS-T and incubated with a peroxidase-conjugated goat anti-human IgG secondary antibody (1:5,000 diluted in PBS-T) (catalog no. SAB3701282; Sigma-Aldrich) for 2 h at room temperature. After being washed 5 times with PBS-T, immunoblots were revealed with the ECL enhanced chemiluminescence Western blotting detection reagent and ImageQuant LAS4000 device (GE Healthcare). Equivalent gels were stained with colloidal Coomassie brilliant blue G-250, following a technical procedure (35). For image analysis, the stained gels were scanned using an ImageScanner III (GE Healthcare), and the gel spots equivalent to those recognized only by antibodies of CL and/or ML patients in the blots were excised manually for protein identification. Three independent preparations, each obtained from separate parasite cultures, were used. One representative assay is shown in this study.
Protein digestion, peptide extraction, and spot handling.
Selected spots were manually excised, and fragments were destained with a solution containing 50% methanol and 2.5% acetic acid. The proteins were reduced in 10 mM DTT and alkylated with 50 mM iodoacetamide solution. After drying, gel fragments were placed on ice in a 50-μl protease solution (20 ng ml−1 of a sequence-grade-modified trypsin in 25 mM ammonium bicarbonate [Promega]) for 30 min. Excess protease solution was removed and replaced by 25 mM ammonium bicarbonate. Digestion was performed for 18 h at 37°C. Peptide extraction was performed twice for 15 min, using 30 μl of a 50% acetonitrile–5% formic acid solution. Trypsin (Promega) digests were concentrated in a speed vacuum (Savant) and submitted to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses on an ion trap time of flight (IT-TOF) instrument. The analyses were performed using an electrospray ion-trap time of flight (ESI-IT-TOF) mass spectrometer (Shimadzu Co., Japan) coupled to a binary ultrafast liquid chromatography (UFLC) system (20A Prominence; Shimadzu Co., Japan). Aliquots were loaded using the autosampler in a Discovery C18 column (2.2 μm; 100.0 by 3.0 mm) and diluted in the following binary solvent system: solvent A, water-formic acid at 999:1 (vol/vol); solvent B, acetonitrile (ACN)-water-formic acid (FA) (900:99:1 vol/vol/vol). The column was eluted at a constant flow rate of 0.2 ml/min with a 5 to 60% gradient of solvent B for 40 min. The eluates were monitored using a Shimadzu SPD-M20A PDA detector. Before introduction into the mass spectrometer, the spray voltage was kept at 4.5 kV and the capillary voltage at 1.76 kV at 200°C. The MS spectra were acquired under positive mode and collected in the m/z range of 80 to 1,850. The MS/MS spectra were acquired according to data-dependent acquisition (DDA) parameters. Instrument control, data acquisition, Mascot generic format (MGF) generation, and data processing were performed using our own solutions (LCMS solution 3.60.361 version; Shimadzu).
Protein identification and database search.
The MGF files generated by Protein Post Run Analyses software (Shimadzu Co., Japan) were analyzed using either the Mascot Server v.2.4 or the Peaks Studio v.7 against a customized database consisting of all UniProt entries of the genus Leishmania (downloaded on 31 July 2014). For this, the following parameters were used: one missed cleavage, mass tolerance of 0.2 Da, fixed modification with carbamidomethylcysteine, and variable modification with oxidized methionine. The results are presented using the InChorus Peaks Studio analysis, which combined the results from both the Mascot and Peaks programs.
Data analysis.
The statistical analyses were performed using GraphPad Prism (version 5.0 for Windows), WinEpiscope (version 2.0 for Windows), and Microsoft Excel (version 10.0). The cutoff was chosen based on the point that provides the maximum values of sensitivity and specificity. The accuracy (AC) was analyzed according to the area under the curve (AUC) relative to the receiver operating characteristic (ROC) curves, considering a 95% confidence interval (95% CI). The ROC curves were plotted with the values from serum samples from patients with CL or ML versus those from the control group (noninfected subjects), according to a sick/nonsick rating method. Fisher's test was used to compare the diagnostic performance among the evaluated antigens. P values of <0.05 were considered statistically significant.
RESULTS
Immunoblot assays and identification of target proteins in stationary promastigote and amastigote-like forms of Leishmania braziliensis.
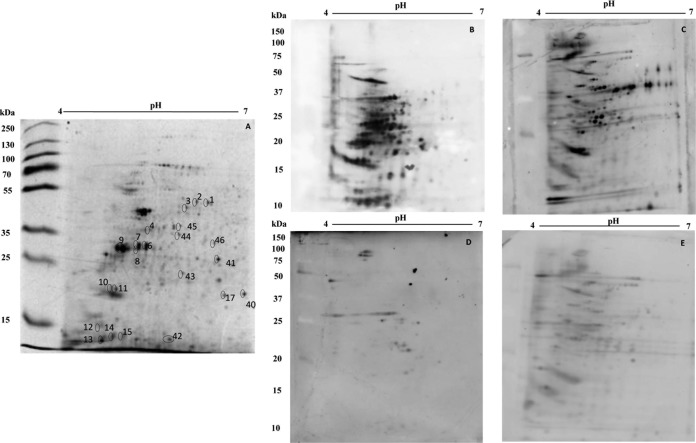
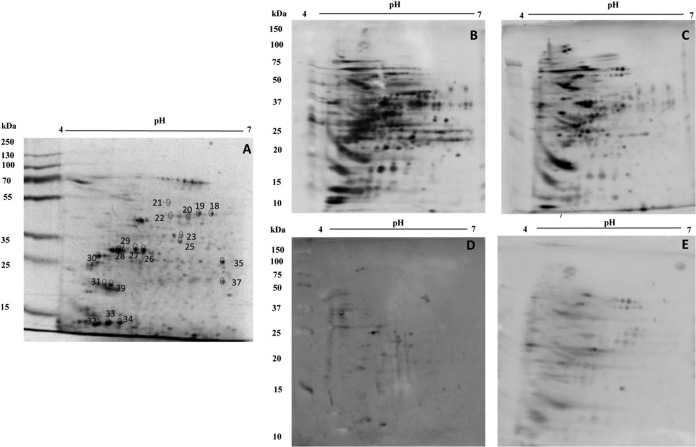
To evaluate the antigenicity of the spots resolved in the 2DE gels of both parasite stages, immunoblots were performed using the different pools of the sera from the patients. Using blots prepared from 2DE gels of promastigote-stage total protein extracts (equivalent to that shown in Fig. 1A), it was possible to verify that about 158 spots were recognized by antibodies present in the sera from ML patients (Fig. 1B). With the sera from CL patients, about 147 spots were recognized in the immunoblots (Fig. 1C). Serum samples from noninfected subjects and from Chagas disease patients were also employed in the assays, and the results showed that 31 and 122 spots, respectively, were identified in the promastigote stage (Fig. 1D and E, respectively). Evaluating the reactive patterns from 2DE blots prepared from gels obtained in the amastigote-like stage of the parasites (a representative stained gel is shown in Fig. 2A), it was possible to verify that about 251 spots were recognized by antibodies of sera from ML patients (Fig. 2B), whereas 197 spots were recognized by sera from CL patients (Fig. 2C). Using the sera from noninfected subjects and those from Chagas disease patients, about 20 and 133 spots, respectively, were recognized in this parasite stage (Fig. 2D and E, respectively). Excluding the cross-reactive spots, a total of 46 and 39 proteins were selected to be identified by mass spectrometry in the promastigote and amastigote-like stages. The 2DE spots profiles obtained were reproducible in terms of the total number of revealed protein spots, and their relative positions and intensities in three independent preparations were determined. After mass spectrometry, 20 proteins were finally identified. A summary of the results is included in Table 1. Briefly, from the 20 identified proteins, 2 hypothetical proteins and 18 proteins with known functions were identified. Six proteins were expressed in the promastigote stage, 4 were found in the amastigote-like stage, and 10 were identified in both parasite stages. Also, 5 proteins were recognized only by sera from ML patients, whereas 15 proteins were recognized by both serum sources. Between them, housekeeping proteins, such as α- and β-tubulins, chaperonins, such as heat shock proteins, and proteins related to parasite virulence, such as enolase (36), actin (37), and paraflagellar rod protein 1D (38), were identified. Proteins evaluated as diagnostic markers, such as heat shock protein 83 (39) and β-tubulin (40), as well as vaccine candidates, such as the paraflagellar rod protein 1D (38) and heat shock protein 70 (41), were also found.
FIG 1.
Two-dimensional profile and immunoproteomic analysis using the stationary-phase promastigote total extracts of Leishmania (Viannia) braziliensis. 2DE was performed using total protein extracts (150 μg) of stationary-phase promastigote-stage parasites (first dimension, IEF, pH range 4 to 7; second dimension, 12% SDS-PAGE). A stained gel is shown in panel A. Blots obtained from equivalent gels were revealed after incubation with pools of sera from mucosal (B) or cutaneous (C) leishmaniasis patients, as well as with pools of sera from noninfected subjects (D) or from patients developing Chagas disease (E). Bound antibodies were detected with conjugated goat anti-human IgG antibody at a 1:5,000 dilution. The x axis represents the tentative isoelectric point (pI), while the y axis represents the approximate molecular mass (kDa), as determined by a commercial gel marker (BenchMark protein ladder). The 46 spots identified after their specific recognition by antibodies in sera from TL patients are marked in the 2DE-stained gel shown in panel A.
FIG 2.
Two-dimensional profile and immunoproteomic analysis using amastigote-like total extracts of Leishmania (Viannia) braziliensis. 2DE was performed with total protein extracts (150 μg) of amastigote-like parasite forms (first dimension, IEF, pH range 4 to 7; second dimension, 12% SDS-PAGE). A stained gel is shown in panel A. Blots prepared from equivalent gels were revealed after incubation with pools of sera from mucosal (B) or cutaneous (C) leishmaniasis patients, as well as with pools of sera from noninfected subjects (D) or from patients developing Chagas disease (E). Bound antibodies were detected with conjugated goat anti-human IgG antibody at a 1:5,000 dilution. The x axis represents the tentative isoelectric point (pI), while the y axis represents the approximate molecular mass (kDa), as determined by a commercial 2DE gel marker (BenchMark protein ladder). The 39 spots identified after their specific recognition by antibodies in sera of TL patients are marked in a 2DE-stained gel shown in panel A.
TABLE 1.
Proteins identified in the total extracts from stationary-phase promastigote and amastigote-like stages from Leishmania braziliensis
| Protein namea | NCBI accession no. | Predicted mol mass (kDa) | Predicted pI | Parasite stageb |
Leishmaniasis typec |
||
|---|---|---|---|---|---|---|---|
| Promastigote | Amastigote-like | CL | ML | ||||
| Enolase | XP_001563419.1 | 46.1 | 5.77 | P | P | + | + |
| Actin | XP_001561807.1 | 21.0 | 5.11 | P | P | + | + |
| Heat shock protein 83 kDa | XP_001567803.1 | 41.4 | 4.66 | P | A | + | + |
| α-Tubulin | CAM45582 | 49.7 | 4.65 | P | P | + | + |
| β-Tubulin | XP_001567862.1 | 49.8 | 4.45 | P | P | + | + |
| Eukaryotic initiation factor 5a | XP_001565563.1 | 17.8 | 4.62 | P | P | + | + |
| Calpain-like cysteine peptidase | XP_001563372.1 | 13,1 | 4.4 | P | P | − | + |
| 14-3-3 protein-like protein | XP_001568962.1 | 29.7 | 4.57 | P | A | − | + |
| Iron superoxide dismutase | XP_001567555.1 | 21.7 | 6.62 | P | A | − | + |
| Paraflagellar rod protein 1D | XP_001567017.1 | 69.3 | 5.21 | A | P | + | + |
| Isocitrate dehydrogenase | XP_001568049.1 | 46.4 | 5.22 | A | P | + | + |
| Cytochrome c oxidase subunit IV | XP_001563117.1 | 39.1 | 5.96 | P | P | + | + |
| Hypothetical protein | XP_001566959.1 | 40.9 | 5.45 | P | P | + | + |
| Translation elongation factor 1β | XP_001568778.1 | 23.0 | 4.32 | A | P | + | + |
| Heat shock protein 70 kDa | XP_001566325.1 | 71.2 | 4.64 | P | P | + | + |
| Putative heat shock protein 70 | XP_001566324.1 | 27.0 | 4.89 | P | P | + | + |
| Tryparedoxin peroxidase | XP_001563558.1 | 22,6 | 6.51 | A | P | + | + |
| Hypothetical protein | XP_001563170.1 | 13.2 | 5.46 | P | A | + | + |
| Proteasome activator protein pa26 | XP_001568187.1 | 24.1 | 4.99 | P | A | − | + |
| Receptor for activated C kinase 1 | XP_001566321.1 | 34.4 | 6.37 | P | A | − | + |
Name of the protein in L. braziliensis.
Parasite stage in which the protein was identified. P, present; A, absent.
Diagnosed by the serum class used in the recognition of the protein. CL, cutaneous leishmaniasis; ML, mucosal leishmaniasis. + positive; −, negative.
Evaluation of some of the identified candidates expressed as recombinant proteins for the serodiagnosis of TL.
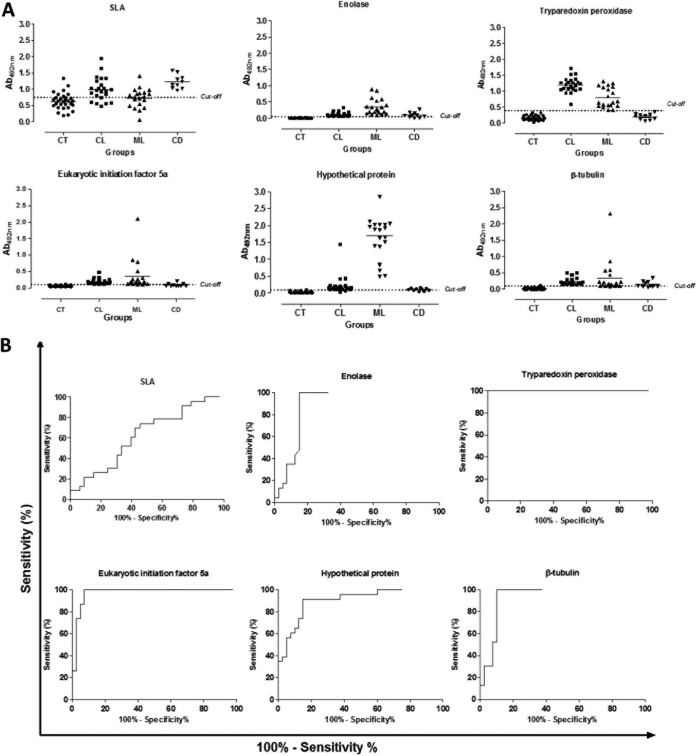
The regions encoding five of the characterized proteins were cloned in a prokaryotic expression vector. These antigens were identified in the immunoblots as protein spots in the promastigote stage (with spot no. 1, 44, 11, and 9 for enolase, hypothetical protein, eukaryotic initiation factor 5a, and β-tubulin, respectively) and amastigote stage (with spot no. 18, 37, 25, 39, and 28 for enolase, tryparedoxin peroxidase, hypothetical protein, eukaryotic initiation factor 5a, and β-tubulin, respectively) of the parasites, and the corresponding recombinant proteins were expressed in bacteria, purified, and employed as antigens to coat ELISA plates in order to validate their use for the TL serodiagnosis. An SLA total extract prepared from L. braziliensis promastigotes was used as a control and comparative antigen in the ELISAs. Serum samples from noninfected control patients and from CL and MCL patients, as well as sera from Chagas disease patients, were employed. The individual optical density (OD) values obtained against the different antigens are shown in Fig. 3A, whereas ROC curves showing the diagnostic value of each antigen are shown in Fig. 3B. The selected proteins, namely, enolase, tryparedoxin peroxidase, hypothetical protein, eukaryotic initiation factor 5a, and β-tubulin, were recognized by both serum sources (CL and ML patients). Of note, cross-reactivity observed between SLA and sera from T. cruzi-infected patients was absent when the individual antigens were employed. Regarding the individual validation of each recombinant antigen (summarized in Table 2), the tryparedoxin peroxidase presented the best results for the serodiagnosis of both CL and ML conditions, since its sensitivity and specificity values were 100% for both cases. As indicated above, this antigen was able to distinguish all of the Chagas disease patients and noninfected subjects from the CL and ML patients. When the other recombinant proteins were evaluated, eukaryotic initiation factor 5a showed sensitivity and specificity values of 100% and 92.5%, respectively. Enolase, β-tubulin, and the hypothetical protein presented sensitivity values of 100, 100, and 95.4%, respectively, whereas their specificity values were 85.0, 82.5, and 85.0%, respectively. The hypothetical protein was able to recognize all sera from patients developing ML, presenting higher OD values than the other diagnostic antigens. As a conclusion, all of them present better diagnostic properties than the SLA (sensitivity and specificity values of 65.1% and 57.5%, respectively) for the serodiagnosis of TL.
FIG 3.
Diagnostic performance of the recombinant proteins against a human serological panel. Serum samples derived from noninfected subjects living in an area of leishmaniasis endemicity (n = 30), as well as sera from cutaneous (n = 23) or mucosal (n = 20) leishmaniasis patients and sera from Chagas disease patients (n = 10), were used in the ELISAs. Reactivities against enolase (1.0 μg per well), tryparedoxin peroxidase (0.5 μg per well), hypothetical protein (0.5 μg per well), eukaryotic initiation factor 5a (0.05 μg per well), β-tubulin (0.01 μg per well), and L. braziliensis SLA (2.0 μg per well) were determined. The mean optical density (OD) value was calculated by subtracting the mean blank OD from the OD mean for each sample by using the values obtained in the ELISAs (A). The cutoff value for each diagnostic antigen was established based on their sensitivity and specificity values, which were obtained by an ROC analysis (B).
TABLE 2.
Diagnostic performance of tryparedoxin peroxidase, eukaryotic initiation factor 5a, enolase, β-tubulin, and recombinant hypothetical protein, as well as L. braziliensis SLA for serodiagnosis of TL
| Antigen | Cutoff valuea | % sensitivity (95% CI) | % specificity (95% CI) | AUC (95% CI) | % AC |
|---|---|---|---|---|---|
| Tryparedoxin peroxidase | 0.374 | 100 (91.8–100) | 100 (91.2–100) | 1.0 (1.0–1.0) | 100 |
| Eukaryotic initiation factor 5a | 0.100 | 100 (91.8–100) | 92.5 (79.6–98.4) | 1.0 (0.9–1.0) | 96.4 |
| Enolase | 0.045 | 100 (91.8–100) | 85.0 (70.2–94.3) | 0.9 (0.9–1.0) | 92.8 |
| β-Tubulin | 0.081 | 100 (91.0–100) | 82.5 (67.2–92.7) | 0.9 (0.9–1.0) | 91.6 |
| Hypothetical protein | 0.089 | 95.4 (84.2–99.4) | 85.0 (70.2–94.3) | 1.0 (0.9–1.0) | 90.4 |
| L. braziliensis SLA | 0.743 | 65.1 (49.1–79.0) | 57.5 (40.9–73.0) | 0.6 (0.5–0.7) | 61.4 |
The cutoff values were obtained using the ROC curves, as well as the sensitivity, specificity, accuracy (AC) and area under the curve (AUC).
DISCUSSION
In the present study, an immunoproteomic approach was applied to screen antigenic L. braziliensis proteins. The purpose was to characterize individual parasite antigens able to be employed for the serodiagnosis of human TL. For this, total extracts from stationary-phase promastigotes and amastigote-like parasites of L. braziliensis resolved in 2DE gels were blotted and tested against serum samples derived from CL and ML patients. For a more refined selection in the identification of the reactive spots, blots were also assayed against pools of sera from noninfected subjects living in areas of disease endemicity, as well as against sera from Chagas disease patients, in order to eliminate cross-reactive candidates.
Nowadays, the diagnosis of TL is based on clinical criteria associated with the laboratory diagnosis (8); however, problems related to the sensitivity of the tests have been described, since a significant percentage of patients are classified as false negative in the serological assays employing antigenic Leishmania preparations, due to the low levels of antileishmanial antibodies encountered in their serum samples (23). In relation to specificity, an important problem for the TL serodiagnosis resides in the cross-reactivity of the antigens with sera from noninfected subjects living in areas of disease endemicity, as well as with serum samples from patients developing related pathologies, such as Chagas disease, which can present cross-reactivity in the serological analysis performed (42). Therefore, there is a necessity to identify new antigens with higher sensitivity and specificity values than total extracts to be used for the improvement in the serodiagnosis of TL. The present study presents promising results, since different L. braziliensis proteins (including two hypothetical ones) were identified as antigenic by their recognition by antibodies of TL patients but not by sera from noninfected subjects or Chagas disease patients.
Proteomic studies have been previously performed with L. braziliensis with the purpose to identify excreted factors, as well as proteins showing variations of expression in different L. braziliensis strains producing diverse clinical forms of the disease (28, 29). The present study is complementary to those performed, since promastigote extracts, as well as amastigote-like-stage proteins, were employed for the identification of the most antigenic proteins of L. braziliensis. Amastigote-like forms were included, since this parasite stage is present in the mammal hosts a few hours after infection and is responsible for the development of active disease in the patients. In addition, the exclusion of the proteins by their cross-reactivity with antibodies from sera of healthy subjects living in areas of endemicity and those from Chagas disease patients represent a refinement of the present proteomic analysis, allowing the discarding of these antigenic proteins in order to be able to diagnose false-positive patients.
The specificity of ELISAs using Leishmania antigenic preparations to diagnose leishmaniasis largely depends on the protein formulation employed, although false-positive and false-negative results have still been encountered in some studies (43–45). In the present study, 20 proteins were identified as possible diagnostic markers for TL. Most of them were expressed in both promastigote and amastigote-like stages of the parasites. As a proof of concept, five of them were selected to be evaluated as candidates in a serological assay. Remarkably, none of them presented cross-reactivity with the sera from Leishmania-noninfected subjects, validating the strategy followed in this research project. The best candidate antigen was the tryparedoxin peroxidase, which presented sensitivity and specificity values of 100% when sera from CL or ML patients were assayed. Tryparedoxin peroxidase belongs to a family of proteins called peroxiredoxins, which are associated with parasite virulence and have a high antigenicity during the active disease. Previous studies have shown the antigenicity of peroxiredoxins in dogs and humans infected by Leishmania infantum or L. braziliensis (46, 47). Khosravi et al. showed that a real-time PCR using the tryparedoxin peroxidase gene was specific for CL diagnosis (48). In this case, the sensitivity and specificity values encountered were 98.7 and 59.8%, respectively. The authors concluded that this gene, when evaluated by a reverse transcription-PCR (RT-PCR) technique, could be considered an effective marker for the diagnosis of the disease. In another study, Santarém et al. showed that a combination between the tryparedoxin peroxidase and K39 proteins was able to improve the VL serodiagnosis (49). However, to the best of our knowledge, an ELISA employing tryparedoxin peroxidase has not been evaluated. Interestingly, the present study showed that it is a viable alternative for the serodiagnosis of TL.
Although enolase had been studied as a vaccine candidate against experimental VL (50, 51), this antigen has not been evaluated as a diagnostic marker for TL, as also occurred for the eukaryotic initiation factor 5a and the hypothetical protein. All of the proteins evaluated here presented sensitivity and specificity values higher than those obtained using SLA from L. braziliensis as an antigen. We concluded that all of them, alone or in a combined form, could be included in future experiments looking for a more sensitive and specific serodiagnosis test of TL. As an interesting alternative, characterization of their immunodominant linear and conformational antigenic determinants may be also a future line of research for the development of such diagnostic tools. The use of synthetic peptides containing the main epitopes of the proteins is emerging as an interesting alternative to recombinant proteins for the serodiagnosis of different diseases, since they are simpler and cheaper to produce (52). Although this approach was not the purpose of the present work, additional studies will certainly be performed to predict the putative B cell epitopes of the new antigenically characterized proteins for their use in ELISAs. The final objective is to obtain a precise, effective, easy to produce, and cheap diagnostic antigen preparation to improve diagnostic performance for the serodiagnosis of TL.
In conclusion, the present study has identified different antigenic L. braziliensis proteins that should be further analyzed. The evaluation of larger human TL serological panels, as well as serum samples obtained from patients developing related pathologies, could be considered for the next steps. This is especially important for those patients living in areas of endemicity who present skin lesions similar to those found in TL patients, such as varicose lesions or fungal skin lesions. We plan to validate not only the 5 selected candidates but also the rest of the 20 identified proteins. In addition, and besides remission of the clinical lesions, the study of the evolution of serology against these proteins during treatment will be a further research pathway toward the development of additional tools for the evaluation of recovery in patients after treatment. In this context, data from the present study could be taken as a proof of concept of the proposed antigens' capacity for TL serodiagnosis and may well serve as a reference for further assays. However, these novel highly accurate recombinant proteins and their use in ELISAs may be promptly applied to the serodiagnosis used in TL due to their simplicity, ease of use, speed, and reproducibility.
ACKNOWLEDGMENTS
This work was supported by grants from Instituto Nacional de Ciência e Tecnologia em Nano-biofarmacêutica (INCT-Nanobiofar), FAPEMIG (CBB-APQ-00496-11 and CBB-APQ-00819-12), and CNPq (APQ-472090/2011-9, APQ-482976/2012-8, and APQ-488237/2013-0). In addition, this study was partially funded in Madrid by a Spanish grant from Ministerio de Economía y Competitividad-FEDER (FISPI14/00366 from the Instituto de Salud Carlos III). M.A.C.F. is a grant recipient of FAPEMIG/CAPES. E.A.F.C., A.P.F., and M.O.C.R. are recipients of grants from CNPq.
REFERENCES
- 1.World Health Organization. 2010. Control of the leishmaniases: report of a meeting of the 399 WHO Expert Committee on the Control of Leishmaniases, Geneva. WHO 400 technical report series 949. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Leishmaniasis Control Team WHO. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Grimaldi G Jr, Tesh RB. 1993. Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev 6:230–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reithinger R, Dujardin J, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect Dis 7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 6.Marzochi MCA, Marzochi KBF. 1994. Tegumentary and visceral leishmaniases in Brazil—emerging anthropozoonosis and possibilities for their control. Cad Saude Publica Rio Janeiro 10:359–375. [DOI] [PubMed] [Google Scholar]
- 7.Silveira FT, Lainson R, Corbett CEP. 2004. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz 99:239–251. doi: 10.1590/S0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 8.Goto H, Lindoso JAL. 2010. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther 8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 9.Vega-López F. 2003. Diagnosis of cutaneous leishmaniasis. Curr Opin Infect Dis 16:97–101. doi: 10.1097/00001432-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Boggild AK, Valencia BM, Veland N, Pilar-Ramos A, Calderon F, Arevalo J, Low DE, Llanos-Cuentas A. 2011. Non-invasive cytology brush PCR diagnostic testing in mucosal leishmaniasis: superior performance to conventional biopsy with histopathology. PLoS One 6:e26395. doi: 10.1371/journal.pone.0026395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunedo SN, Thomaz-Soccol V, Castro EA, Telles JE. 2012. Immunocytochemical and immunohistochemical methods as auxiliary techniques for histopathological diagnosis of cutaneous leishmaniasis. Acta Histochem 114:252–258. doi: 10.1016/j.acthis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Alves CF, Figueiredo MM, Souza CC, Machado-Coelho GL, Melo MN, Tafuri WL, Raso P, Soares RP, Tafuri WL. 2013. American tegumentary leishmaniasis: effectiveness of an immunohistochemical protocol for the detection of Leishmania in skin. PLoS One 8:e63343. doi: 10.1371/journal.pone.0063343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza AP, Soto M, Costa JML, Boaventura VS, de Oliveira CI, Cristal JR, Barral-Netto M, Barral A. 2013. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One 8:e66110. doi: 10.1371/journal.pone.0066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayrink W, Coelho GL, Guimarães TM, Andrade HM, Castro-Peres E, Costa CA, Toledo VP. 2006. Immuno-biochemical evaluations of phenol and thimerosal as antigen preservatives in Montenegro skin test. Acta Trop 98:87–93. doi: 10.1016/j.actatropica.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Perinoto AC, Maki RM, Colhone MC, Santos FR, Migliaccio V, Daghastanli KR, Stabeli RG, Ciancaglini P, Paulovich FV, Oliveira MC, Oliveira ON Jr, Zucolotto V. 2010. Biosensors for efficient diagnosis of leishmaniasis: innovations in bioanalytics for a neglected disease. Anal Chem 82:9763–9768. doi: 10.1021/ac101920t. [DOI] [PubMed] [Google Scholar]
- 16.Malchiodi EL, Chiaramonte MG, Taranto NJ, Zwirner NW, Margni RA. 1994. Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp: use of immunoblotting and ELISA with a purified antigen (Ag163B6). Clin Exp Immunol 97:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito MEF, Mendonça MG, Gomes YM, Jardim ML, Abath FGC. 2000. Identification of potentially diagnostic Leishmania braziliensis antigens in human cutaneous leishmaniasis by immunoblot analysis. Clin Diagn Lab Immunol 7:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colmenares M, Kar S, Goldsmith-Pestana K, McMahon-Pratt D. 2002. Population mechanisms genetics of pathogenesis: differences amongst Leishmania species. Trans R Soc Trop Med Hyg 96:S3–S7. doi: 10.1016/S0035-9203(02)90044-1. [DOI] [PubMed] [Google Scholar]
- 19.Kubar J, Fragaki K. 2005. Recombinant DNA-derived Leishmania proteins: from the laboratory to the field. Lancet Infect Dis 5:107–114. doi: 10.1016/S1473-3099(05)70085-2. [DOI] [PubMed] [Google Scholar]
- 20.Menezes-Souza D, Antônio T, Mendes DO, Carolina A, Leão DA, Souza-Gomes M, Fujiwara RT, Bartholomeu DC. 2015. Linear B-cell epitope mapping of MAPK3 and MAPK4 from Leishmania braziliensis: implications for the serodiagnosis of human and canine leishmaniasis. Appl Microbiol Biotechnol 99:1323–1336. doi: 10.1007/s00253-014-6168-7. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez Y, Salinas GH, Palma G, Valderraa LB, Santrich CV, Saravia NG. 1991. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg 45:281–289. [DOI] [PubMed] [Google Scholar]
- 22.Gomes-Silva A, Pereira-Carvalho R, Fagundes-Silva G, Oliveira-Neto MP, Da-Cruz AM. 2009. Homeostasis of specific immune response in clinically cured cutaneous leishmaniasis subjects due to Leishmania (Viannia) braziliensis. Rev Soc Bras Med Trop 42:147–150. [Google Scholar]
- 23.Fagundes-Silva GA, Vieira-Goncalves R, Nepomuceno MP, de Souza MA, Favoreto S Jr, Oliveira-Neto MP, Da-Cruz AM, Gomes-Silva A. 2012. Decrease in anti-Leishmania IgG3 and IgG1 after cutaneous leishmaniasis lesion healing is correlated with the time of clinical cure. Parasite Immunol 34:486–491. doi: 10.1111/j.1365-3024.2012.01379.x. [DOI] [PubMed] [Google Scholar]
- 24.Delgado O, Guevara P, Silva S, Belfort E, Ramirez JL. 1996. Follow-up of a human acidental infection by Leishmania (Viannia) braziliensis using conventional immunologic techniques and polymerase chain reaction. Am J Trop Med Hyg 55:267–272. [DOI] [PubMed] [Google Scholar]
- 25.Brito MEF, Mendonça MG, Gomes YM, Jardim ML, Abath FGC. 2001. Dynamics of the antibody response in patients with therapeutic os spontaneous cure of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 95:203–206. doi: 10.1016/S0035-9203(01)90168-3. [DOI] [PubMed] [Google Scholar]
- 26.Junqueira Pedras M, Orsini M, Castro M, Passos VMA, Rabelo A. 2003. Antibody subclasse profile against Leishmania braziliensis and Leishmania amazonensis in the diagnosis and follow-up of mucosal leishmaniasis. Diagn Microbiol Infect Dis 47:477–485. doi: 10.1016/S0732-8893(03)00141-X. [DOI] [PubMed] [Google Scholar]
- 27.Castellano LR, Filho DC, Argiro L, Dessein H, Prata A, Dessein A, Rodrigues V. 2009. Th1/Th2 immune responses are associated with active cutaneous leishmaniasis and clinical cure is associated with strong interferon gamma production. Hum Immunol 70:383–390. doi: 10.1016/j.humimm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Cuervo P, Jesus JB, Junqueira M, Mendonça-Lima L, González LJ, Betancourt L, Grimaldi G Jr, Domont GB, Fernandes O, Cupolillo E. 2007. Proteome analysis of Leishmania (Viannia) braziliensis by two-dimensional gel electrophoresis and mass spectrometry. Mol Biochem Parasitol 154:6–21. doi: 10.1016/j.molbiopara.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Cuervo P, De Jesus JB, Saboia-Vahia L, Mendonça-Lima L, Domont GB, Cupolillo E. 2009. Proteomic characterization of the released/secreted proteins of Leishmania (Viannia) braziliensis promastigotes. J Proteomics 73:79–92. doi: 10.1016/j.jprot.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Coelho EA, Tavares CA, Carvalho FA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP. 2003. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994. doi: 10.1128/IAI.71.7.3988-3994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle PS, Engel JC, Pimenta PF, da Silva PP, Dwyer DM. 1991. Leishmania donovani: long-term culture of axenic amastigotes at 37°C. Exp Parasitol 73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 32.Lewis TS, Hunt JB, Aveline LD, Jonscher KR, Louie DF, Yeh JM, Nahreini TS, Resing KA, Ahn NG. 2000. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol Cell 6:1343–1354. doi: 10.1016/S1097-2765(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Melo MN, Mayrink W, da Costa CA, Magalhães PA, Dias M, Williams P, Araujo FG, Coelho MV, Batista SM. 1977. Standardization of the Montenegro antigen. Rev Inst Med Trop Sao Paulo 19:161–164. [PubMed] [Google Scholar]
- 35.Neuhoff V, Arold N, Taube D, Ehrhardt W. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie brilliant blue G-250 and R-250. Electrophoresis 9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 36.Quiñones W, Peña P, Domingo-Sananes M, Cáceres A, Michels PA, Avilan L, Concepción JL. 2007. Leishmania mexicana: molecular cloning and characterization of enolase. Exp Parasitol 116:241–251. doi: 10.1016/j.exppara.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Azevedo E, Oliveira LT, Castro-Lima AK, Terra R, Dutra PM, Salerno VP. 2012. Interactions between Leishmania braziliensis and macrophages are dependent on the cytoskeleton and myosin Va. J Parasitol Res 2012:275436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunger-Glaser I, Seebeck T. 1997. Deletion of the genes for the paraflagellar rod protein PFR-A in Trypanosoma brucei is probably lethal. Mol Biochem Parasitol 90:347–351. doi: 10.1016/S0166-6851(97)00139-4. [DOI] [PubMed] [Google Scholar]
- 39.Celeste BJ, Arroyo-Sanchez MC, Ramos-Sanchez EM, Castro LG, Lima-Costa FA, Goto H. 2014. Recombinant Leishmania infantum heat shock protein 83 for the serodiagnosis of cutaneous, mucosal, and visceral leishmaniases. Am J Trop Med Hyg 90:860–865. doi: 10.4269/ajtmh.13-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pateraki E, Portocala R, Labrousse H, Guesdon JL. 1983. Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect Immun 42:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira GG, Magalhaes FB, Teixeira MC, Pereira AM, Pinheiro CG, Santos LR, Nascimento MB, Bedor CN, Albuquerque AL, dos Santos WL, Gomes YM, Moreira ED Jr, Brito ME, Pontes-de-Carvalho LC, Melo Neto OP. 2011. Characterization of novel Leishmania infantum recombinant proteins encoded by genes from five families with distinct capacities for serodiagnosis of canine and human visceral leishmaniasis. Am J Trop Med Hyg 85:1025–1034. doi: 10.4269/ajtmh.2011.11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vexenat AC, Santana JM, Teixeira AR. 1996. Cross-reactivity of antibodies in human infections by the kinetoplastid protozoa Trypanosoma cruzi, Leishmania chagasi and Leishmania (Viannia) braziliensis. Rev Inst Med Trop São Paulo 38:177. [DOI] [PubMed] [Google Scholar]
- 43.Coelho EAF, Ramírez L, Costa MAF, Coelho VTS, Martins VT, Chávez-Fumagalli MA, Oliveira DM, Tavares CA, Bonay P, Nieto CG, Abánades DR, Alonso C, Soto M. 2009. Specific serodiagnosis of canine visceral leishmaniasis using Leishmania species ribosomal protein extracts. Clin Vaccine Immunol 16:1774–1780. doi: 10.1128/CVI.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chávez-Fumagalli MA, Martins VT, Testasicca MCS, Lage DP, Costa LE, Lage PS, Duarte MC, Ker HG, Ribeiro TG, Carvalho FA, Régis WC, Reis AB, Tavares CA, Soto M, Fernandes AP, Coelho EA. 2013. Sensitive and specific serodiagnosis of Leishmania infantum infection in dogs by using peptides selected from hypothetical proteins identified by an immunoproteomic approach. Clin Vaccine Immunol 20:835–841. doi: 10.1128/CVI.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins VT, Chávez-Fumagalli MA, Costa LE, Canavaci AM, Lage PS, Lage DP, Duarte MC, Valadares DG, Magalhães RD, Ribeiro TG, Nagem RA, Darocha WD, Régis WC, Soto M, Coelho EA, Fernandes AP, Tavares CA. 2013. Antigenicity and protective efficacy of a Leishmania amastigote-specific protein, member of the super-oxygenase family, against visceral leishmaniasis. PLoS Negl Trop Dis 7:e2148. doi: 10.1371/journal.pntd.0002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santarem N, Tomas A, Ouaissi A, Tavares J, Ferreira N, Manso A, Campino L, Correia JM, Cordeiro-da-Silva A. 2005. Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunol Lett 101:18–23. doi: 10.1016/j.imlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Menezes-Souza D, Antônio T, Mendes DO, Nagem RA, Oliveira-Santos TT, Silva AL, Santoro M, Guimarães-Carvalho S, Coelho EA, Bartholomeu DC, Fujiwara RT. 2014. Mapping B-cell epitopes for the peroxidoxin of Leishmania (Viannia) braziliensis and its potential for the clinical diagnosis of tegumentary and visceral leishmaniasis. PLoS One 9:e99216. doi: 10.1371/journal.pone.0099216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khosravi S, Hejazi SH, Hashemzadeh M, Eslami G, Darani HY. 2012. Molecular diagnosis of Old World leishmaniasis: real-time PCR based on tryparedoxin peroxidase gene for the detection and identification of Leishmania spp. J Vector Borne Dis 49:15–18. [PubMed] [Google Scholar]
- 49.Santarem N, Silvestre R, Cardoso L, Schallig H, Reed SG, Cordeiro-da-Silva A. 2010. Application of an improved enzyme-linked immunosorbent assay method for serological diagnosis of canine leishmaniasis. J Clin Microbiol 48:1866–1874. doi: 10.1128/JCM.02402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumari S, Samant M, Misra P, Khare P, Sisodia B, Shasany AK, Dube A. 2008. Th1-stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine 26:5700–5711. doi: 10.1016/j.vaccine.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Gupta R, Kumar V, Kushawaha PK, Tripathi CP, Joshi S, Sahasrabuddhe AA, Mitra K, Sundar S, Siddiqi MI, Dube A. 2014. Characterization of glycolytic enzymes, rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS One 9:e86073. doi: 10.1371/journal.pone.0086073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noya O, Patarroyo ME, Guzmán F, Alarcón de Noya B. 2003. Immunodiagnosis of parasitic diseases with synthetic peptides. Curr Protein Pept Sci 4:299–308. doi: 10.2174/1389203033487153. [DOI] [PubMed] [Google Scholar]