Abstract
Objective:
To conduct a systematic review and meta-analysis of randomized placebo-controlled trials of mirtazapine for the treatment of antipsychotic-induced acute akathisia (AIAA).
Methods:
Studies were identified using online searches of PUBMED/MEDLINE and Cochrane database (CENTRAL), along with websites recording trial information such as www.clinicaltrials.gov, www.controlled-trials.com, and www.clinicalstudyresults.org. The study eligibility criteria were randomized, double-blind clinical trials comparing mirtazapine with placebo for AIAA with standardized rating for akathisia as outcome measure. The methodological quality of included trials was assessed using the Jadad Scale. Separate meta-analyses were undertaken for each outcome (response rate and complete remission) and treatment effects were expressed as Mantel–Haenszel risk ratio (RR). Fixed-effect meta-analysis was performed as heterogeneity was not significant. Number need to treat (NNT) as a measure of relative treatment effectiveness was calculated.
Results:
A systematic review of the literature revealed six studies that had assessed mirtazapine for the treatment of AIAA. Of these, two studies (n = 86) met the review inclusion criteria and were included in the final analysis. A meta-analysis was performed to see the effect size of response rate and complete remission. For response rate, RR was 6.67 [95% confidence interval (CI) 2.14–20.78], favoring mirtazapine compared with placebo, and the overall effect was significant (p = 0.001, NNT 4, 95% CI 2.6–8.6). For complete remission, RR was 6.20 (95% CI 1.74–22.08), favoring mirtazapine compared with placebo, and the overall effect was significant (p = 0.005, NNT 5, 95% CI 2.9–11.6).
Conclusions:
Although limited to only two studies and small sample, existing data support the efficacy of mirtazapine for the treatment of AIAA, with one in four patients showing partial response and one in five patients showing complete remission.
Keywords: akathisia, meta-analysis, mirtazapine, systematic review
Introduction
Akathisia is a clinical syndrome characterized by the subjective sense of unease or restlessness, or observable motor manifestations including shuffling or tramping movements of the legs and feet [Barnes and Braude, 1985]. It is commonly associated with antipsychotic medications, both first generation and atypical [Kumar and Sachdev, 2009], as well as antidepressants, including tricyclics and selective serotonin reuptake inhibitors (SSRIs). Estimates of the prevalence of akathisia in people treated with antipsychotics vary from 20% to 75%, occurring more frequently in the first 3 months of treatment [Ayd, 1961; Sachdev, 1995].
There are several clinical implications of identifying akathisia. Its presence suggests higher rates of psychopathology and poor response to pharmacotherapy [Van Putten et al. 1984; Newcomer et al. 1994; Duncan et al. 2000]. It is also identified as a predictor for development of tardive dyskinesia [Goswami and Channabasavanna, 1984]. Furthermore, the marked distress associated with akathisia has been associated with impulsive suicide attempts [Shear et al. 1983; Drake and Ehrlich, 1985; Wirshing et al. 1992; Hansen and Kingdom, 2006]. All these can contribute to noncompliance with antipsychotic drug treatment leading to increased risk for relapse [Barnes, 2003]. All these factors warrant early identification and treatment.
Antipsychotic-induced akathisia has been described as acute or tardive: the former occurs within 6 weeks of starting antipsychotics, whereas the later occurs after 3 months [Sachdev, 1995]. There is evidence to suggest differences in the clinical manifestation and response to treatment in acute and tardive akathisia. The pathophysiology of antipsychotic-induced acute akathisia (AIAA) is unknown. Involvement of dopaminergic and serotonergic pathways has been suggested as a possible mechanism. β blockers, benzodiazepines, and anticholinergics are currently recommended as the first-line treatment options in AIAA [Miller and Fleischhacker, 2000]. The high rate of nonresponse and their side effects prompted the search for effective anti-AIAA treatment alternatives. Recently, agents with marked serotonin 5-HT2A antagonism (ritanserin, cyproheptadine, and mianserin) were found efficacious for AIAA [Miller et al. 1990, 1992; Weiss et al. 1995; Poyurovsky and Weizman, 1997, 2001b; Stryjer et al. 2004].
Mirtazapine is a novel antidepressant that acts centrally to increase both noradrenergic and serotonergic neurotransmission. It is structurally and pharmacologically similar to mianserin and exhibits marked 5-HT2A antagonism. Common side effects include sedation, weight gain and increased appetite. Recent studies have shown that mirtazapine is useful for the treatment of AIAA [Poyurovsky and Weizman, 2001a; Wilson, 2005; Ranjan et al. 2006; Poyurovsky et al. 2003, 2006, 2014], although a few case reports of akathisia, both acute and tardive, induced by mirtazapine have been reported [Girishchandra et al. 2002; Gulsun and Doruk, 2008; Ozyildirim and Kosecioglu, 2009; Markoula et al. 2010]. As the effect of first-line agents for AIAA is variable, we reviewed existing literature to identify if enough evidence is available for mirtazapine. We conducted a systematic review and meta-analysis with an objective to determine whether mirtazapine is effective for the treatment of AIAA.
Methods
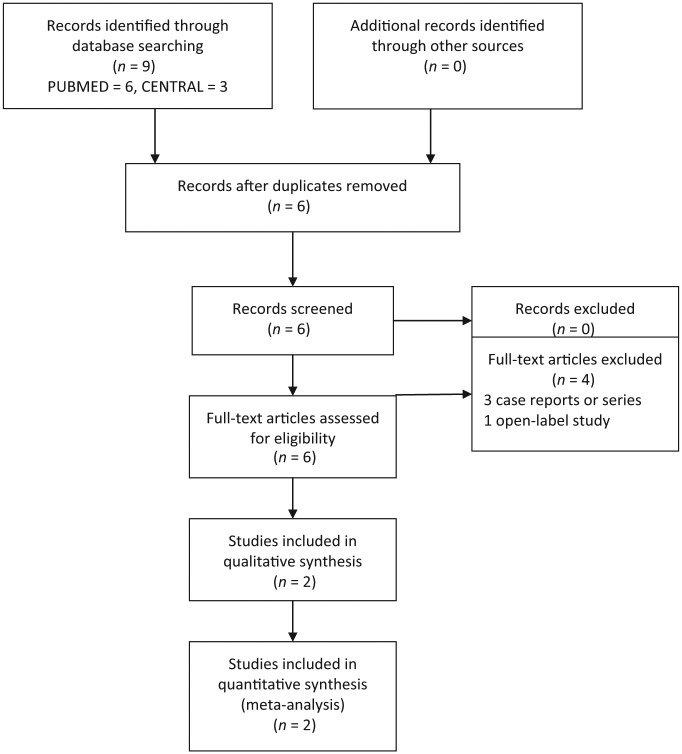
In our systematic review and meta-analysis, we adhered to the recent update of preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [Moher et al. 2009]. The flow of studies is summarized in Figure 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2009 flow diagram.
Data sources and search strategy
Studies were identified using online searches of PUBMED/MEDLINE and Cochrane database (CENTRAL). Also, websites recording trial information such as www.clinicaltrials.gov, www.controlled-trials.com, and www.clinicalstudyresults.org were searched for relevant studies. Searches were conducted using combination of terms ‘antipsychotics’, ‘neuroleptic’, ‘akathisia’, and ‘mirtazapine’. We inspected the reference lists of all identified studies, including existing reviews for relevant citations. The search was restricted to publications in the English language.
Study selection: inclusion criteria
One reviewer (SKP) initially evaluated the abstracts from the literature search. The following criteria were used to identify the studies:
Randomized, double-blind clinical trials comparing mirtazapine with placebo for AIAA.
Outcome measures including standardized rating on a scale for akathisia (e.g. Barnes Akathisia Rating Scale).
Data extraction
Two reviewers (SKP and SK) decided, independently, whether individual studies met the inclusion criteria. Any discrepancy was resolved after discussion with RVB. A standardized form was used, and extracted data which included patient and study characteristics, outcome measures, and study results.
Assessment of methodological quality of studies
The methodological quality of included trials in this review was assessed using the Jadad Scale [Jadad et al. 1996]. It includes three items: (1) Was the study described as randomized? (2) Was the study described as double blind? (3) Was there a description of withdrawals and drop outs? Scoring was done as follows: one point for a positive answer and one point deducted if either the randomization or the blinding/masking procedures was inadequate. Cutoff of two points on the Jadad Scale was considered.
Quantitative data synthesis
Meta-analyses were undertaken to estimate overall treatment effects where the trials were considered to be similar enough to combine using RevMan 5 version. This decision was based on assessing similarity of trial characteristics as well as results. A separate meta-analysis was undertaken for response rate (at least two-point reduction in the Barnes Akathisia Scale (BAS) Global Scale) and complete remission (0 or 1 on BAS Global Scale) as outcome measures. Treatment effects were expressed as Mantel–Haenszel risk ratio (RR) as outcome was categorical with 95% confidence interval (95% CI). Homogeneity among studies was tested using Cochran’s Q test and I2 statistic, in which more than 50% indicates a moderate amount of heterogeneity [Higgins et al. 2003]. If significant statistical heterogeneity was detected (Cochran’s Q test p <0.1 or I2 value > 50%), random effects estimates were calculated. Otherwise, the fixed-effect model was used for analysis. When overall results were significant, the number need to treat (NNT) as a measure of relative treatment effectiveness was calculated on an intent-to-treat basis.
Results
Studies included
The combined search strategies identified six papers on use of mirtazapine in AIAA after removing duplications (Figure 1). Among the three papers in CENTRAL, two [Poyurovsky et al. 2002, 2003] were identical studies based on sample, methodology and results, but the Poyurovsky et al. [2003] paper used intent-to-treat analysis and reported results from 26 patients. Both the Poyurovsky studies were also identified through a PUBMED search [Poyurovsky et al. 2003, 2006]. Four studies were excluded as they were case reports or case series [Poyurovsky and Weizman, 2001a; Wilson, 2005; Ranjan et al. 2006; Poyurovsky et al. 2014]. In a case study, Poyurovsky and Weizman observed a reduction of AIAA (that did not improve with trial of biperiden 4 mg twice daily for 5 days, and subsequently diazepam 10 mg for 3 days) after the second day, with the addition of a morning dose of 15 mg mirtazapine [Poyurovsky and Weizman, 2001a]. There was recurrence of akathisia with discontinuation of mirtazapine, which improved after restarting medication. It was tolerated well with mild transient sedation as an adverse effect. Wilson found low-dose mirtazapine (7.5 mg) reduced akathisia with antipsychotics in three patients having bipolar disorder, without induction of mania [Wilson, 2005]. Improvement was noted after 2–7 days, and only case 3 could be weaned off mirtazapine over a month without recurrence, whereas case 1 could be maintained on 3.75 mg dose. In another case series (n = 5), Ranjan and colleagues reported reduction in AIAA with the addition of 15 mg mirtazapine, with an additional benefit for depressive symptoms [Ranjan et al. 2006]. Improvement was noted after 3 days in three patients, and in the other two patients after 1–2 weeks. In an open-related trial, Poyurovsky and colleagues studied mirtazapine 15 mg daily for the treatment of aripiprazole-related akathisia. They found significant improvement in five of eight (62.5%) patients, and partial improvement in another patient. There was mild transient sedation in three patients, without any aggravation of psychosis [Poyurovsky et al. 2014]. Finally, two studies [Poyurovsky et al. 2003, 2006] met the review inclusion criteria (total 86 subjects) and were included in the final analysis. Characteristics of included studies are summarized in Table 1. In one study, only mirtazapine and placebo arm values were included in the meta-analysis [Poyurovsky et al. 2006].
Table 1.
Characteristics of included studies.
| Study | Methods | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Poyurovsky et al. [2003] | Allocation: randomized | Diagnosis: schizophrenia N = 26 | 1. Mirtazapine 15 mg daily, N = 13 | BAS, PANSS, HAM-D, SAS |
| Blinding: doubleDuration: 5 days | 2. Placebo, N = 13 | |||
| Poyurovsky et al. [2006] | Allocation: randomized | Diagnosis: schizophrenia, delusional disorder, major depressive disorder with psychotic features N = 60 | 1. Mirtazapine 15 mg daily, N = 30 | BAS, BPRS, HAM-D, SAS |
| Blinding: double | 2. Placebo, N = 30 | |||
| Duration: 7 days |
BAS, Barnes Akathisia Scale; BPRS, Brief Psychiatric Rating Scale; HAM-D, Hamilton Rating Scale for Depression; PANSS, Positive and Negative Syndrome Scale; SAS, Simpson–Angus Scale.
Study quality
Both of the studies were described as randomized and were double blind. Dropout rates were mentioned in both the studies, and varied from 11.2% to 14.2%. Concealment of allocation was not adequately reported in both studies. Therefore, as it was unclear how randomization sequences were kept concealed, it is likely that the studies are prone to at least a moderate degree of bias [Juni et al. 2001].
Meta-analysis
Forest plots for meta-analyses for response rate (at least two-point reduction in BAS Global Scale) and complete remission (0 or 1 on BAS Global Scale) are presented in Figures 2 and 3. For response rate, test for heterogeneity was not significant (p = 0.95, I2 = 0%); therefore the fixed-effects model was used. Mantel–Haenszel RR for response rate was 6.67 (95% CI 2.14–20.78), favoring mirtazapine compared with placebo, and the overall effect was significant (p = 0.001). NNT for response rate in the mirtazapine group compared with placebo was calculated as 4 (95% CI 2.6–8.6). For complete remission of akathisia, the test for heterogeneity was not significant (p = 0.62, I2 = 0%); therefore the fixed-effects model was used. The Mantel–Haenszel RR for complete remission was 6.20 (95% CI 1.74–22.08), favoring mirtazapine compared with placebo, and the overall treatment effect was significant (p = 0.005). NNT for complete remission in the mirtazapine group compared with placebo was calculated as 5 (95% CI 2.9–11.6). Mirtazapine was well tolerated in these studies, with sedation as the most common adverse effect.
Figure 2.
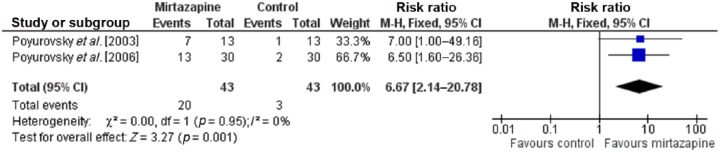
Forest plot showing response rate (at least two-point reduction in BAS Global Scale) in randomized controlled trials comparing mirtazapine with placebo for antipsychotic-induced acute akathisia (N = 86). CI, confidence interval; M-H, Mantel–Haenszel.
Figure 3.
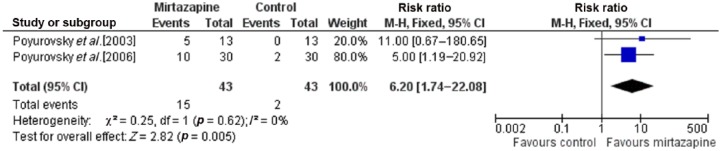
Forest plot showing complete remission (0 or 1 on BAS Global Scale) in randomized controlled trials comparing mirtazapine and placebo for antipsychotic-induced acute akathisia (N = 86). CI, confidence interval; M-H, Mantel–Haenszel.
Discussion
There is a paucity of studies examining the efficacy of mirtazapine for the treatment of AIAA (only two studies, N = 86). Nevertheless, existing data support the efficacy of mirtazapine for the treatment of AIAA, with RR 6.67 (95% CI 2.14–20.78) for response and 6.20 (95% CI 1.74–22.08) for complete remission. The effect size (NNT) for the efficacy was high, with one in four patients showing partial response and one in five patients showing complete remission. The dose of mirtazapine used in both studies was 15 mg, given as a morning dose, and the most common adverse effect reported was sedation. Considering the long half life of mirtazapine (20–40 h), it will be worthwhile to study whether bedtime dosing of mirtazapine will be effective for the treatment of AIAA with minimal sedation.
Traditionally, β blockers have been advocated as first-line treatment of AIAA. A meta-analysis [Lima et al. 2004] found three small randomized controlled trials (RCTs) (total N = 51), but this was considered as insufficient to recommend β blockers for akathisia. Another meta-analysis [Lima et al. 2002] found two small RCTs (total N = 26) which showed benzodiazepines may reduce the symptoms of AIAA using the outcome criterion of ‘at least 50% remission’ (two RCTs, N = 26, RR 0.09, 95% CI 0.01–0.6). But for the outcome of ‘complete remission’, pooled data showed no significant difference between the benzodiazepine and control groups (two RCTs, N = 26, RR 0.86, 95% CI 0.6–1.2). Also there was no evidence in favor of anticholinergic agents for antipsychotic-induced acute akathisia in another meta-analysis [Rathbone and Soares-Weiser, 2006].
Our review is limited by the number of studies included in the meta-analysis. The small number of studies did not allow us to conduct tests for publication bias. Also a sensitivity analysis was not performed in our study. No heterogeneity was found for the study variables across both the studies; therefore the fixed effects model was used. In both the studies [Poyurovsky et al. 2003, 2006], the duration of treatment varied from 5 to 7 days, therefore the longer term effects of mirtazapine are not known. We suggest that all future studies should respect standards of measuring outcomes and of reporting data in order to enhance the comparability of study results. In addition, binary outcomes (response and remission) should be reported as they are easier to interpret and clinically relevant. Details regarding the allocation sequence and allocation concealment should be clearly described in all studies to prevent bias.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Samir Kumar Praharaj, Department of Psychiatry, Kasturba Medical College, Manipal, Karnataka, 576104, India.
Sreejayan Kongasseri, Department of Psychiatry, Kasturba Medical College, Manipal, Karnataka, India.
Rishikesh V. Behere, Department of Psychiatry, Kasturba Medical College, Manipal, Karnataka, India
Podila Satya Venkata Narasimha Sharma, Department of Psychiatry, Kasturba Medical College, Manipal, Karnataka, India.
References
- Ayd F., Jr (1961) A survey of drug-induced extrapyramidal reactions. JAMA 175: 1054–1060. [DOI] [PubMed] [Google Scholar]
- Barnes T. (2003) The Barnes Akathisia Rating Scale – revisited. J Psychopharmacol 17: 365–370. [DOI] [PubMed] [Google Scholar]
- Barnes T., Braude W. (1985) Akathisia variants and tardive dyskinesia. Arch Gen Psychiatry 42: 874–878. [DOI] [PubMed] [Google Scholar]
- Drake R., Ehrlich J. (1985) Suicide attempts associated with akathisia. Am J Psychiatry 142: 499–501. [DOI] [PubMed] [Google Scholar]
- Duncan E., Adler L., Stephanides M., Sanfilipo M., Angrist B. (2000) Akathisia and exacerbation of psychopathology: a preliminary report. Clin Neuropharmacol 23: 169–173. [DOI] [PubMed] [Google Scholar]
- Girishchandra B., Johnson L., Cresp R., Orr K. (2002) Mirtazapine-induced akathisia. Med J Aust 176: 242. [DOI] [PubMed] [Google Scholar]
- Goswami U., Channabasavanna S. (1984) Is akathisia a forerunner of tardive dyskinesia? A clinical report with brief review of literature. Clin Neurol Neurosurg 86: 107–110. [DOI] [PubMed] [Google Scholar]
- Gulsun M., Doruk A. (2008) Mirtazapine-induced akathisia. J Clin Psychopharmacol 28: 467. [DOI] [PubMed] [Google Scholar]
- Hansen L., Kingdom D. (2006) Akathisia as a risk factor for suicide. Br J Psychiatry 188: 192. [DOI] [PubMed] [Google Scholar]
- Higgins J., Thompson S., Deeks J., Altman D. (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A., Moore R., Carroll D., Jenkinson C., Reynolds D., Gavaghan D., et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Juni P., Altman D., Egger M. (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sachdev P. (2009) Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry 22: 293–299. [DOI] [PubMed] [Google Scholar]
- Lima A., Bacalcthuk J., Barnes T., Soares-Weiser K. (2004) Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 4: CD001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A., Soares-Weiser K., Bacaltchuk J., Barnes T. (2002) Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 1: CD001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoula S., Konitsiotis S., Chatzistefanidis D., Lagos G., Kyritsis A. (2010) Akathisia induced by mirtazapine after 20 years of continuous treatment. Clin Neuropharmacol 33: 50–51. [DOI] [PubMed] [Google Scholar]
- Miller C., Fleischhacker W. (2000) Managing antipsychotic-induced acute and chronic akathisia. Drug Saf 22: 73–81. [DOI] [PubMed] [Google Scholar]
- Miller C., Fleischhacker W., Ehrmann H., Kane J. (1990) Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol Bull 26: 373–376. [PubMed] [Google Scholar]
- Miller C., Hummer M., Pycha R., Fleischhacker W. (1992) The effect of ritanserin on treatment-resistant neuroleptic induced akathisia: case reports. Prog Neuropsychopharmacol Biol Psychiatry 16: 247–251. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer J., Miller L., Faustman W., Wetzel M., Vogler G., Csernansky J. (1994) Correlations between akathisia and residual psychopathology: a by-product of neuroleptic-induced dysphoria. Br J Psychiatry 164: 834–838. [DOI] [PubMed] [Google Scholar]
- Ozyildirim I., Kosecioglu S. (2009) Mirtazapine induced tardive akathisia: a case report. Eur Psychiatry 24: 507.19540728 [Google Scholar]
- Poyurovsky M., Bergman J., Pashinian A., Weizman A. (2014) Beneficial effect of low-dose mirtazapine in acute aripiprazole-induced akathisia. Int Clin Psychopharmacol 29: 296–298. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Epshtein S., Fuchs C., Schneidman M., Weizman R., Weizman A. (2002) Efficacy of low-dose mirtazapine in neuroleptic-induced akathisia: a double-blind randomized placebo-controlled pilot study. Eur Neuropsychopharmacol 12: 300. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Epshtein S., Fuchs C., Schneidman M., Weizman R., Weizman A. (2003) Efficacy of low-dose mirtazapine in neuroleptic-induced akathisia: a double-blind randomized placebo-controlled pilot study. J Clin Psychopharmacol 23: 305–308. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Pashinian A., Weizman R., Fuchs C., Weizman A. (2006) Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol Psychiatry 9: 1071–1077. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Weizman A. (1997) Serotonergic agents in the treatment of acute neuroleptic-induced akathisia: open-label study of buspirone and mianserin. Int Clin Psychopharmacol 12: 263–268. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Weizman A. (2001a) Mirtazapine for neuroleptic-induced akathisia. Am J Psychiatry 158: 819. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M., Weizman A. (2001b) Serotonin-based pharmacotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br J Psychiatry 179: 4–8. [DOI] [PubMed] [Google Scholar]
- Ranjan S., Chandra P., Chaturvedi S., Prabhu S., Gupta A. (2006) Atypical antipsychotic-induced akathisia with depression: therapeutic role of mirtazapine. Ann Pharmacother 40: 771–774. [DOI] [PubMed] [Google Scholar]
- Rathbone J., Soares-Weiser K. (2006) Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 4: CD003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P. (1995) The epidemiology of drug-induced akathisia: Part I. Acute akathisia. Schizophr Bull 21: 431–449. [DOI] [PubMed] [Google Scholar]
- Shear M., Frances A., Weiden P. (1983) Suicide associated with akathisia and depot fluphenazine treatment. J Clin Psychopharmacol 3: 235–236. [DOI] [PubMed] [Google Scholar]
- Stryjer R., Grupper D., Strous R., Poyurovsky M., Weizman A. (2004) Mianserin for the rapid improvement of chronic akathisia in a schizophrenia patient. Eur Psychiatry 19: 237–238. [DOI] [PubMed] [Google Scholar]
- Van Putten T., May P., Marder S. (1984) Response to antipsychotic medication: the doctor’s and the consumer’s view. Am J Psychiatry 141: 16–19. [DOI] [PubMed] [Google Scholar]
- Weiss D., Aizenberg D., Hermesh H., Zemishlany Z., Munitz H., Radwan M., et al. (1995) Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry 167: 483–486. [DOI] [PubMed] [Google Scholar]
- Wilson M., 2nd (2005) Mirtazapine for akathisia in bipolar disorder. J Clin Psychopharmacol 25: 394–395. [DOI] [PubMed] [Google Scholar]
- Wirshing W., Van Putten T., Rosenberg J., Marder S., Ames D., Hicks-Gray T. (1992) Fluoxetine, akathisia, and suicidality: is there a causal connection? Arch Gen Psychiatry 49: 580–581. [DOI] [PubMed] [Google Scholar]