Abstract
The pneumococcal enzyme-linked immunosorbent assay (ELISA) reference standard serum, lot 89SF, has been in use since 1990 and was replaced in 2013 with a new reference standard, 007sp, that is projected to be available for the next 25 years. 007sp was generated under an FDA-approved clinical protocol; 278 adult volunteers were immunized with the 23-valent unconjugated polysaccharide vaccine Pneumovax II, and a unit of blood was obtained twice from each immunized subject within 120 days following immunization. Pooled serum was prepared from the plasma of 262 subjects, filled at 6 ml per vial, and lyophilized. Five independent laboratories participated in bridging the serotype-specific IgG assignments for 89SF to the new reference standard, 007sp, to establish equivalent reference values for 13 pneumococcal capsular serotypes (1,3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) by using the WHO reference ELISA. In a second study involving three laboratories, a similar protocol was used to assign weight-based IgG concentrations in micrograms per ml to 007sp of seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) also present in the 23-valent pneumococcal unconjugated polysaccharide vaccine. In addition, the IgG assignments for a 12-member WHO quality control (QC) serum panel were also extended to cover these seven serotypes. Agreement was excellent, with a concordance correlation coefficient (rc) of >0.996 when each laboratory was compared to the assigned values for the 12 WHO QC serum samples. There are four remaining pneumococcal serotypes (2, 9N, 17F, and 20) found in Pneumovax II for which IgG assignments exist for 89SF and remain to be bridged.
INTRODUCTION
A Streptococcus pneumoniae Human Reference Standard, lot 89SF, greatly facilitated the standardization of enzyme-linked immunosorbent assay (ELISA) methodologies during a critical period when the first pneumococcal polysaccharide conjugate vaccines were being evaluated for licensure. Lot 89SF was used in serotype-specific ELISAs designed to measure IgG antibody specific for individual pneumococcal capsular polysaccharides. Serotype-specific weight-based values for IgG, IgA, and IgM were originally derived for serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F for lot 89SF by Quataert et al. (1). Assignments for the additional serotypes in the 23-valent pneumococcal polysaccharide vaccine were subsequently bridged from the assignments for the original 11 serotypes (2). Because of dwindling supplies of 89SF, a new reference standard, 007sp, was developed and described in 2011 (3). This serum was generated under an FDA-approved clinical protocol in which 278 adult volunteers were immunized with the 23-valent unconjugated polysaccharide vaccine Pneumovax II. A unit of blood was obtained twice from each immunized subject within 120 days following immunization. Pooled serum was prepared from the plasma, filled at 6 ml per vial, and lyophilized. Five independent laboratories participated in bridging the serotype-specific IgG assignments for 89SF to the new reference standard, 007sp, to establish equivalent reference values for 13 pneumococcal capsular serotypes (1,3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) by using the WHO reference ELISA (3). 007sp has replaced 89SF (which is no longer distributed) and been routinely used in pneumococcal assays globally since 2011.
With the ongoing requirement to evaluate Pneumovax II and the ongoing development of additional extended valency conjugate vaccines, it has been imperative to assign values to 007sp for additional serotypes (4, 5). This report describes the efforts undertaken to establish the serotype-specific IgG concentrations for 007sp to seven more serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F), to validate its performance as a standard, and to assign values to a set of 12 existing World Health Organization (WHO) quality control (QC) serum samples for the serotypes.
MATERIALS AND METHODS
Collection of human serum samples.
The collection of serum samples has been described in detail and published previously (3). Briefly, 278 volunteers were vaccinated once with Pneumovax II, and serum was collected on two occasions postvaccination. Serological and virological testing showed the serum samples to be free from the hepatitis B and C viruses, Treponema pallidum, and human immunodeficiency virus. Serum samples from 262 volunteers were pooled and then aliquoted at 6 ml per vial and lyophilized, while serum samples from the remaining 16 donors were separately aliquoted to create a new panel of individually calibrated serum samples for use in functional assays.
An existing WHO QC serum panel, established previously by D. Goldblatt (University College of London Institute of Child Health) by immunizing adults with pneumococcal polysaccharide vaccine and distributed by the National Institute for Biological Standards and Control (Potters Bar, Hertfordshire, United Kingdom), was supplied for serotype-specific IgG assignment of the 12 QC serum panel members.
Laboratory methods.
Three laboratories participated in the assignment (Pfizer Vaccine Research and Development, Pearl River, NY; Institute of Child Health, University College London, London, United Kingdom; Universitätsklinikum Erlangen Kinder- und Jugendklinik, Erlangen, Germany). The assignment of weight-based units followed the protocol established for the initial assignment, which can be found in the reference materials section at http://www.vaccine.uab.edu. In the first phase of the study, serotype-specific IgG assignments for seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) were established by calibrating reference standard 007sp under double absorbent assay conditions against 89SF under single absorbent conditions using the standardized pneumococcal reference ELISA (the “WHO ELISA”) (6). The ELISA protocol followed by the participating laboratories can be found at http://www.vaccine.uab.edu/ELISA%20Protocol.pdf. The only deviation from the WHO protocol is that absorption of 007sp with cell wall polysaccharide (CPS) (7) was undertaken by using two absorbents prepared from unencapsulated S. pneumoniae mutant strains incorporating both mono- and disubstituted CPS (8, 9) rather than CPS and purified 22F capsular polysaccharide. The use of 22F as an absorbent was not possible, as 22F concentrations were being assigned, so for these experiments, all of the serum samples were absorbed in the same manner for all of the serotypes tested. Briefly, independent sets of serial dilutions of reference standard 007sp (supplied by the Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, MD) were made from four independent 007sp vials, and all of the samples were serially diluted in duplicate. Four sets of independently prepared 007sp serial dilutions (seven-point serial dilutions) were tested as unknown samples on each ELISA plate. Ten assay plates were run to generate a total of 40 data points per serotype for 007sp from each of the participating laboratories. Each plate also contained serial dilutions of 89SF and a QC serum, both run in duplicate. The ELISA procedure was carried out for each serotype, and the raw optical density measurements were sent to Pfizer's testing laboratories for analysis with SAS software (version 9.4).
In the second phase of the study, a panel of 12 existing WHO QC serum samples was assayed and quantified with both 007sp and 89SF as the reference standards. Three WHO QC serum samples, as well as 007sp and 89SF, were run in duplicate on each ELISA plate of a five-plate replicate series two times for each serotype, yielding up to 10 reported values for each serotype from each laboratory for final analyses. The performance of 007sp was assessed by comparing the calculated concentrations obtained with 007sp to those obtained with 89SF as the reference standard.
Statistical analysis.
During each phase of this study and the selected repeated assays, there were about 40 determinations of antibody concentrations for 007sp for each serotype from each laboratory. Antibody concentrations were estimated for the seven serotypes with a linear mixed-effects analysis of variance (ANOVA) model. All models were fitted independently by serotype and included the laboratory and batch as random effects. Ninety-five percent confidence intervals (95% CIs) were estimated by serotype, accounting for the variance components between the laboratories and between batches within a laboratory and residual variability. Data were analyzed after (common) log transformation of ELISA IgG concentrations. The means of the log concentrations for each serotype were calculated for each laboratory and used to assess agreement and precision among the three laboratories. Agreement is defined as the closeness of the (log) concentration between two laboratories for each of the seven serotypes and is measured with Lin's concordance correlation coefficient (rc), which is a combination of Lin's coefficient of accuracy (Ca) (10) and Pearson's correlation coefficient (r).
For each serotype using 007sp, antibody concentrations estimated by using ANOVA models adjusting for laboratory were obtained by back-transforming the estimated log-transformed concentration and associated 95% CI. These concentrations served as the “assigned” values for each serotype using 007sp.
Once assignments for the seven serotypes were finalized for 007sp, concentrations were determined for the 12 WHO QC serum samples. Through the two phases of the study, each laboratory contributed at least five IgG concentration estimates for each WHO QC serum sample for each serotype. The 12 WHO reference serum samples do not have known ELISA IgG concentrations or assignments, and hence, “consensus” ELISA IgG concentrations were estimated from the present data by using an ANOVA mixed-effects model. Scatter plots and box plots were employed to assess and evaluate the ability of the three laboratories to produce consistent estimates of antibody concentrations for each serotype by using 007sp.
RESULTS
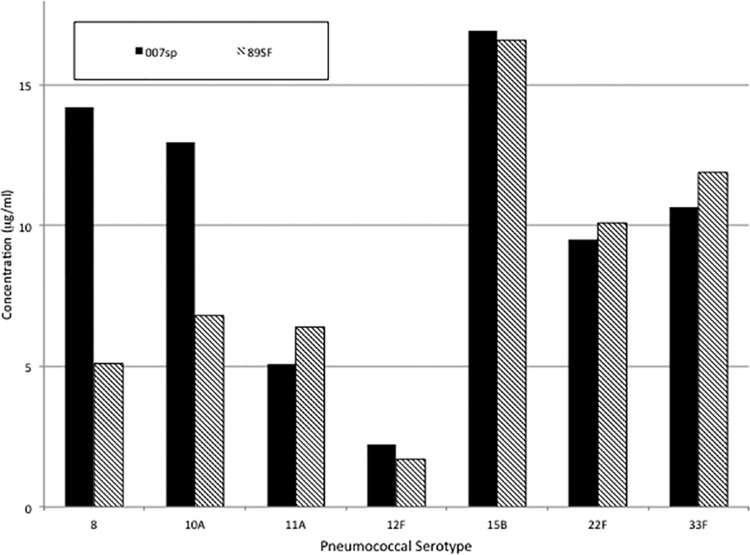
To assess the consistency of the laboratories, the mean of the log concentrations of 007sp for each serotype was calculated for each laboratory and used to assess the level of agreement among the laboratories. Figure 1 displays scatter plots between all pairs of laboratories with the concordance correlation coefficient listed within each plot. The solid diagonal line provides a reference for perfect agreement (slope = 1, intercept = 0). These statistics indicate a high level of agreement, with rc exceeding 0.994 for all plots. For the same data, the Pearson correlation coefficient was ≥0.998 and the accuracy coefficient (Ca) was ≥0.995 in each case. ANOVA models were used to estimate IgG antibody concentrations for each of the serotypes in 007sp. Final point estimates and CIs were obtained by back-transforming the estimated log-transformed concentrations and associated 95% CIs. These IgG concentrations are the “assigned” values for each serotype using 007sp and are shown in Table 1. These values were derived by the double absorption of 007sp with both monosubstituted and disubstituted CPS (7, 9), and thus in the future, when used as a standard, both standard and unknown test samples should be double absorbed. The IgG values assigned to 007sp compared to the original values assigned to 89SF are shown in Fig. 2.
FIG 1.
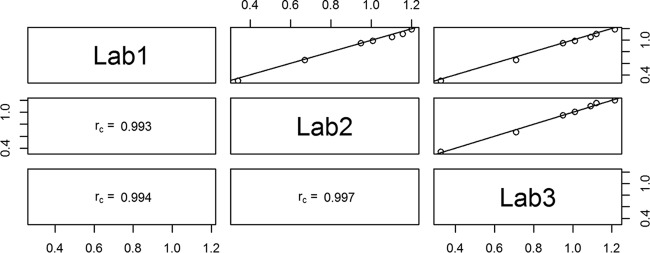
Scatter plots showing the correlation of IgG antibody concentrations between laboratories for the seven serotypes using 007sp with the log IgG concentration of the values represented on the x and y axes. The three panels in the lower left corner show the rc value for each comparison. For example, rc = 0.994 for the comparison between laboratories 1 and 2. The three panels in the upper right corner show a graphical representation of the comparison of each pair of laboratories. Each point on the charts represents the mean of approximately 80 log IgG antibody concentrations for 007sp for a given serotype from each laboratory being compared. The solid diagonal line indicates theoretical perfect agreement, where the slope is 1 and the intercept is 0.
TABLE 1.
Assigned IgG antibody concentrations for 007sp
| Type | ELISA IgG concn (μg/ml) | Lower 95% CI | Upper 95% CI | n |
|---|---|---|---|---|
| 8 | 14.24 | 13.26 | 15.30 | 119 |
| 10A | 12.98 | 12.16 | 13.85 | 115 |
| 11A | 5.08 | 4.36 | 5.91 | 117 |
| 12F | 2.21 | 2.09 | 2.33 | 120 |
| 15B | 16.94 | 16.04 | 17.88 | 117 |
| 22F | 9.50 | 9.04 | 9.99 | 117 |
| 33F | 10.66 | 10.18 | 11.16 | 117 |
FIG 2.
Bar graph showing a visual comparison of the original published 89SF IgG assignments and the new 007sp IgG assignments for seven serotypes.
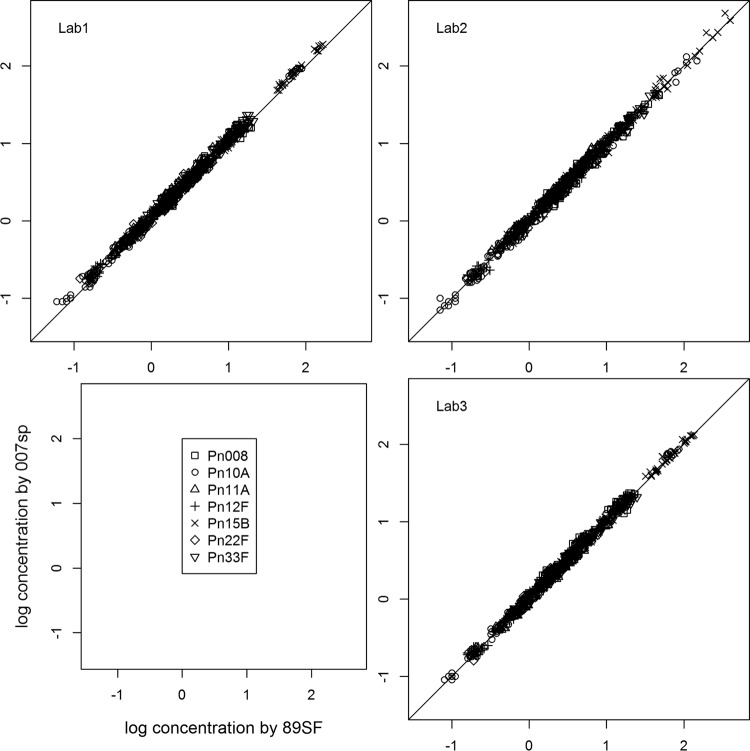
Serum IgG concentrations were determined by ELISA for the 12-member WHO QC serum panel by using both 89SF and 007sp as the reference standards. Table 2 presents the assigned values for the QC serum panel (n = ≥25 for each estimate), while Fig. 3 and 4 display the scatter plots and box plots for the seven serotypes analyzed. These plots illustrate the agreement of the seven estimated assigned IgG values for 007sp compared to lot 89SF for each WHO QC serum and serotype.
TABLE 2.
Assigned values for 12 pneumococcal WHO QC serum samples as determined with the new pneumococcal reference standard 007spa
| WHO calibration serum | Assigned value for pneumococcal capsular serotype: |
||||||
|---|---|---|---|---|---|---|---|
| 8 | 10A | 11A | 12F | 15B | 22F | 33F | |
| 728 | 2.54 | 0.09 | 2.60 | 1.73 | 3.42 | 3.95 | 8.69 |
| 732 | 1.49 | 0.47 | 0.61 | 0.58 | 1.57 | 0.20 | 2.90 |
| 736 | 4.07 | 0.19 | 0.87 | 3.93 | 0.63 | 0.90 | 3.31 |
| 746 | 2.27 | 1.68 | 2.84 | 1.51 | 12.69 | 0.66 | 0.78 |
| 756 | 19.86 | 1.01 | 3.42 | 1.97 | 49.31 | 4.42 | 16.83 |
| 758 | 2.46 | 85.86 | 8.99 | 6.00 | 74.23 | 2.23 | 9.99 |
| 760 | 17.15 | 13.00 | 5.48 | 4.03 | 10.43 | 6.33 | 16.43 |
| 762 | 1.83 | 0.19 | 2.01 | 0.24 | 5.66 | 0.98 | 1.07 |
| 770 | 14.08 | 0.57 | 2.01 | 1.22 | 170.62 | 2.26 | 21.20 |
| 772 | 6.22 | 5.85 | 3.49 | 0.68 | 2.67 | 2.17 | 3.51 |
| 774 | 4.68 | 2.02 | 0.78 | 0.22 | 3.66 | 5.12 | 1.87 |
| 776 | 2.10 | 0.37 | 0.42 | 0.20 | 10.94 | 2.42 | 1.59 |
n = ≥25 for each estimate.
FIG 3.
Scatter plots showing, for each laboratory, the correlation between the IgG concentration estimates for the panel of 12 WHO QC serum samples using 007sp (vertical scale) versus 89SF (horizontal scale) as the reference standards for the seven serotypes analyzed (n = >10 for each for the 12 QC serum from each laboratory). The panel in the lower left corner is the key that identifies the serotype by the symbol used in the charts, as well as the labels for the x and y axes.
FIG 4.
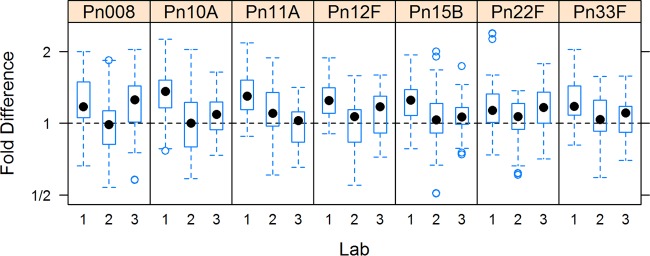
Box plots illustrating the deviation of the 007sp estimates from those obtained by using 89SF for the seven serotypes of the panel of 12 WHO QC serum samples analyzed (n = ≥10 for each QC serum from each laboratory, total n = ≥120). In these plots, the box is defined by the 25th and 75th percentiles of the distribution; the dot within the box represents the median or 50th percentile. Vertical lines extend to the most extreme observation that is less than 1.5 times the interquartile range (75th to 25th percentiles), empty circles correspond to individual assay values that are progressively distant from the bulk of the data. Data above the dotted horizontal line indicate that 007sp estimates are greater than the estimates made with 89SF as the reference standard. On the vertical axis, 2 indicates a point where the 007sp estimate was twice the 89SF estimate. A value of 1/2 indicates that the 89SF estimate was two times the 007sp estimate. Boxes centered on the horizontal dotted line indicate good agreement between the 007sp and 89SF estimates.
The scatter plots (Fig. 3) show the high degree of agreement and correlation among the calculated (log) IgG concentrations for the panel of 12 WHO QC serum samples by using 007sp (vertical scale) versus 89SF (horizontal scale) as the reference standard. A perfect level of agreement would yield a straight line with slope of 1 and intercept at 0, and in general, all of the data points cluster tightly about this line of identity.
The box plots (Fig. 4) illustrate the deviation of the 007sp-based estimates from those obtained with 89SF as the reference standard for the 12 WHO QC serum samples. The IgG concentrations calculated with 007sp as the reference standard are largely within 2-fold (1/2 to 2.0) of those calculated with lot 89SF as the reference standard.
Table 3 presents Ca, r, and rc, which measure the agreement between pairs of laboratories and between laboratories and consensus ELISA IgG concentrations for the WHO QC serum samples. In order to form paired data between the labs for these comparisons, the 10 or more serotype-specific replicate IgG concentrations generated in each laboratory were replaced with a single predicted value obtained from a mixed-model ANOVA. Agreement was high, with all values ≥0.99.
TABLE 3.
Comparison of ELISA IgG concentrations between laboratories and laboratory-to-consensus assigned values for WHO QC serum samplesa
| Laboratory(ies) and statistic | Value for laboratory: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1b | |||
| Ca | 1.0 | 0.993 | 0.998 |
| r | 1.0 | 0.996 | 0.995 |
| rc | 1.0 | 0.990 | 0.994 |
| 95% CI | 0.985, 0.993 | 0.990, 0.996 | |
| 2b | |||
| Ca | 1.0 | 0.997 | |
| r | 1.0 | 0.996 | |
| rc | 1.0 | 0.993 | |
| 95% CI | 0.990, 0.995 | ||
| 3b | |||
| Ca | 1.0 | ||
| r | 1.0 | ||
| rc | 1.0 | ||
| 95% CI | |||
| Allb | |||
| Ca | 0.998 | 0.998 | 1.000 |
| r | 0.999 | 0.999 | 0.999 |
| rc | 0.997 | 0.997 | 0.998 |
| 95% CI | 0.996, 0.998 | 0.995, 0.998 | 0.997, 0.999 |
Consensus ELISA (log) IgG concentrations were estimated within a serotype by the use of a mixed-effects ANOVA model. Predicted ELISA (log) IgG concentrations were obtained for each laboratory by sample within a serotype for each of the replicate observations by use of a mixed-effects ANOVA model.
n = 84.
DISCUSSION
In this report, we describe the assignment of IgG concentrations in weight-based microgram-per-milliliter units to the human antipneumococcal reference standard serum 007sp and a panel of 12 pneumococcal QC serum samples for seven serotypes. This new standard was developed in 2009 and 2010 to replace limited stocks of the original reference standard, 89SF. Assignment for additional serotypes is required, as the original reference standard, 89SF, which had values assigned for the 23 serotypes in Pneumovax II, is no longer available (007sp is exclusively distributed via the FDA). However, studies evaluating Pneumovax II are still undertaken and new conjugate vaccines are currently under development incorporating additional serotypes found in Pneumovax II. In addition, 89SF was the standard used for the serological evaluation of serum samples derived from infants immunized with PCV7 in the efficacy trial that led to licensure of the vaccine (11). Bridging back to 89SF and ensuring that ELISA performance remains comparable to the original measurements in the Kaiser Permanente study (11) help maintain a link/bridge to PCV7 efficacy. Assignment of the weight-based antibody concentrations to human pneumococcal reference standard 007sp was originally performed for the 13 serotypes represented in currently licensed conjugate vaccines (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) (3). By using established laboratories and a well-characterized ELISA procedure (12) that was performed by all of the participating laboratories, it was possible to assign weight-based units to 007sp by running 007sp alongside a standard curve of 89SF and treating 007sp as the unknown. Very high levels of agreement between the participating laboratories for the weight-based units of IgG specific for 13 serotypes in 007sp were achieved. Having accepted concentrations for an existing standard has significantly simplified the assignment process.
As in the original assignment study, we were able to further validate the values obtained and the performance of 007sp as a standard during the process of assigning serotype-specific IgG values (micrograms per milliliter) to a panel of 12 WHO QC serum samples previously prepared from the serum samples of pneumococcal polysaccharide-vaccinated adults. Concordance was high among laboratories (Table 3) and between results for laboratories and consensus ELISA IgG concentrations. With the adherence to the uniform application of the WHO ELISA (12) in the present study, we were able to achieve high levels of precision and accuracy in the values assigned to seven additional serotypes for 007sp and the WHO QC serum samples.
ANOVA mixed modeling is a flexible framework that allows estimation of ELISA IgG concentrations for 007sp and the 12 WHO QC serum samples for each serotype by laboratory. These models may be used to compare and contrast results within and among laboratories. Random-effects ANOVA models allowed us to reduce the replicate measurements to a single predicted value that was then used to measure levels of consistency among the laboratories. While we were able to estimate serotype-specific concentrations for 007sp through a bridge to 89SF (Table 1), the actual ELISA IgG concentrations for the WHO QC serum samples used in this study were unknown, so it was not possible to compare “true” values. The ANOVA mixed model provided a mechanism for estimating consensus values, which served as assigned values for these serum samples (Table 2).
Establishing a new reference serum for the pneumococcus was essential for ongoing efforts to evaluate pneumococcal vaccines and to maintain the link with the original serology performed as part of the pivotal efficacy studies conducted prior to licensure. The high degree of agreement between the 007sp-based and 89SF-based estimates in the original assignment exercise (3) has inspired confidence in the validity of the 007sp assignments. In the study described in this paper, a similar high level of agreement has been observed. We have noted differences in the overall concentration of serotype-specific IgG in 007sp compared to 89-SF for two of the seven serotypes evaluated (8 and 10A). These differences are to be expected, as 89-SF was prepared in the late 1980s from the plasma of 17 adult volunteers immunized with a 23-valent polysaccharide vaccine made by Lederle and thus was never a true “population-based” reference serum, while 007sp was prepared from pooled plasma from 278 individuals immunized with a 23-valent vaccine manufactured by Merck.
The new reference standard, 007sp, is available in large quantities and should provide continuity for the foreseeable future. Its performance in ELISA suggests that it would not affect the operation of validated assays currently established in serology laboratories. A project is under way to bridge the IgG assignments for 89SF to the new reference standard, 007sp, for the remaining four serotypes that are less prevalent in young pediatric populations but are also included in the 23-valent polysaccharide vaccine Pneumovax II (13, 14). No serotype-specific values for IgA, IgM, or the IgG subclasses are available at the moment. IgG assignments may also be needed for other serotypes in the future to evaluate pneumococcal vaccines containing emerging serotypes not present in the 23-valent pneumococcal polysaccharide vaccine. Functional pneumococcal assays are assuming increasing importance in the evaluation of new vaccines, especially for use in adult populations, and the new set of FDA opsonophagocytic activity (OPA) assay calibration serum samples should prove as valuable as the WHO QC serum samples have been for the ELISA. A multilaboratory OPA assay study is currently being analyzed to assign OPA titers in due course, and the OPA calibration serum samples will be available for distribution by CBER once assignments have been made.
ACKNOWLEDGMENTS
We thank Roger French, Director, Pfizer Biotechnology Clinical Development Statistics, for statistical support.
D.G.'s laboratory receives research and contract support for serology from Merck and GSK. D.G. participates in occasional advisory boards and provides advice to GSK and Merck. C.Y.T., S.M., L.M., M.S., and P.C.G. are employees of Pfizer.
This paper is dedicated to the memory of Milan Blake, who initiated the 007sp development in 2006 but tragically died unexpectedly prior to the project's completion.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00437-15.
REFERENCES
- 1.Quataert SA, Kirch CS, Wiedl LJ, Phipps DC, Strohmeyer S, Cimino CO, Skuse J, Madore DV. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol 2:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quataert SA, Rittenhouse-Olson K, Kirch CS, Hu B, Secor S, Strong N, Madore DV. 2004. Assignment of weight-based antibody units for 13 serotypes to a human antipneumococcal standard reference serum, lot 89-S(F). Clin Diagn Lab Immunol 11:1064–1069. doi: 10.1128/CDLI.11.6.1064-1069.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldblatt D, Plikaytis BD, Akkoyunlu M, Antonello J, Ashton L, Blake M, Burton R, Care R, Durant N, Feavers I, Fernsten P, Fievet F, Giardina P, Jansen K, Katz L, Kierstead L, Lee L, Lin J, Maisonneuve J, Nahm MH, Raab J, Romero-Steiner S, Rose C, Schmidt D, Stapleton J, Carlone GM. 2011. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol 18:1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFetridge R, Meulen AS, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, Marchese RD, Zacholski DM, Watson WJ, Stek JE, Hartzel JS, Musey LK. 2015. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 33:2793–2799. doi: 10.1016/j.vaccine.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Sobanjo-ter Meulen A, Vesikari T, Malacaman EA, Shapiro SA, Dallas MJ, Hoover PA, McFetridge R, Stek JE, Marchese RD, Hartzel J, Watson WJ, Musey LK. 2015. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 34:186–194. doi: 10.1097/INF.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 6.Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, Kayhty H, Jonsdottir I, Nahm MH. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 10:514–519. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concepcion NF, Frasch CE. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 8:266–272. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong NM, Quataert SA ZN, Koster M, Madore DV. 1996. Comparison of pneumococcal absorbent preparations used in human anti-pneumococcal polysaccharide (PNPS) antibody (AB) ELISAs. Pediatr Res 39:186. doi: 10.1203/00006450-199604001-01122. [DOI] [Google Scholar]
- 9.Karlsson C, Jansson PE, Skov Sørensen UB. 1999. The pneumococcal common antigen C-polysaccharide occurs in different forms. Mono-substituted or di-substituted with phosphocholine. Eur J Biochem 265:1091–1097. [DOI] [PubMed] [Google Scholar]
- 10.Lin LI. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 11.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 19:187–195. [DOI] [PubMed] [Google Scholar]
- 12.Plikaytis BD, Goldblatt D, Frasch CE, Blondeau C, Bybel MJ, Giebink GS, Jonsdottir I, Kayhty H, Konradsen HB, Madore DV, Nahm MH, Schulman CA, Holder PF, Lezhava T, Elie CM, Carlone GM. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J Clin Microbiol 38:2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, Miller L, Scherzinger K, Thomas A, Farley MM, Zell ER, Taylor TH Jr, Pondo T, Rodgers L, McGee L, Beall B, Jorgensen JH, Whitney CG. 2015. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 15:301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. 2015. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]