Abstract
Over 35% of children in a region of malaria endemicity are infected with Epstein-Barr virus (EBV) by 6 months of age. This susceptibility may be linked to impaired transplacental transfer of antibodies. In this study, we determined the effect of malaria exposure during pregnancy on the transfer of EBV-specific maternal antibodies in a region of western Kenya that experiences endemic malaria. Pregnant mothers were recruited and followed up until delivery to determine levels of neonatal malaria exposure. Levels of EBV lytic (viral capsid antigen [VCA], Z transcriptional activator [Zta], and early diffuse antigen complex [EAd]) and EBV latent (EBV nuclear antigen-1 (EBNA1]) and tetanus-specific IgG antibodies were measured in 70 paired maternal and cord blood samples using a Luminex-bead-based assay. A high proportion (63%) of the infants were exposed to malaria in utero. Levels of EBV- and tetanus-specific antibodies were similar in malaria-infected mothers and in mothers who had no detectable malaria infection. Malaria-exposed neonates had significantly lower levels of anti-EBNA1, anti-Zta, and anti-EAd antibodies than were seen in their mothers. In utero malaria exposure resulted in significant reductions in transplacental transfer of anti-VCA-p18 and anti-EBNA1 antibodies of 13% and 22%, respectively. Neonates received significantly low levels of anti-Zta and anti-EAd antibodies irrespective of malaria exposure levels. In multivariate analysis, in utero malaria exposure was associated with a significant reduction in the transfer of anti-VCA-p18 and anti-EBNA1 antibodies to the neonates (P = 0.0234 and P = 0.0017, respectively). Malaria during pregnancy results in differential levels of transfer of EBV-specific antibodies from the mother to the fetus. The impaired transplacental transfer of some antibodies may lead to the malaria-exposed neonates being susceptible to early EBV infection.
INTRODUCTION
Endemic Burkitt's lymphoma (eBL) is a distinct form of non-Hodgkin's lymphoma and is the most common pediatric malignancy in regions of malaria endemicity of sub-Saharan Africa (1). Both infection with Epstein-Barr virus (EBV) and repeated episodes of Plasmodium falciparum malaria are known risk factors for eBL (2), but the mechanism(s) by which these two agents interact to promote the emergence of malignant B cell clones has not been elucidated. Recently, we found that infants from a region of malaria endemicity of western Kenya were infected with EBV by 6 months of age (3). Living in regions of malaria endemicity was a predictor of this early age of primary infection. This aberrant primary EBV infection may set the stage for lymphoma development, as previously hypothesized (4–6).
The lytic and latent phases of the EBV life cycle induce distinct antibodies in response to specific lytic and latent antigens. Anti-EBV nuclear antigen-1 (EBNA1) antibodies are produced against EBNA1, the only antigen expressed in latently infected memory B cells and in eBL tumors (7). Anti-viral capsid antigen (anti-VCA), anti-early antigen (anti-EA), and anti-immediate-early protein (anti-Zta) antibodies are produced against their respective target lytic antigens (8). Clinically, elevated levels of anti-VCA and anti-EBNA1 immunoglobulin G (IgG) antibodies have been used as evidence of past infection (9), while the presence of IgG antibodies to the EBV early antigens (EAd and Zta) generally reflects recent or reactivated infections (10, 11). Although EBV-specific antibody patterns reflect the dynamics of EBV activity in adults, few studies have addressed this issue in infants and children (3, 12–15) or in newborns (16, 17). More importantly, no comparison to maternal antibody levels has been made and there has been no analysis of how maternal malaria infections impact transplacental transfer of EBV-specific antibodies.
Mothers transfer pathogen-specific antibodies to their infants during pregnancy. These passive antibodies from the mothers provide protection to the infants before they develop de novo antibodies (18). These antibodies are mainly acquired through transplacental transfer. The transport of maternally derived IgG across the placenta is mediated by Fc receptors of IgG, including FcγR and FcRn (19–21). It is an active and selective process whereby neonatal FcR binds IgG, crosses the syncytiotrophoblast, and releases IgG into the endothelium of fetal capillaries.
Maternal factors such as placental malaria, human immunodeficiency virus (HIV) infection, maternal hypergammaglobulinemia, and preterm birth have been shown to inhibit the efficient mother-to-child transfer of pathogen-specific antibodies (22–27). For example, in a study in the rural coastal area of Kenya, placental malaria infection as well as HIV infection was associated with a significant reduction in the transfer of anti-tetanus IgG antibodies to the neonates (26). In a rural Gambian population, placental malaria infection was associated with a significant reduction in transplacental transfer of antibodies against herpes simplex virus (HSV), varicella-zoster virus (VZV), and respiratory syncytial virus (RSV) (27). Maternal HIV infection was associated with reduced transplacental transfer of IgG antibodies against tetanus toxoid (TT), measles virus, and varicella-zoster virus (22). Together, these studies suggest that maternal infections during pregnancy can interfere with the efficient vertical transfer of pathogen-specific antibodies, potentially leaving infant susceptible to infections early in life. Transplacental transfer of EBV-specific maternal antibodies to their infants in the context of maternal malaria infection has not been investigated.
Given that over 35% of infants from a region of malaria endemicity can be infected with EBV during infancy (3), when maternally acquired antibodies should typically protect them against EBV infection, we tested the hypothesis that maternal malaria infection reduced the transplacental transfer of EBV-specific maternal antibodies to the neonates. In addition, the above-mentioned studies looked at antibody transfer in the context of placental malaria infection as determined by the presence of malaria parasites during delivery. The long-term effect of maternal malaria infection (e.g., infection with malaria at any time during pregnancy) on antibody transfer remains poorly understood. This study investigated the effect of maternal malaria infection during pregnancy on the efficiency of transplacental transfer of well-characterized EBV-specific antibodies (e.g., VCA, EBNA1, EAd, and Zta) and tetanus toxoid-specific antibodies in mother-child pairs from a region of western Kenya where malaria transmission is high.
MATERIALS AND METHODS
Study population.
This study was conducted at the antenatal clinic (ANC) and maternity ward of Chulaimbo Sub-District Hospital in Kisumu West District. This hospital mainly serves a rural population that experiences holoendemic malaria with two seasonal peaks: June to August and November to December (28). The inclusion criteria specified pregnant women of any gravidity, gestation for <24 weeks, having a normal full blood count, HIV-negative status, residency within a 10-km distance of the hospital, and willingness to return to the hospital for delivery, follow-up clinical procedures, and laboratory testing. Gestational age was evaluated by measurement of fundal height and history (the last menstrual period). All pregnant women were enrolled in a 6-month period from June to November 2011. The demographics of this study population have been previously described (29). Briefly, at enrollment, demographic and antenatal care data were captured. A total of 200 pregnant women were enrolled. Of these, 25 were HIV positive and were excluded from these analyses. The remaining 175 pregnant women who were HIV-1 negative were followed up monthly through antenatal visits (up to 4 follow-up visits per mother) until delivery. Of these, 93 pregnant women delivered at the hospital and 70 of them provided a complete mother-child pair of plasma samples that were analyzed in the current study.
All the women included in the study were tested for HIV as a part of programs investigating mother-to-child-transmission (MTCT) of HIV in accordance with the Kenya Ministry of Health national guidelines. All the mothers received an average of two doses of sulfadoxine-pyrimethamine (SP) that were administered by directly observed therapy during the ANC visits as part of the recommendation of the Kenya Ministry of Health for malaria prophylaxis. Approvals for this study were obtained from the Kenya Medical Research Institute (KEMRI) Ethical Review Committee and the Ethical Review Board of the State University of New York (SUNY) Upstate Medical University Hospital, USA. Written informed consent was obtained from all the mothers.
Sample collection and preservation.
During the ANC visits and within 12 h of delivery, 200 to 500 μl of maternal venous blood was collected by venipuncture or finger prick and placed into EDTA Microtainers (BD, Franklin Lakes, NJ). After delivery, ∼500 μl of cord blood, representative of the pool of blood circulating in the neonates, was collected from the umbilical vein into EDTA Microtainers (BD, Franklin Lakes, NJ). All samples were immediately transported to the SUNY Upstate University laboratory at KEMRI's Centre for Global Health Research, and plasma was separated from whole blood and stored at −80°C until antibody assays were performed.
Malaria diagnosis.
Malaria parasite load and plasmodium species were determined in maternal and cord blood samples as previously described (29). An infant was considered exposed to malaria in utero if any of the blood smear and/or quantitative (Q)-PCR results determined during any of the ANC visits or any of the samples of maternal blood at delivery or samples of cord blood were positive for P. falciparum. An infant was considered not malaria exposed if the blood smear and/or Q-PCR results at any of the ANC visits or maternal blood samples at delivery or cord blood samples were negative.
EBV peptide antigens and Luminex-based suspension bead assays.
EBV-specific antibodies were detected using 5 synthetic peptides covering immunodominant epitopes of the viral capsid antigen VCA-p18 subunit, VCA gp125 subunit, EBV nuclear antigen 1 (EBNA1), early diffuse antigen complex (EAd), and immediate-early protein Z transcriptional activator (Zta) (13, 30, 31). EBV gp125 is another major capsid immunogen of VCA that is independent of p18. Tetanus toxoid (TT) antigen (Calbiochem, Darmstadt, Germany) was used as a control since mothers are routinely immunized against tetanus during pregnancy. The choices of the above-mentioned antigens were based on their well-characterized serological profiles as previously described (13, 32, 33).
To detect EBV- and TT-specific IgG using the panel of EBV peptides and TT described above, we used a Luminex-bead-based suspension assay and a previously described protocol (13). Plasma was diluted 1:100 prior to testing. At least 75 beads per region were acquired on a Bioplex reader (Bio-Rad, Hercules, CA), and results were expressed as mean fluorescence intensity (MFI) values.
VCA- and EBNA1-specific IgG subclass ELISA.
EBV-specific IgG subclass antibody distributions were analyzed in the mother-child pairs of plasma samples using two synthetic peptides covering the immunodominant epitopes of VCA-p18 and EBNA1 by enzyme-linked immunosorbent assay (ELISA) as previously described (13, 32) with modifications. Peroxidase-conjugated sheep antibodies specific to the different human IgG subclasses (Invitrogen, Camarillo, CA) were added and the plates developed using tetramethylbenzidine (TMB) substrate. Plasma samples were diluted 1:100. The optical densities (ODs) were measured at 490 nm on a Bio-Rad microplate reader using Microplate Manager version 6 (Bio-Rad, Hercules, CA). Antibody levels for IgG subclass responses were expressed in arbitrary units (AU), which were calculated by dividing the test sample ODs by the mean OD from EBV negative-control sera plus 2 standard deviations (SDs).
Total IgG ELISA.
Determination of total IgG in maternal plasma was done using a human IgG total ELISA kit from eBioscience (San Diego, CA) following the manufacturer's instructions. The plates (Molecular Devices, Sunnyvale, CA) were read at 450 nm, and the concentration of total IgG (in milligrams per milliliter) in plasma was extrapolated from the standard curve.
Statistical analysis.
The differences in the levels of EBV- and TT-specific IgG antibodies between malaria-exposed and nonexposed neonates as well as between malaria-infected and uninfected mothers were determined using the Mann-Whitney U test. Placental transfer was measured as the ratio of the level of specific antibody in cord blood to that in the maternal blood, i.e., the cord blood/maternal blood ratio (CMR) (26, 27). The correlation between the levels of maternal EBV- and TT-specific antibodies in relation to those in cord blood was calculated using the Spearman correlation test. Linear regression analysis was used to assess the effect of a number of variables such as maternal age, parity, birth weight (BW), and gestational age at first malaria exposure on the transplacental transfer of EBV-specific antibodies. In multivariate analysis, we adjusted for maternal age, hypergammaglobulinemia, and gestational age at exposure as potential confounders, as they are known or suspected risk factors affecting outcomes. Statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software) and Stata statistical software version 11 (StataCorp). For all statistical analyses, a two-sided P value of ≤0.05 was considered significant.
RESULTS
Characteristics of the study population.
Plasma from 70 mother-child pairs was analyzed in this study. A neonate was considered exposed to malaria if P. falciparum malaria was detected in maternal venous blood at any ANC visit, in the placenta or venous blood at delivery, or in cord blood. A neonate was considered nonexposed if P. falciparum malaria was not detected in the maternal venous blood at any ANC visit, at delivery, or in cord blood. It is possible that we did not capture all malaria infections in the mother, but for the purpose of this comparison, we labeled this group nonexposed. Using this criterion, 63% (44/70) of the neonates in this study were classified as having been exposed to malaria in utero (Table 1).
TABLE 1.
General characteristics of the mothers and their infantsa
| Factor | Values for indicated subject malaria exposure condition |
P value | |
|---|---|---|---|
| Exposed | Unexposed | ||
| Maternal characteristics | |||
| n | 44 (63) | 26 (37) | 0.041 |
| Age, yr (mean) [SD] | 22.36 [± 6.10] | 21.77 [± 6.79] | 0.566 |
| <20 yr | 18 (41) | 12 (46) | 0.790 |
| >20 yr | 26 (59) | 14 (54) | 0.763 |
| Gestational age | |||
| <37 wks | 8 (18) | 4 (15) | 0.900 |
| >37 wks | 33 (75) | 18 (69) | 0.648 |
| Gravida | |||
| Primigravida | 16 (36) | 7 (30) | 0.784 |
| Secundigravida | 12 (28) | 12 (40) | 0.548 |
| Multigravida | 16 (36) | 7 (30) | 0.784 |
| TT vaccine (current pregnancy) | 42 (95) | 24 (92) | 0.627 |
| Bed net use | 32 (73) | 20 (77) | 0.750 |
| EBV seroprevalence (%) | 44 (100) | 26 (100) | 1.000 |
| Mean total IgG [SD] (mg/ml) | 29.15 [± 10.12] | 25.29 [± 8.93] | 0.112 |
| Neonate characteristics | |||
| Mean birth wt (g) [SD] | 3,171 [± 444] | 3,146 [± 420] | 0.923 |
| Time of malaria exposure | |||
| Early | 31 (70) | ||
| Late | 13 (30) | ||
Data are presented as number (percent) unless otherwise stated. Differences in proportions between the groups were determined by the Fisher exact test. Early exposure to malaria was defined as <26 weeks gestation; late exposure was defined as >26 weeks gestation.
The demographic and clinical characteristics of this study population are shown in Table 1. The mean maternal age in the malaria-exposed group was 22.36 years (SD, ±6.1), whereas that in the nonexposed group was 21.77 years (SD, ±6.79). To determine if the mothers were infected with EBV, anti-VCA-p18 IgG antibody levels were measured in the enrollment ANC plasma samples by ELISA (13, 32). All the women in this study were EBV seropositive. A total of 95% of the pregnant mothers in the malaria-exposed group and 92% in the malaria-nonexposed group had received tetanus toxoid (TT) vaccine during the current pregnancy. The proportions of primigravid mothers in the malaria-exposed group and in the nonexposed group were 36% and 30%, respectively. Only four neonates weighed less than 2.5 kg, which is defined as representing low birth weight (LBW). There were 2 (5%) infants with LBW in the malaria-exposed group and 2 (8%) in the malaria-nonexposed group. Hypergammaglobulinemia was defined as representing levels of total IgG greater than 30 mg/ml. Seven (27%) mothers in the malaria-uninfected group and 16 (36%) mothers in the malaria-infected group had hypergammaglobulinemia.
Comparison of levels of EBV- and TT-specific antibodies in mothers and their neonates.
We first compared the levels of EBV- and TT-specific antibodies in mothers and their paired neonates to determine if malaria exposure during pregnancy affected the levels of antibodies in the mothers or in the cord blood of the infants. To do this, we used a Luminex-bead-based assay that allowed us to measure antibodies to EBV lytic (e.g., VCA-p18, VCA-gp125, EAd, and Zta) and latent (e.g., EBNA1) antigens as well as TT antigen (13). We included the TT antigen as a reference antigen, as there are numerous studies evaluating the transplacental transfer of anti-TT antibody (22, 26, 34, 35). The levels of EBV-specific anti-VCA-p18, anti-VCA-gp125, and anti-TT antibodies were comparable in the mothers and their neonates irrespective of malaria exposure status (Fig. 1). In contrast, both the malaria-exposed and malaria-nonexposed neonates had significantly lower levels of anti-Zta and anti-EAd antibodies in the cord blood than were seen in their mothers (both P < 0.001). Interestingly, malaria-exposed neonates had significantly lower levels of anti-EBNA1 antibodies than were seen in their mothers (P < 0.001), whereas there was no significant difference in the levels of anti-EBNA1 antibodies between the mothers and their neonates that were not exposed to malaria (Fig. 1). Because the pregnant mothers were infected with malaria at different time points during pregnancy, we compared the levels of anti-EBV and anti-TT antibodies in neonates who were exposed to malaria early (<26 weeks gestation) versus late (>26 weeks gestation) in pregnancy and found no significant difference in the levels of anti-VCA-p18, anti-EBNA1, anti-Zta, anti-EAd, anti-VCA-gp125, and anti-TT between the two groups of neonates (data not shown).
FIG 1.
Relative levels of EBV- and TT-specific IgG antibodies in malaria-exposed and nonexposed mothers and infants. Plasma was diluted at 1:100 and tested using a Luminex-bead-based assay. The mean fluorescence intensity (MFI) of 75 Luminex beads for each of the antigens tested is indicated on the y axis. Significant P values of paired t tests are indicated in the figures. Horizontal bars represent median values.
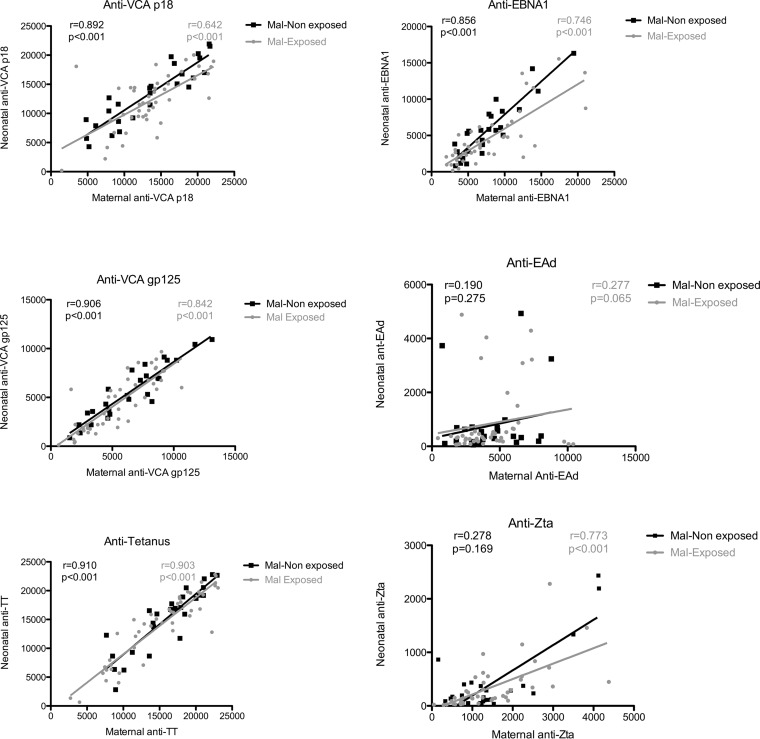
Since we observed similar levels of anti-VCA-p18, anti-VCA-gp125, and anti-TT in the mothers and their neonates, we then correlated the levels of anti-EBV- and anti-TT-specific antibodies in maternal venous blood to the levels in their neonates to determine if the maternal antibody level is a predictor of neonatal antibody levels. We observed a significant positive correlation between levels of maternal and neonatal anti-VCA-p18, anti-EBNA1, anti-gp125, and anti-TT antibodies regardless of malaria exposure (Fig. 2). However, there was no significant correlation in the levels of anti-Zta and anti-EAd antibodies in the malaria-nonexposed group.
FIG 2.
Correlation between maternal and infant anti-EBV- and anti-TT-specific antibodies in malaria (Mal)-exposed and nonexposed groups. The correlation between maternal and infant antibody levels was assessed by Spearman correlation, where P < 0.05 was considered significant, and only significant P values are shown in the figure.
Neonates born to mothers with malaria during pregnancy have reduced transplacental transfer of anti-VCA-p18 and anti-EBNA1 antibodies.
We next determined if there was a reduction in EBV-specific antibody transfer due to exposure to malaria in utero. Placental transfer was measured as the ratio of the level of antibody in cord blood to that in the maternal venous blood at delivery, i.e., the cord blood/maternal blood ratio (CMR). Transplacental transfer of antibodies to VCA-p18 and EBNA1 from malaria-infected mothers to their exposed neonates was significantly reduced (by 13.40% and 21.65%, respectively) (Table 2). There was no significant reduction in transplacental transfer of anti-Zta, anti-EAd, and anti-TT from the mothers to the neonates due to malaria exposure in utero (P = 0.950, P = 0.349, and P = 0.458, respectively), although there was a trend to a more significant reduction of anti-VCA gp125 (P = 0.064) (Table 2).
TABLE 2.
Transplacental transfer of EBV- and TT-specific antibodies from the mother to the neonatea
| Antibody | CMR for indicated subject malaria exposure condition |
% reduction | P value | |
|---|---|---|---|---|
| Exposed | Nonexposed | |||
| Anti-VCA-p18 | 0.862 (0.754–1.001) | 0.996 (0.829–1.182) | 13.40 | 0.023 |
| Anti-EBNA1 | 0.546 (0.426–0.809) | 0.762 (0.558–1.012) | 21.65 | 0.002 |
| Anti-Zta | 0.151 (0.080–0.313) | 0.153 (0.074–0.320) | 0.25 | 0.950 |
| Anti-EAd | 0.102 (0.048–0.176) | 0.068 (0.035–0.166) | −3.40 | 0.349 |
| Anti-VCA-gp125 | 0.697 (0.552–0.979) | 0.850 (0.680–0.972) | 15.25 | 0.064 |
| Anti-TT | 0.949 (0.816–0.103) | 0.981 (0.854–1.040) | 3.20 | 0.458 |
Data represent the median CMR (cord blood/maternal blood ratio; i.e., ratio of the level of the specific antibody in cord blood to that in the maternal blood of the mother). Interquartile ranges are shown in parentheses. Maternal and cord blood plasma samples were tested for the presence of anti-EBV- and anti-TT-specific antibodies by Luminex-bead-based assay. The CMR was used to determine levels of placental transfer of antibodies. Percentage reduction due to malaria was determined by the following formula: (CMR of nonexposed subject − CMR of exposed subject) × 100. Statistical differences of P ≤ 0.05 are considered significant as determined by the Mann-Whitney test. Boldface data indicate a significant difference in the transfer of EBV-specific antibodies between the two groups.
When we performed a multivariate analysis to determine the effect of maternal malaria infection on transplacental transfer of anti-EBV and anti-TT antibodies while adjusting for potential confounding factors such as maternal age, hypergammaglobulinemia status, and gestational age at first malaria exposure, we observed that transfer of anti-VCA-p18 and anti-EBNA1 antibodies to the neonates from mothers who had malaria infection during pregnancy was significantly lower than that seen with those who did not have malaria during pregnancy (P = 0.009 and P = 0.042, respectively) (Table 3). No significant reductions were observed for the other EBV-specific antibodies or for TT-specific antibodies.
TABLE 3.
Multivariate linear regression analysis of the transplacental transfer of EBV- and TT-specific antibodiesa
| Outcome | Intercept | SE | Mean difference | SE | P value |
|---|---|---|---|---|---|
| Log anti-VCA-p18 | 0.014 | 0.330 | −0.573 | 0.212 | 0.009 |
| Log anti-EBNA1 | 0.703 | 0.395 | −0.526 | 0.253 | 0.042 |
| Log anti-Zta | −2.065 | 0.749 | −0.644 | 0.480 | 0.185 |
| Log anti-EAd | −2.681 | 0.998 | −0.466 | 0.640 | 0.469 |
| Log anti-VCA-gp125 | 0.029 | 0.367 | −0.215 | 0.235 | 0.365 |
| Log anti-TT | 0.213 | 0.270 | −0.143 | 0.173 | 0.410 |
The CMR data were log transformed, and then values were adjusted for neonatal values plus maternal age, hypergammaglobulinemia status, and gestational age at exposure. Boldface data indicate a significant association between maternal malaria infection during pregnancy and the transfer of the EBV-specific antibodies.
VCA-p18- and EBNA1-specific IgG subclass distributions in maternal venous blood and cord blood.
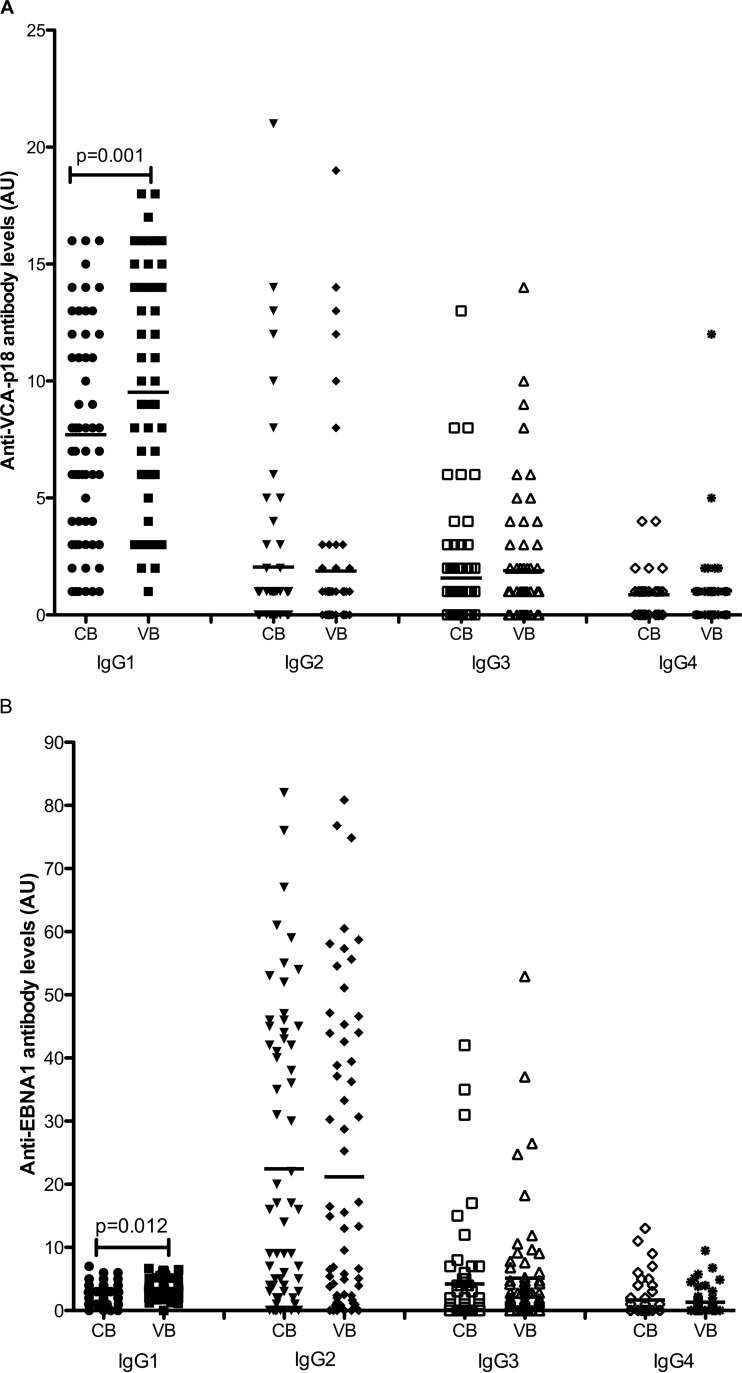
One possible explanation for the reduced transplacental transfer of anti-VCA and anti-EBNA antibodies could be differences in the IgG subclass distributions of these antibodies during pregnancy, potentially due to viral reactivation. To test this possibility, we assessed IgG subclass reactivity to EBNA1 and VCAp18 antigens in the maternal venous blood and in the infant cord blood. Anti-VCA-p18 IgG1 and IgG4 were detected in all the maternal and cord blood samples. A total of 42% and 40% of the cord blood samples had detectable anti-VCA-p18 IgG2 and IgG3 antibodies, respectively. EBNA1 IgG1 was detected in 98% and 88% of the maternal and cord blood samples, respectively. A total of 83% of maternal and cord blood samples had detectable EBNA1 IgG2, while EBNA1 IgG3 was detected in 71% and 59% of the maternal and cord blood samples. A total of 35% and 36% of maternal blood and cord blood had detectable EBNA1 IgG4, respectively.
Next, we evaluated the levels of anti-VCAp18 and anti-EBNA1 IgG subclass levels in maternal venous blood and cord blood samples. Levels of VCA-p18- and EBNA1-specific IgG1 were significantly lower in cord blood than in maternal venous blood (P = 0.001 and 0.012, respectively). There were comparable levels of VCA-p18-specific IgG2, IgG3, and IgG4 in maternal venous blood and cord blood. Similarly, we did not observe any significant difference in the levels of EBNA1-specific IgG2, IgG3, and IgG4 between maternal venous blood and cord blood (all P > 0.05) (Fig. 3). There were higher overall levels of anti-EBNA1 IgG2 antibodies than of the other IgG isotypes.
FIG 3.
Levels of EBV-specific IgG subclass in venous blood of mothers and their neonates. Anti-VCA-p18-specific (A) and anti-EBNA1-specific (B) IgG subclass antibodies in cord blood (CB) and maternal venous blood (VB) at delivery were measured by ELISA. Arbitrary units were calculated by dividing the test sample ODs by the mean OD plus 2 standard deviations (SDs) of the negative-control sera. Horizontal bars represent the means, and only significant P values are shown in the figure.
DISCUSSION
This study tested the hypothesis that exposure to P. falciparum malaria in utero would interfere with the transplacental transfer of EBV-specific antibodies from the mother to the neonate. Indeed, the data presented in this study demonstrate that transfer of IgG antibodies to the EBV lytic VCA-p18 antigen and latent EBNA1 antigen from the mother to the neonate was significantly reduced following malaria exposure in utero and that this effect remained significant even after adjusting for other confounding factors. We also observed that, regardless of malaria exposure during pregnancy, neonates had significantly lower levels of EBV lytic anti-Zta and anti-EAd IgG antibodies.
A number of conditions are known to affect the maternal-fetal transfer of IgG antibodies, including HIV infection, placental malaria, and maternal hypergammaglobulinemia (23, 26, 27, 34). In this study, only HIV-negative mothers were enrolled, and we adjusted for hypergammaglobulinemia in our multivariate analysis, with results indicating that the effect of reduced transfer of anti-VCA-p18 and anti-EBNA1 antibodies was likely due to maternal malaria infection during pregnancy. It is not clear why this effect is selective for only two of the EBV antigens tested. However, we have previously shown that malaria infection during pregnancy results in EBV reactivation (29). It is possible that there was a shift in antibody isotype responses during EBV reactivation in the mothers such that a different isotype of IgG was dominant and was less efficiently transferred to the neonates. It is known that antigens that mainly elicit IgG1 or IgG3 responses are transported across the placenta more efficiently than either IgG2 or IgG4 (36, 37), with a consequence of reduced transplacental transfer of antibodies of these specificities. A previous study found that the IgG subclass distribution to VCA in acutely infected individuals or during viral reactivation was predominantly IgG1, no IgG2 was detected, and the presence of IgG3 was indicative of viral reactivation (12). Consistent with that study, we found that IgG1 was the dominant subclass of IgG in anti-VCA and anti-EBNA antibodies detected in maternal venous blood and infant cord blood. Thus, the reduction in transplacental transfer was likely due to decreased IgG1 transfer and not to a shift in IgG subclasses.
Antibodies to the EBV EAd lytic antigen have long been used clinically as a marker of EBV reactivation, as they are short-lived (38). More recently, studies conducted with another EBV lytic antigen, Zta, have also shown utility in indicating viral reactivation (10, 13). All the mothers in this study had both anti-Zta and anti-EAd antibodies at the time of delivery indicative of viral reactivation. This is consistent with studies that have demonstrated that pregnancy induced viral reactivation (39, 40). One surprising finding from this study is that, irrespective of malaria exposure, antibodies against Zta and EAd are not efficiently transported across the placenta into fetal circulation, resulting in low levels of anti-Zta and anti-EAd antibodies in the neonates. One possible explanation is the low maternal levels of anti-Zta and anti-EAd antibodies. This observation is supported by data from a previous study that demonstrated that levels of maternal antibodies to varicella-zoster virus represented the most important predictor of the neonatal antibody levels (41). The low maternal levels of anti-EAd antibodies could have been due to the fact that the levels of anti-EAd antibodies fall relatively quickly after infection (42). Since the majority of IgG is transported across the placenta during the last trimester of pregnancy (43), the rapid decay of anti-EAd antibodies may result in very few antibodies being transferred to the neonates, as reported. This is in contrast to the high levels of IgG antibodies against both VCA-p18 and EBNA1 that we observed and that persist for life in healthy carriers (42). This could also explain the lack of correlation between maternal and neonatal anti-EAd antibodies observed in the current study. Since some neonates in both the malaria-exposed and nonexposed groups had elevated anti-EAd IgG levels suggestive of active neonatal infection, EBV DNA was assessed in all the cord blood samples, but the results were found to be negative (data not shown).
Another plausible explanation for the low levels of anti-Zta and anti-EAd IgG antibodies in cord blood is the competitive binding of antibodies to the neonatal Fc receptors. The transfer of antibodies across the placenta is a selective process that is dependent on neonatal Fc receptors (FcRn). For antibodies to be transferred across the placenta, they have to bind to the FcRn and be transported across the syncytiotrophoblast and into the fetal circulation (20, 43). Antibodies that are expressed at high levels in the mother may be competing with the antibodies that are expressed at low levels, such as anti-Zta and anti-EAd, for binding to the limited number of FcRn receptors, thereby reducing the transfer of anti-Zta and anti-EAd antibodies to the infants. However, this observation warrants further studies to investigate the mechanism. Lastly, the low levels of cord blood anti-Zta and anti-EAd antibodies could be explained by the fact that these antibodies may have been bound to the specific antigen and trapped within the placenta as immune complexes (44) and, as a consequence, not transported into the fetal circulation.
The finding from this study that maternal malaria infection during pregnancy did not affect the transplacental transfer of TT-specific antibodies is consistent with previous studies that demonstrated that placental malaria does not have an effect on the transplacental transfer of anti-TT antibodies (22, 35). However, these results are in contrast to other studies that demonstrated that placental malaria results in reduced transplacental transfer of anti-TT antibodies (26, 34). However, all of these previous studies focused on placental malaria as determined by the presence of malaria parasites in the mother at birth. In contrast, we assessed the presence of malaria infection at any time during pregnancy; thus, our results are not directly comparable with the results from previous studies.
A strength of this study was the longitudinal study design, which allowed us to actively follow pregnant mothers through pregnancy up to delivery, whereas other studies have focused mainly on placental malaria due to their cross-sectional study design. Our approach allowed us to identify peripheral malaria infections of the mother during pregnancy rather than only malaria infection at delivery. In addition, we used a Q-PCR method that was more sensitive (compared to methods employing blood smears) to determine the malaria infection status of the pregnant mothers at enrollment, at subsequent follow-up, and at delivery and the malaria infection status of cord blood. However, whether the mothers had single or repetitive infections with malaria was not analyzed, which represents a limitation of the study. We did not analyze mothers with placental malaria separately from those with malaria infection at any time during pregnancy because there were so few mothers with placental malaria in this cohort. An additional limitation of our study was the modest number of mother-child pairs included in these analyses. Finally, while anti-VCA, anti-EBNA1, anti-EAd, and anti-Zta antibodies are well described during infection with EBV, they are not known to be neutralizing antibodies. In contrast, antibodies against the EBV gp350 protein are neutralizing but have not been well characterized in human populations. Future studies will need to be done to analyze the levels of anti-gp350 antibodies in this cohort.
In summary, malaria infection during pregnancy results in impaired transplacental transfer of a subset of EBV-specific antibodies. Inadequate transfer of anti-EBV antibodies from mothers to the neonates may predispose the infants to early EBV infection. Infection with EBV in infancy leads to poor control of the virus and has been hypothesized to be a risk factor for Burkitt's lymphoma. It remains to be determined whether this reduced transplacental transfer of anti-EBV antibodies predisposes the neonates to EBV infection in early infancy. This report demonstrates the need for improved measures to prevent maternal malaria infections during pregnancy.
ACKNOWLEDGMENTS
We acknowledge the Chulaimbo Sub-District Hospital for allowing us to use their facilities to perform this study. We also thank our clinical officers and data entry and field staff members involved in the project and M. M. Majiwa for his invaluable insights. We are particularly grateful to the mothers and their infants for participating in this study.
The manuscript was submitted for publication with the permission of the Director of KEMRI.
We do not have any commercial or other association that might pose a conflict of interest.
Funding was provided through the National Cancer Institute at the National Institutes of Health (CA102667 [R.R.]). S.O. is a Ph.D. Research Fellow supported by a D43 training grant (NCI 153707). The funders had no role in study design, in data collection and interpretation, or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Rickinson A. 2002. Epstein-Barr virus. Virus Res 82:109–113. [DOI] [PubMed] [Google Scholar]
- 2.Rochford R, Cannon MJ, Moormann AM. 2005. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol 3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 3.Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, Moormann AM, Ploutz-Snyder R, Rochford R. 2012. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 205:906–913. doi: 10.1093/infdis/jir872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabay PA, Preciado MV. 2013. EBV primary infection in childhood and its relation to B-cell lymphoma development: a mini-review from a developing region. Int J Cancer 133:1286–1292. doi: 10.1002/ijc.27858. [DOI] [PubMed] [Google Scholar]
- 5.de-Thé G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. 1978. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 6.Orem J, Sandin S, Mbidde E, Mangen FW, Middeldorp J, Weiderpass E. 2014. Epstein-Barr virus viral load and serology in childhood non-Hodgkin's lymphoma and chronic inflammatory conditions in Uganda: implications for disease risk and characteristics. J Med Virol 86:1796–1803. doi: 10.1002/jmv.23988. [DOI] [PubMed] [Google Scholar]
- 7.Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat Rev Cancer 4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 8.Dardari R, Menezes J, Drouet E, Joab I, Benider A, Bakkali H, Kanouni L, Jouhadi H, Benjaafar N, El Gueddari B, Hassar M, Khyatti M. 2008. Analyses of the prognostic significance of the Epstein-Barr virus transactivator ZEBRA protein and diagnostic value of its two synthetic peptides in nasopharyngeal carcinoma. J Clin Virol 41:96–103. doi: 10.1016/j.jcv.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 9.De Paschale M, Clerici P. 2012. Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol 1:31–43. doi: 10.5501/wjv.v1.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middeldorp JM. 2015. Epstein-Barr virus specific humoral immune responses in health and disease. Springer Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 11.Henle W, Henle G. 1981. Epstein-Barr virus-specific serology in immunologically compromised individuals. Cancer Res 41:4222–4225. [PubMed] [Google Scholar]
- 12.Linde A, Andersson J, Lundgren G, Wahren B. 1987. Subclass reactivity to Epstein-Barr virus capsid antigen in primary and reactivated EBV infections. J Med Virol 21:109–121. doi: 10.1002/jmv.1890210203. [DOI] [PubMed] [Google Scholar]
- 13.Piriou E, Kimmel R, Chelimo K, Middeldorp JM, Odada PS, Ploutz-Snyder R, Moormann AM, Rochford R. 2009. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol 81:1088–1093. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggar RJ, Henle G, Bocker J, Lennette ET, Fleisher G, Henle W. 1978. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer 22:244–250. [DOI] [PubMed] [Google Scholar]
- 15.Biggar RJ, Henle W, Fleisher G, Bocker J, Lennette ET, Henle G. 1978. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 22:239–243. [DOI] [PubMed] [Google Scholar]
- 16.Joncas J, Boucher J, Granger-Julien M, Filion C. 1974. Epstein-Barr virus infection in the neonatal period and in childhood. Can Med Assoc J 110:33–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KH, Tam JS, Peiris JS, Seto WH, Ng MH. 2001. Epstein-Barr virus (EBV) infection in infancy. J Clin Virol 21:57–62. doi: 10.1016/S1386-6532(01)00149-4. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes-Pinto I, Hart CA. 1997. Transplacental antibody transfer and neonatal immunity. Br J Hosp Med 58:317–319. [PubMed] [Google Scholar]
- 19.Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE. 1995. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol 154:6246–6251. [PubMed] [Google Scholar]
- 20.Simister NE, Story CM. 1997. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol 37:1–23. [DOI] [PubMed] [Google Scholar]
- 21.Story CM, Mikulska JE, Simister NE. 1994. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med 180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Moraes-Pinto MI, Almeida AC, Kenj G, Filgueiras TE, Tobias W, Santos AM, Carneiro-Sampaio MM, Farhat CK, Milligan PJ, Johnson PM, Hart CA. 1996. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 173:1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 23.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, Milligan PJ, Wesumperuma L, Broadhead RL, Brabin BJ, Johnson PM, Hart CA. 1998. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed 79:F202–F205. doi: 10.1136/fn.79.3.F202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott S, Cumberland P, Shulman CE, Cousens S, Cohen BJ, Brown DW, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts F. 2005. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis 191:1854–1860. doi: 10.1086/429963. [DOI] [PubMed] [Google Scholar]
- 25.Wesumperuma HL, Perera AJ, Pharoah PO, Hart CA. 1999. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol 93:169–177. doi: 10.1080/00034989958654. [DOI] [PubMed] [Google Scholar]
- 26.Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT. 2007. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 196:550–557. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 27.Okoko BJ, Wesumperuma LH, Ota MO, Pinder M, Banya W, Gomez SF, McAdam KP, Hart AC. 2001. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis 184:627–632. doi: 10.1086/322808. [DOI] [PubMed] [Google Scholar]
- 28.Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, Mbogo C, Minakawa N, Zhou G, Yan G. 2006. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol 43:200–206. doi: 10.1603/0022-2585(2006)043[0200:PDOMVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Daud II, Ogolla S, Amolo AS, Namuyenga E, Simbiri K, Bukusi EA, Ng'ang'a ZW, Ploutz-Snyder R, Sumba PO, Dent A, Rochford R. 2015. Plasmodium falciparum infection is associated with Epstein-Barr virus reactivation in pregnant women living in malaria holoendemic area of western Kenya. Matern Child Health J 19:606–614. doi: 10.1007/s10995-014-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middeldorp JM, Meloen RH. 1988. Epitope-mapping on the Epstein-Barr virus major capsid protein using systematic synthesis of overlapping oligopeptides. J Virol Methods 21:147–159. doi: 10.1016/0166-0934(88)90061-4. [DOI] [PubMed] [Google Scholar]
- 31.van Grunsven WM, Spaan WJ, Middeldorp JM. 1994. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis 170:13–19. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Fachiroh J, Paramita DK, Hariwiyanto B, Harijadi A, Dahlia HL, Indrasari SR, Kusumo H, Zeng YS, Schouten T, Mubarika S, Middeldorp JM. 2006. Single-assay combination of Epstein-Barr virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening. J Clin Microbiol 44:1459–1467. doi: 10.1128/JCM.44.4.1459-1467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu AD, Mo HY, Xie YB, Peng RJ, Bei JX, Peng J, Li MY, Chen LZ, Feng QS, Jia WH, Zeng YX. 2008. Evaluation of a multianalyte profiling assay and an enzyme-linked immunosorbent assay for serological examination of Epstein-Barr virus-specific antibody responses in diagnosis of nasopharyngeal carcinoma. Clin Vaccine Immunol 15:1684–1688. doi: 10.1128/CVI.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brair ME, Brabin BJ, Milligan P, Maxwell S, Hart CA. 1994. Reduced transfer of tetanus antibodies with placental malaria. Lancet 343:208–209. doi: 10.1016/S0140-6736(94)90991-1. [DOI] [PubMed] [Google Scholar]
- 35.Okoko BJ, Wesuperuma LH, Ota MO, Banya WA, Pinder M, Gomez FS, Osinusi K, Hart AC. 2001. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr 19:59–65. [PubMed] [Google Scholar]
- 36.Malek A, Sager R, Schneider H. 1994. Maternal-fetal transport of immunoglobulin G and its subclasses during the third trimester of human pregnancy. Am J Reprod Immunol 32:8–14. doi: 10.1111/j.1600-0897.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 37.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. 1994. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol 1:667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman MA, Kingsley LA, Atchison RW, Belle S, Breinig MC, Ho M, Rinaldo CR Jr. 1991. Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. J Clin Microbiol 29:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeri S, Baker AM, Boggess KA. 2010. Prevalence of Epstein-Barr virus reactivation in pregnancy. Am J Perinatol 27:715–719. doi: 10.1055/s-0030-1253098. [DOI] [PubMed] [Google Scholar]
- 40.Purtilo DT, Sakamoto K. 1982. Reactivation of Epstein-Barr virus in pregnant women: social factors, and immune competence as determinants of lymphoproliferative diseases—a hypothesis. Med Hypotheses 8:401–408. doi: 10.1016/0306-9877(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 41.van Der Zwet WC, Vandenbroucke-Grauls CM, van Elburg RM, Cranendonk A, Zaaijer HL. 2002. Neonatal antibody titers against varicella-zoster virus in relation to gestational age, birth weight, and maternal titer. Pediatrics 109:79–85. doi: 10.1542/peds.109.1.79. [DOI] [PubMed] [Google Scholar]
- 42.Miller G. 1990. The switch between latency and replication of Epstein-Barr virus. J Infect Dis 161:833–844. doi: 10.1093/infdis/161.5.833. [DOI] [PubMed] [Google Scholar]
- 43.Saji F, Samejima Y, Kamiura S, Koyama M. 1999. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 4:81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 44.Simister NE. 1998. Human placental Fc receptors and the trapping of immune complexes. Vaccine 16:1451–1455. doi: 10.1016/S0264-410X(98)00107-8. [DOI] [PubMed] [Google Scholar]