Abstract
No previous studies have assessed the persistence of immune responses in individuals with diabetes. We conducted this study to evaluate the long-term immunogenicity and safety of an influenza vaccine in type 2 diabetic subjects compared with nondiabetic controls. A randomized and controlled study was conducted at two university hospitals during the 2012-2013 influenza season. The study vaccine was a standard-dose trivalent subunit inactivated intramuscular vaccine. Serum hemagglutination-inhibiting (HI) antibodies were measured at the time of vaccination and 1 month and 6 months after vaccination. Local and systemic reactions were recorded for 7 days. A total of 105 diabetic patients and 108 controls were included in the analysis. One month after vaccination, both the diabetic and nondiabetic groups satisfied all of the criteria of the Committee for Medical Products for Human Use (CHMP), and the immunogenicity profiles were statistically similar between the two groups. Although the vaccine was well tolerated, and all adverse reactions were mild to moderate, there was a tendency toward a reduced incidence of local reactions in the diabetic group. All values in the long-term immunogenicity profiles were statistically similar between the two groups, except for the seroprotection rate for the A/H1N1 influenza virus strain, which was significantly lower in the elderly diabetic group than that in the elderly nondiabetic group. However, in multivariate analysis, long-term immunogenicity was associated with age and prevaccination titer, regardless of diabetes status. (This study has been registered at CRIS [https://cris.nih.go.kr/cris/en/] under registration no. KCT0001423.)
INTRODUCTION
Although the available data are limited, diabetic individuals may be more susceptible to influenza infections than nondiabetic individuals (1). In addition, individuals with diabetes are at increased risk of severe influenza virus infection and its complications compared to nondiabetic persons (2–4). Such phenomena are thought to be mediated by impairments in cellular and humoral immunity, which include reduced T cell responses, decreased neutrophil function, and B cell disorders (5). For this reason, annual influenza vaccination is universally recommended for patients with diabetes.
To achieve protection against influenza virus infection, vaccinations should elicit a sufficient antibody response. Many studies have shown that diabetic individuals have an immune response to influenza vaccination similar to that of healthy controls, while a few studies have reported suboptimal responses in diabetic subjects (6–12). Immunogenicity should be maintained throughout the entire seasonal epidemic; therefore, an evaluation of long-term immunogenicity is essential before the current conventional vaccination program can be recommended. However, no study has assessed long-term immunogenicity in individuals with diabetes.
If the immune responses and safety profiles prove to be unsatisfactory with the conventional influenza vaccine, immunogenicity-enhancing strategies, including high-dose, booster, and adjuvant use or use of an intradermal route, may need to be considered. We conducted this study to evaluate the long-term immunogenicity and safety of the influenza vaccine in type 2 diabetic subjects in comparison with those in nondiabetic controls.
MATERIALS AND METHODS
Ethics statement.
This study (clinical trial registration no. KCT0001423) was approved by the institutional review board (IRB) of each hospital, Korea University Guro Hospital, Inha University Hospital, and Kangnam Sacred Heart Hospital, all of which are located in the Republic of Korea. This study was also performed in accordance with the Helsinki Declaration and Good Clinical Practices.
Study subjects and vaccine.
This multicenter, randomized, and controlled study was conducted during the 2012-2013 influenza season. Adults ≥19 years of age with type 2 diabetes who were not immunized with the 2012-2013 influenza vaccine were recruited during the preinfluenza period. Adults without diabetes were also recruited as study controls. Informed consent was obtained from all participants. Exclusion criteria included a known allergy to eggs, presentation of any febrile illness of ≥37.5°C on the day of vaccination, any history of a hypersensitive reaction to a previous influenza vaccination, any other vaccinations within the past month, use of immunosuppressive agents, having received blood products or immunoglobulins during the previous 3 months, and any other conditions that might interfere with the study results. The study vaccine was a standard-dose trivalent subunit inactivated intramuscular vaccine (Agrippal S1; Novartis Vaccines and Diagnostics S. R. L., Italy). The vaccine contained an A/California/7/2009 (H1N1)-like strain, an A/Victoria/361/2011 (H3N2)-like strain, and a B/Brisbane/60/2008-like strain, as recommended by the WHO during 2012-2013 influenza season.
Antibody assay.
Blood samples were taken from all participants prior to vaccination and at 1 month and 6 months after vaccination. Hemagglutination-inhibiting (HI) antibodies against each of the three antigen components were measured using a standard microtiter assay (13). In brief, serum was treated with a receptor-destroying enzyme (Sigma, St. Louis, MO, USA). Serum dilutions ranging from 1:5 to 1:5,120 were then prepared. HI titers were read after a 0.5% suspension of washed chicken erythrocytes was added.
The antibody response was interpreted according to the criteria of the Committee for Medical Products for Human Use (CHMP). The geometric mean titer (GMT), seroprotection rate (proportion of participants with an HI titer of ≥1:40), seroconversion rate (proportion of participants with a ≥4-fold increase in titer from baseline or a postvaccination HI titer of ≥1:40 if the baseline titer was <1:40), and mean fold increase (MFI) (GMT ratio of postvaccination HI titer to prevaccination HI titer) were calculated. The vaccine approval criteria in adults <60 years were a seroprotection rate of >70%, seroconversion rate of >40%, and MFI of >2.5.
Safety assessment.
At the time of vaccination, participants were provided with a thermometer, ruler, and diary and were asked to monitor any local or systemic reactions for 7 days. The diary was based on the Food and Drug Association (FDA) Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials (14). The contents of the diary included details about body temperature, pain, tenderness, redness and induration diameter at the injection site, and the severity of systemic symptoms, such as headache, malaise, chills, muscle aches, arthralgia, and any other adverse events. Redness and induration diameter were considered mild if the diameter was 1 to 4 mm, moderate if the diameter was 5 to 9 mm, and severe if the diameter was ≥10 mm.
Statistical analysis.
An analysis of covariance (ANCOVA) model was used to compare the postvaccination results adjusted according to prevaccination levels. Comparisons of the seroprotection rate, seroconversion rate, and mean fold increase according to age and diabetes status were performed. For subgroup analyses, subjects were divided into age groups of ≤60 years and >60 years. The chi-square or Fisher's exact test was used for bivariate analyses. Mean comparisons were performed by the Student t test or Mann-Whitney test. Univariate analysis was used to identify independent factors associated with seroprotection and seroconversion at 6 months postvaccination. Additionally, multivariate logistic regression analysis was performed. The variables studied included gender, age, smoking history, comorbidity, prevaccination GMT, duration of diabetes, hemoglobin A1c (HbA1c) level, and insulin use. All P values were two-sided and accepted as significant at values of <0.05. All analyses were performed using the SPSS 18.0 software (SPSS Korea, Seoul, Republic of Korea).
RESULTS
Study participant characteristics.
A total of 118 patients with diabetes and 118 nondiabetic controls were enrolled. During the study period, 13 diabetic participants and 10 controls did not complete a second or third visit. Therefore, data were available for 105 (89.0%) diabetic patients and 108 (91.5%) controls. Table 1 shows the baseline characteristics of the diabetic and nondiabetic groups. The majority of the participants were female, and females were more common in the nondiabetic group (P < 0.001). The mean age was statistically similar between the two groups (63 and 60 years for the diabetic and nondiabetic groups, respectively). The proportion of the elderly (age, >60 years) subjects was nearly 60% in each group, and the proportions did not differ between the groups. Smokers predominated among the diabetic patients. Diabetic patients had more comorbidities than the nondiabetic controls (P < 0.001). Chronic heart disease and chronic renal disease were more frequent in diabetic patients. Among diabetes patients, the mean duration of diabetes was 8.8 years, and the mean HbA1c level was 7.1 mmol/mol.
TABLE 1.
Baseline characteristics of study participants
| Characteristic | Diabetic patients (n = 105) | Nondiabetic controls (n = 108) | P value |
|---|---|---|---|
| Male (no. [%]) | 51 (48.6) | 21 (29.2) | <0.001 |
| Age (mean ± SD) (yr) | 63.0 ± 9.7 | 60.0 ± 14.1 | 0.072 |
| >60 years (no. [%]) | 63 (60.0) | 67 (62.0) | 0.780 |
| Current smoker (no. [%]) | 23 (21.9) | 8 (7.4) | 0.003 |
| Comorbidity (no. [%])a | 32 (30.5) | 7 (6.5) | <0.001 |
| Chronic heart disease | 18 (17.1) | 2 (1.9) | <0.001 |
| Cerebrovascular disease | 5 (4.8) | 4 (3.7) | 0.746 |
| Chronic lung disease | 2 (1.9) | 0 (0) | 0.242 |
| Chronic renal disease | 6 (5.7) | 0 (0) | 0.013 |
| Chronic liver disease | 3 (2.9) | 0 (0) | 0.118 |
| Solid tumor | 4 (3.8) | 2 (1.9) | 0.441 |
| Duration of diabetes (mean ± SD) (yr) | 8.8 ± 7.3 | ||
| HbA1c (mean ± SD) (mmol/mol) | 7.1 ± 1.3 | ||
| Insulin use (no. [%]) | 90 (85.7) |
Some patients had several comorbidities.
Immunogenicity.
The GMTs and seroprotection data prevaccination and at 1 month and 6 months postvaccination for all three strains are presented in Table 2. Prevaccination, the GMTs and seroprotection rates for the A/H3N2 and the B strains were similar between the two groups. However, the values for the A/H1N1 strain were significantly lower in the diabetic group than those in the nondiabetic controls. One month after vaccination, the GMTs and seroprotection rates had increased significantly in both groups for all three virus strains (P < 0.001). However, differences were not found between the two groups. Six months postvaccination, the GMTs and seroprotection rates for the A/H1N1 and B strains had decreased significantly but showed a tendency to remain higher than the prevaccination levels. The GMTs and seroprotection rates for A/H3N2 remained higher until 6 months postvaccination in both groups.
TABLE 2.
Antibody responses as measured with the hemagglutination inhibition assay
| Value by time period | A/H1N1 virus |
A/H3N2 virus |
B virus |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Diabetic patients (n = 105) | Nondiabetic controls (n = 108) | P value | Diabetic patients (n = 105) | Nondiabetic controls (n = 108) | P value | Diabetic patients (n = 105) | Nondiabetic controls (n = 108) | P value | |
| Prevaccination | |||||||||
| Geometric mean titer (95% CI) | 16.2 (13.1–20.0) | 26.7 (21.3–33.4) | 0.001 | 78.4 (61.4–100.1) | 80.0 (62.9–101.8) | 0.909 | 11.4 (9.9–13.1) | 13.7 (11.9–15.8) | 0.075 |
| Antibody titer ≥40 (no. [%]) | 25 (23.8) | 47 (43.5) | 0.002 | 82 (78.1) | 83 (76.9) | 0.828 | 12 (11.4) | 17 (15.7) | 0.426 |
| 1 mo postvaccination | |||||||||
| Geometric mean titer (95% CI) | 63.3 (51.3–78.2) | 67.9 (55.1–83.5) | 0.649 | 282.3 (222.5–358.2) | 270.8 (217.5–337.1) | 0.800 | 39.9 (33.2–48.0) | 33.2 (27.8–39.9) | 0.170 |
| Seroprotection (no. [%]) | 73 (69.5) | 83 (76.9) | 0.227 | 104 (99.0) | 106 (98.1) | 0.578 | 59 (56.2) | 65 (60.2) | 0.555 |
| Seroconversion (no. [%]) | 57 (54.3) | 44 (40.7) | 0.048 | 49 (46.7) | 53 (49.1) | 0.725 | 57 (54.3) | 56 (51.9) | 0.722 |
| Mean fold increase (95% CI) | 3.4 (2.7–4.3) | 2.9 (2.3–3.6) | 0.324 | 3.6 (2.8–4.6) | 2.7 (2.7–4.2) | 0.708 | 3.3 (2.7–4.1) | 2.6 (2.2–3.0) | 0.066 |
| 6 mo postvaccination | |||||||||
| Geometric mean titer (95% CI) | 33.3 (27.5–38.0) | 34.8 (29.7–40.8) | 0.530 | 161.1 (127.1–204.2) | 159.0 (128.9–196.0) | 0.935 | 22.7 (19.6–26.2) | 18.6 (16.2–21.5) | 0.061 |
| Seroprotection (no. [%]) | 46 (43.8) | 64 (59.3) | 0.028 | 100 (95.2) | 103 (95.4) | 1.000 | 33 (31.4) | 37 (34.3) | 0.600 |
| Seroconversion (no. [%]) | 28 (26.7) | 21 (19.4) | 0.211 | 36 (34.3) | 32 (29.6) | 0.466 | 34 (32.4) | 26 (24.1) | 0.178 |
| Mean fold increase (95% CI) | 1.7 (1.4–2.0) | 1.5 (1.3–1.8) | 0.523 | 2.1 (1.6–2.6) | 2.0 (1.7–2.4) | 0.801 | 1.9 (1.6–2.2) | 1.5 (1.3–1.7) | 0.021 |
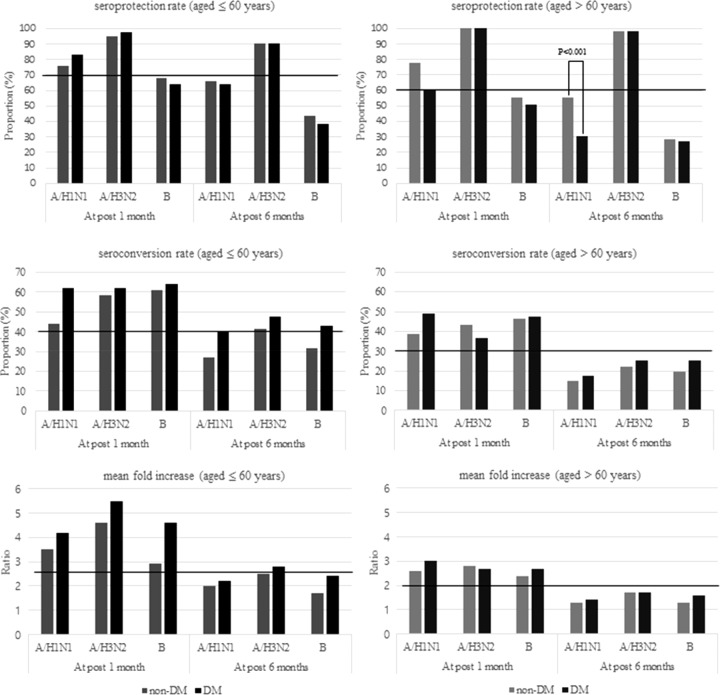
The immunogenicity profiles according to age group are shown in Fig. 1. One month postvaccination, both the diabetic group and the nondiabetic group satisfied all CHMP criteria for the A/H1N1 and A/H3N2 strains. Each value in the immunogenicity profile was statistically similar between the two groups, regardless of age group. Six months after vaccination, the immunogenicity profiles in the elderly groups were somewhat unsatisfactory. In contrast, the initial immune responses were better preserved in the young adult groups. In all groups, seroprotection rates remained high (≥90%) for the A/H3N2 strain only. None of the values of the CHMP criteria were different between the diabetic and nondiabetic groups, regardless of age group. The exception to this was the seroprotection rate for the A/H1N1 strain, which was significantly lower in the elderly diabetic group than that in the elderly nondiabetic group.
FIG 1.
Comparison of seroprotection rate, seroconversion rate, and mean fold increase (MFI) according to the age and diabetes status. Horizontal lines indicate the cutoff value for each CHMP criterion.
Factors associated with long-term seroprotection and seroconversion.
Multivariate analysis was performed to find factors that influenced differences in seroprotection rates and seroconversion rates (Table 3). On multivariate analysis, age and prevaccination GMT were found to be related to long-term seroprotection and seroconversion. Increasing age was related to the failure of both long-term seroprotection and seroconversion. High prevaccination GMT was the predictor of the maintenance of long-term seroprotection, and low prevaccination GMT was independently associated with long-term seroconversion. No other factor, including diabetes, was identified as an independent factor of long-term seroprotection and seroconversion. For the strain A/H3N2, advanced age and high prevaccination GMT were also the predictors of long-term immunogenicity: the seroprotection prevaccination GMT odds ratio was 59.80 (95% confidence interval [CI], 7.35 to 486.65), the old age odds ratio was 0.96 (95% CI, 0.92 to 0.99), and the seroconversion prevaccination GMT odds ratio was 0.01 (95% CI, 0 to 0.10), and the old age odds ratio was 0.95 (95% CI, 0.93 to 0.97). For the B strain, no factor was identified as a predictor in the multivariate analysis. However, age and prevaccination GMT showed significant differences in the univariate analysis: the seroprotection prevaccination GMT had a P value of <0.001 and an age P value of 0.005, and the seroconversion prevaccination GMT had a P value of 0.041 and an age P value of 0.005. The individuals who achieved seroprotection and seroconversion were younger, and prevaccination titers were higher in the positive seroprotection group and lower in the positive seroconversion group.
TABLE 3.
Factors related to seroprotection and seroconversion for A/H1N1 at 6 months postvaccination
| Factor | Seroprotection |
Seroconversion |
||||||
|---|---|---|---|---|---|---|---|---|
| Negative (n = 103) | Positive (n = 110) | Odds ratio (95% CI) | P value | Negative (n = 164) | Positive (n = 49) | Odds ratio (95% CI) | P value | |
| Male (no. [%]) | 35 (34.0) | 37 (33.6) | 49 (29.9) | 23 (46.9) | ||||
| Age (mean ± SD) (yr) | 64.6 ± 1.0 | 58.6 ± 13.4 | 0.95 (0.92–0.98) | 0.001 | 62.2 ± 12.2 | 59.1 ± 11.9 | 0.97 (0.94–0.99) | 0.018 |
| Current smoker (no. [%]) | 13 (12.6) | 18 (16.4) | 24 (14.6) | 7 (14.3) | ||||
| Comorbidity (no. [%])a | ||||||||
| Diabetes | 59 (57.3) | 46 (41.8) | 77 (47.0) | 28 (57.1) | ||||
| Chronic heart disease | 10 (9.7) | 10 (9.1) | 13 (7.9) | 7 (14.3) | ||||
| Cerebrovascular disease | 6 (5.8) | 3 (2.7) | 8 (4.9) | 1 (2.0) | ||||
| Chronic lung disease | 1 (1.0) | 1 (0.9) | 1 (0.6) | 1 (2.0) | ||||
| Chronic renal disease | 3 (2.9) | 3 (2.7) | 3 (1.8) | 3 (6.1) | 5.21 (0.96–28.3) | 0.056 | ||
| Chronic liver disease | 1 (1.0) | 2 (1.8) | 2 (1.2) | 1 (2.0) | ||||
| Solid tumor | 2 (1.9) | 4 (3.6) | 5 (3.0) | 1 (2.0) | ||||
| Prevaccination A/H1N1 GMT (mean ± SD) | 10.6 ± 1.9 | 39.5 ± 3.2 | 34.99 (12.64–96.84) | <0.001 | 24.5 ± 3.4 | 12.2 ± 2.0 | 0.21 (0.09–0.48) | <0.001 |
| Participants with diabetes | ||||||||
| Duration of diabetes (mean ± SD) (yr) | 10.3 ± 7.1 | 7.0 ± 7.3 | 9.7 ± 7.3 | 7.3 ± 7.0 | ||||
| HbA1c (mean ± SD) (mmol/mol) | 7.1 ± 1.5 | 6.8 ± 1.0 | 7.0 ± 1.4 | 7.0 ± 1.1 | ||||
| Insulin use (no. [%]) | 31 (70.5) | 15 (78.9) | 39 (75.0) | 7 (63.6) | ||||
Some patients had several comorbidities.
Safety.
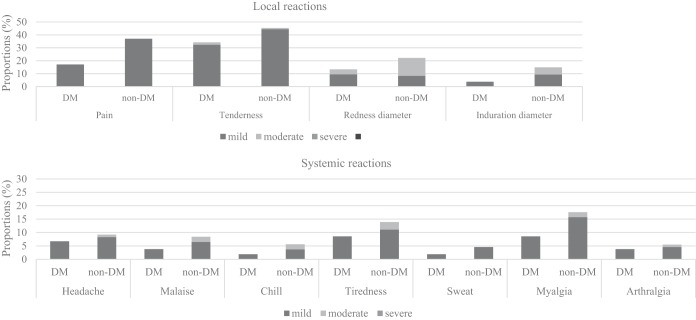
The overall incidences of local and systemic reactions are reported in Fig. 2. The vaccines were well tolerated, and all reactions were mild to moderate. The most frequent local reaction was tenderness at the injection site, which was reported by 34.3% of the participants in the diabetes group and 45.3% in the nondiabetic group (P < 0.001). Other common local reactions were pain, redness, and swelling, all of which occurred less frequently in the diabetic group. The most common systemic reaction was myalgia (diabetes group, 8.6%; nondiabetes group, 15.7%), followed by tiredness, headache, malaise, chills, and arthralgia. The number of participants who experienced any systemic reaction was 20 (19.0%) in the diabetes group and 29 (26.9%) in the nondiabetes group (P = 0.195). The average number of systemic reactions per person was 0.35 in the diabetes group and 0.75 in the nondiabetes controls (P = 0.061). There was a tendency toward a lower incidence of systemic reactions in the diabetic group, but the frequency of each systemic reaction was not significantly different.
FIG 2.
Solicited local and systemic adverse reactions within 7 days after vaccination. All local reactions were statistically different between the diabetes mellitus (DM) group and non-DM group. All systemic reactions were statistically similar between the DM group and non-DM group.
DISCUSSION
Diabetes is associated with impaired T cell function and disorders of humoral immunity (15). To our knowledge, only seven studies have been published regarding the immunogenicity of influenza vaccination among diabetic patients (6–12). Of them, six studies concluded that the immune response was similar between diabetic individuals and healthy controls. This study also revealed that the conventional intramuscular vaccine was sufficient to elicit a satisfactory immune reaction in diabetic individuals, and it demonstrates that immunogenicity was not grossly different from that in nondiabetic persons. Although the profiles did not totally satisfy the CHMP criteria at 6 months postvaccination, the HI antibody responses showed a trend toward persistence in young adults. Consistent with a previous study, age appeared to be an important factor influencing immune response and persistence (16). The seroprotection rate for the A/H3N2 strain was especially high. About 2 months after the vaccinations, the seasonal influenza epidemic started, and A/H3N2 was the dominant strain. Exposure to circulating A/H3N2 may have influenced the high reactivity and persistence of immunogenicity for the A/H3N2 strain.
The differences in the immunogenicity profiles between the diabetic and the nondiabetic groups appeared 6 months after vaccination. The seroprotection rate was significantly lower in the elderly diabetes group than in the elderly nondiabetic group. To find factors affecting this, we conducted a further comparison according to seroprotection status. In this analysis, prevaccination GMTs and age were important factors. Diabetes itself was not related to long-term immunogenicity. Furthermore, glucose control status (HbA1c level) and duration of diabetes were not related to the observed difference. To date, there has been only one study that evaluated demographic and clinical factors influencing humoral immune responses in diabetic individuals (7). Even though the study evaluated only short-term immunogenicity 1 month postvaccination and analyzed the predictive factors only among the patients with diabetes, the study concluded that old age and a longer duration of diabetes were related to a failure to achieve seroprotection. However, the study did not correct the values for multiple comparisons. The longer duration of diabetes might be significantly related to increasing age. A quantitative review study reported that the antibody response to the influenza vaccine is lower in elderly individuals (17). This observation has been explained by the gradual deterioration of the immune system with age, termed immunosenescence. Our study also noted that long-term immunogenicity was significantly lower in the elderly, regardless of diabetes status.
Our study also found that prevaccination GMTs significantly influenced long-term seroprotection. This effect may be explained by booster effects. Preexisting immune memory might strengthen the immune reaction and persistence of vaccination. The A/H3N2 strain was the dominant circulating strain during the 3 years prior to this study. Thus, the overall prevaccination titers for the A/H1N1 strain seemed to be relatively low in our participants. Interestingly, the values were significantly lower in diabetic individuals than those in nondiabetic participants. Although statistical significance was not demonstrated, similar trends have been noted in previous studies. The differences might be due to differences in influenza immunization status and history of influenza infection in the years preceding these studies. Another explanation may be related to B cell dysfunction in diabetic individuals. Either diabetic individuals had lower antibody responses to past infections, or their antibody responses had fallen faster than those of nondiabetic subjects. Although a few studies have reported that there were no differences in B cell responses to influenza vaccination between diabetic individuals and healthy controls, immune reactions were measured only at 1 month postvaccination (6, 8). Long-term studies of B cell responses are needed to explain the low prevaccination titers and weak persistence of immunogenicity in elderly individuals with diabetes.
Regarding seroconversion, high prevaccination GMT was found to be associated with a failure of long-term seroconversion. The result was in accordance with those of previous studies (18, 19). However, it is not clear why this phenomenon occurs. To satisfy the criteria for seroconversion, a 4-fold increase in titer is needed. Thus, high prevaccination titer might be a limiting factor for initial seroconversion. Otherwise, it is likely that postvaccination GMT level seems to be placed mostly under the cutoff value of seroconversion criteria despite the greater strength of the immune reaction with a high prevaccination titer.
In the present study, local reactions were less frequently reported by diabetic individuals than by nondiabetics, although systemic reactions were reported at similar rates in the two groups. Because pain is subjective, these differences may not be comprehensively explained. Sensory impairment is common in diabetic individuals due to diabetic neuropathy, which might influence local sensitivity to an injection. Otherwise, the low frequency of local reactions might suggest that the diabetic subjects have dampened innate immune inflammatory responses but have normal humoral responses to immunization.
This study has some limitations. First, the sample size may have been too small to assess statistical differences. When performing this study, there were no previous studies with a similar study design. Therefore, we were limited in our ability to calculate a statistically powerful sample size. Second, the post hoc analysis of differences in seroprotection was not specified in advance in the trial protocol. The seroprotection rate was significantly lower only for A/H1N1 in the elderly diabetes group than in the elderly nondiabetic group. To explain the differences, we additionally conducted a post hoc analysis. Third, it would be useful to see if there was a correlation between the number of medications subjects were taking and their vaccine responses. However, we did not collect these data while conducting the research. The missed confounder might influence the results.
In this comparative study, we observed that the conventional intramuscular vaccine met the CHMP criteria and was safe for diabetic individuals. The immunogenicity profiles were not different from those of nondiabetic persons. This study also demonstrated that a long-term antibody response to the influenza vaccine was associated with age and prevaccination GMT, regardless of diabetes status. Those with advanced age and low prevaccination titer showed unsatisfactory seroprotection at 6 months postvaccination.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project Ministry of Health & Welfare Republic of Korea (grant A103001).
We declare no conflicts of interest.
REFERENCES
- 1.Reading PC, Allison J, Crouch EC, Anders EM. 1998. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol 72:6884–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouter KP, Diepersloot RJ, van Romunde LK, Uitslager R, Masurel N, Hoekstra JB, Erkelens DW. 1991. Effect of epidemic influenza on ketoacidosis, pneumonia and death in diabetes mellitus: a hospital register survey of 1976–1979 in The Netherlands. Diabetes Res Clin Pract 12:61–68. [DOI] [PubMed] [Google Scholar]
- 3.Allard R, Leclerc P, Tremblay C, Tannenbaum TN. 2010. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 33:1491–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valdez R, Narayan KM, Geiss LS, Engelgau MM. 1999. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white U.S. adults. Am J Public Health 89:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casqueiro J, Casqueiro J, Alves C. 2012. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab 16(Suppl 1):S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pozzilli P, Gale EA, Visalli N, Baroni M, Crovari P, Frighi V, Cavallo MG, Andreani D. 1986. The immune response to influenza vaccination in diabetic patients. Diabetologia 29:850–854. [DOI] [PubMed] [Google Scholar]
- 7.Nam JS, Kim AR, Yoon JC, Byun Y, Kim SA, Kim KR, Cho S, Seong BL, Ahn CW, Lee JM. 2011. The humoral immune response to the inactivated influenza A (H1N1) 2009 monovalent vaccine in patients with type 2 diabetes mellitus in Korea. Diabet Med 28:815–817. [DOI] [PubMed] [Google Scholar]
- 8.Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Ryan JG, Blomberg BB. 2013. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine 31:3603–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diepersloot RJ, Bouter KP, Beyer WE, Hoekstra JB, Masurel N. 1987. Humoral immune response and delayed type hypersensitivity to influenza vaccine in patients with diabetes mellitus. Diabetologia 30:397–401. [DOI] [PubMed] [Google Scholar]
- 10.Feery BJ, Hartman LJ, Hampson AW, Proietto J. 1983. Influenza immunization in adults with diabetes mellitus. Diabetes Care 6:475–478. [DOI] [PubMed] [Google Scholar]
- 11.McElhaney JE, Pinkoski MJ, Au D, Lechelt KE, Bleackley RC, Meneilly GS. 1996. Helper and cytotoxic T lymphocyte responses to influenza vaccination in healthy compared to diabetic elderly. Vaccine 14:539–544. [DOI] [PubMed] [Google Scholar]
- 12.el-Madhun AS, Cox RJ, Seime A, Søvik O, Haaheim LR. 1998. Systemic and local immune responses after parenteral influenza vaccination in juvenile diabetic patients and healthy controls: results from a pilot study. Vaccine 16:156–160. [DOI] [PubMed] [Google Scholar]
- 13.Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. 2006. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine 24:2417–2422. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. 2014. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm. [Google Scholar]
- 15.Heymann AD, Shapiro Y, Chodick G, Shalev V, Kokia E, Kramer E, Shemer J. 2004. Reduced hospitalizations and death associated with influenza vaccination among patients with and without diabetes. Diabetes Care 27:2581–2584. [DOI] [PubMed] [Google Scholar]
- 16.Song JY, Cheong HJ, Seo YB, Kim IS, Noh JY, Choi WS, Lee J, Jeong HW, Kee SY, Kim WJ. 2013. Long-term immunogenicity of the pandemic influenza A/H1N1 2009 vaccine among health care workers: influence of prior seasonal influenza vaccination. Clin Vaccine Immunol 20:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin K, Viboud C, Simonsen L. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169. [DOI] [PubMed] [Google Scholar]
- 18.Seidman JC, Richard SA, Viboud C, Miller MA. 2012. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses 6:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. 2010. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine 28:3929–3935. [DOI] [PubMed] [Google Scholar]