Abstract
Background/Aims
Gastroparesis-like syndrome (GLS) is defined as gastroparesis-like symptoms with normal gastric scintigraphy. While the efficacy of gastric electrical stimulation (GES) in gastroparesis is well known, the utility of GES in GLS is largely unknown. Our aim was to clarify the role of GES in GLS. We implanted consecutive patients with symptoms of gastroparesis with temporary gastric electrical stimulation and observed changes in gastric scintigraphy and total symptom score.
Methods
Five hundred and fifty-one patients suffering from symptoms of gastroparesis (nausea, vomiting, bloating/distension, anorexia/early satiety, and abdominal pain) with negative endoscopy underwent gastric scintigraphy with analysis of 1) solid radio-nuclide gastric emptying at 1, 2, and 4 hours (% remaining); 2) area under the gastric emptying curve (AUC) at 1, 2, and 4 hours; and 3) total gastric emptying test (GET) (the sum of 1, 2, and 4 hour values). Patients were stratified into: delayed gastric emptying, normal gastric emptying, and rapid gastric emptying (Appendix). Of the 551 patients in the larger cohort, 379 had implantation of temporary gastric electrical stimulation (tGES). Gastrointestinal symptoms and gastric emptying were com -pared pre and post tGES implantation.
Results
After tGES, 2 hour gastric retention decreased (P < 0.01) for the delayed patients, and increased (P < 0.001) for normal and rapid patients. These changes were accompanied by improvements (P < 0.001) in vomiting, nausea, and total symptom scores in all 3 subgroups.
Conclusions
Gastric electrical stimulation may be an effective therapy for treating the symptoms of gastroparesis with normal gastric emptying. Further exploration of endoscopic electrical stimulation as a treatment for gastroparesis-like symptoms with non-delayed gastric emptying is needed.
Keywords: Abdominal pain, Electrical stimulation, Gastroparesis, Nausea, Vomiting
Introduction
Gastroparesis (Gp) is a motility disorder characterized by delayed gastric emptying in the absence of mechanical obstruction. The presentation can vary, but typical symptomatology includes: nausea, vomiting, bloating, early satiety, and abdominal pain.1–3 If untreated, chronic gastroparesis can result in significant morbidity and mortality including Mallory-Weiss tears, severe gastritis, and life-threatening electrolyte derangements.4,5 Interestingly, patients with symptoms of Gp may have normal gastric emptying scans: in a study of 425 patients with chronic nausea and vomiting, 106 (25%) had normal gastric emptying despite symptoms of Gp.6 These patients with gastroparesis symptomatology but with non-delayed emptying are defined as suffering from gastroparesis-like syndrome (GLS). GLS is being increasingly recognized as a discrete yet pathological process.6
The benefits of gastric electrical stimulation (GES) in Gp individuals have been extensively documented. Patients ten years post-implantation have demonstrated improved nutritional scores, improved symptom scores, and decreased mortality compared to medically managed patients.7 Symptom improvement has also been reported with temporary gastric electrical stimulation, a non-surgical adaption of GES using stimulating electrodes placed in the stomach endoscopically.8 The long-term results with GES have also been shown to correlate with short-term results of temporary gastric electrical stimulation (tGES).9 The effects of gastric stimulation in non-delayed gastric emptying, however, have not been precisely reviewed as compared to delayed emptying. Our study thus aims to explore the effect of tGES in a prospective study of patients with symptoms of gastroparesis with non-delayed gastric emptying.
Materials and Methods
Regulatory Approval
For this prospective study, Institutional Review Board approval was obtained from the University of Mississippi Institutional Review Board Panel. Patients/insurers paid for all costs, including temporary GES implantation and gastric scintigraphy studies with consecutive patients signing a consent for the protocol. The temporary GES system used in this study has been previously described10 and includes an FDA approved temporary cardiac pacing lead (Medtronic model 6416), and an FDA approved implantable pulse generator (Medtronic 3116). Briefly, patients undergo upper endoscopy with a standard 140 cm long endoscope with a 7F accessory channel. Implantation of the temporary lead was done at junction of antrum and body of stomach into the stomach mucosa with a counter-clockwise turning motion and placement of 3-endoscopic clips. The lead was connected to an external pulse generator. Both components (lead and generator) are used off-label in this study.
Primary and Secondary Outcomes
Primary outcomes included measurement of gastric scintigraphy (gastric emptying test [GET], total gastic emptying [TGE], and area under the curve [AUC]) and total symptoms score (TSS) after insertion of tGES. Secondary outcomes are the symptom improvements.
Study Patients
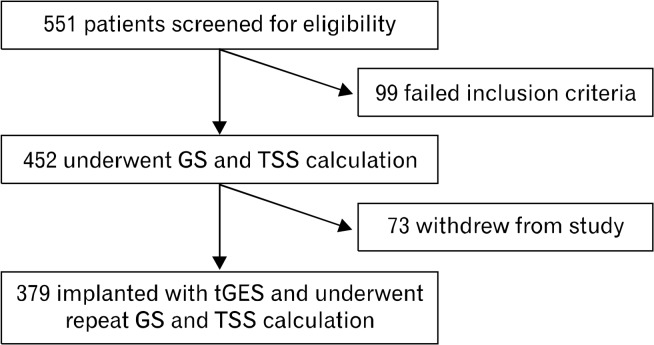
We enrolled 551 consecutive patients who presented to our clinic with symptoms of nausea, vomiting, abdominal pain, and bloating, with non-obstructive esophagogastroduodenoscopy. These patients were considered potential candidates for permanent GES and signed informed consent to participate in the study. The median age was 47 years (110 males and 441 females). After initial enrollment, patients were excluded from final data analysis if they did not meet inclusion criteria for the Humanitarian Use Device (Table 1) or if the patient requested to be removed from the study (Fig. 1).
Table 1.
Inclusion and Exclusion Criteria for Humanitarian Use Device
| Inclusion Criteria |
|
|
| Patients’ gastroparesis symptoms of diabetic, surgically related or idiopathic etiology |
| Patients aged 18–70 years old |
| Symptoms of gastroparesis for ≥ 1 year |
| Refractory or intolerant to prokinetic drug classes (cholinergic agonists, motilin receptor agonists, dopamine receptor antagonists) |
| Refractory or intolerant to antiemetic drug classes (antihistamines and phenothiazines, serotonin receptor antagonists, dopamine receptor antagonists) |
| Chronic vomiting or nausea or severe dyspepsia like syndrome consistent with gastroparesis irrespective of gastric emptying test (GET) values |
|
|
| Exclusion Criteria |
|
|
| Non-candidates for endoscopic or surgical procedures and/or anesthesia due to physical or mental conditions |
| Pregnancy |
Figure 1.
Study design. GS, gastric scintigraphy; TSS, total symptoms score; tGES, temporary gastric electrical stimulation.
Gastric Scintigraphy
Each of the 551 patients underwent gastric scintigraphy as previously described.11 We recorded values as (1) gastric retention at 1, 2, and 4 hours,11 (2) TGE: the sum of 1, 2, and 4 hours, ie, similar to ejection fraction for the heart, and it is calculated as a global measure as a sum of gastric emptying at 1, 2, and 4 hours,12 and (3) total area under the curve (TAUC)13 as demonstrated in prior studies.12 All patients were subsequently stratified as delayed gastric emptying and non-delayed gastric emptying. Non-delayed gastric emptying was further stratified as normal emptying and rapid gastric emptying, which was defined after a sub-analysis of Tougas et al11 revealed the top 10% of emptiers had 1 hour GET < 37%. The analysis and methodology of rapid gastric emptying with Tougas et al11 data can be seen in Appendix.
Patients, regardless of gastric emptying results, then underwent implantation with tGES as previously described,10 with patients undergoing test stimulation implantation for a period of 5 days.
Symptom Scores and Gastric Scintigraphy
For symptom scores, each patient recorded gastroparesis symptoms on a 5 point scale (0 = no symptom, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe [debilitating] symptoms), in a patient reported outcome (PRO) diary.8 The daily diary, which was averaged over the 5 days, provided a method for noting the daily absence/presence/severity of vomiting, nausea, anorexia/early satiety, bloating/distension, and abdominal pain. Study staff summed the 5 daily ratings, averaging frequency and severity for each, to calculate each patient’s TSS.8 The percent gastric retention,11,12 AUC,12,13 TGE, and TSS were analyzed before and after placement of the tGES. Initial gastric scinitigraphy results were used to stratify patients into delayed gastric emptying, rapid gastric emptying (Appendix), and normal gastric emptying. As mentioned above, at the end of the fifth day, patients’ TSS and gastric scintigraphy were re-collected.
Statistical Methods
Patient symptoms (vomiting, nausea, early satiety, bloating, and abdominal pain) severity were assessed on a scale of 0–5 using a standardized questionnaire. TSS was computed as the sum of the 5 individual symptom scores. TGE and TAUC were computed from the patient GET data. Pre and post implantation scores for TSS, GET, TGE, and TAUC are reported as mean and standard error and compared using Student’s t test.
Results
Our 551 consecutive patients had the following etiologies for their Gp symptomatology: diabetic in 169 patients (31%), post-surgical in 38 patients (6.9%), and idiopathic in 320 patients (58%) with 24 patients (4.4%) believed to have “other” etiologies (including known specific neuropathies and multi-system atrophy). Patients subsequently underwent gastric scintigraphy. Of the 551 patients enrolled, 99 could not complete their gastric emptying test (patients could not tolerate meal, vomiting during study). The remaining 452 patients were subsequently stratified into rapid, normal, and delayed gastric emptying cohorts based upon their performance on GET with parameters as described above.
Out of the 551 patients, the 452 patients who met inclusion criteria had gastric scintigraphy (GS) that revealed 42 patients with rapid emptying (9.3%), 273 patients with delayed emptying (60.5%), and 137 patients with normal solid emptying (30.4%). The predominant symptom of all patients was nausea (3.3 symptom score), followed by: early satiety (3.0), then abdominal pain (3.0), bloating (2.8), and finally vomiting (1.4). Sub-dividing the patient cohort into normal, delayed, and rapid gastric emptying demonstrated similar order of symptoms for all 3 groups. The normal cohort reported bloating as the third most common symptom along with abdominal pain (Table 2).
Table 2.
Baseline Characteristics of 551 Consecutive Patients With Gastroparesis Symptomatology and of 451 Patients Undergoing Gastric Emptying Test
| Variable | All patients (N = 551) | Normal (n = 137) ∑451 | Delayed (n = 273) ∑451 | Rapid (n = 42) ∑451 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| N | % | N | % | N | % | N | % | ||
| Demographics | Male | 110 | 20 | 27 | 20 | 53 | 19 | 15 | 36 |
| White | 448 | 81 | 115 | 84 | 221 | 81 | 31 | 74 | |
| Diabetics | 169 | 31 | 44 | 32 | 94 | 34 | 11 | 26 | |
| Idiopathic | 320 | 58 | 79 | 58 | 150 | 55 | 24 | 57 | |
| Post surgical | 38 | 7 | 8 | 5.8 | 22 | 8.1 | 3 | 7.1 | |
| Age (mean ± SD) | 47.4 ± 4.3 | 46.7 ± 14.9 | 47.6 ± 14.3 | 49.1 ± 14.2 | |||||
| Symptom Scores | Vomiting | 2.4 | 1.5 | 2.4 | 1.4 | 2.4 | 1.3 | 2.1 | 1.3 |
| Nausea | 3.3 | 1.0 | 3.4 | 0.9 | 3.4 | 0.8 | 3.1 | 1.0 | |
| Anorexia early satiety | 3.0 | 1.2 | 3.1 | 1.1 | 3.0 | 1.1 | 2.8 | 1.2 | |
| Bloating | 2.8 | 1.3 | 3.0 | 1.1 | 2.9 | 1.1 | 2.7 | 1.4 | |
| Abdominal pain | 3.0 | 1.2 | 3.0 | 1.2 | 3.0 | 1.1 | 2.8 | 1.4 | |
| Total symptoms score | 14.5 | 3.7 | 14.9 | 3.4 | 14.6 | 3.3 | 13.4 | 4.1 | |
| GET measurements (N = 451) | Gastric emptying test 1 hour (% remaining) | 67.4 | 21.4 | 59.6 | 13.8 | 77.8 | 15.3 | 25.2 | 11.8 |
| Gastric emptying test 2 hour | 45.3 | 25.4 | 28.7 | 13.7 | 59.1 | 20.8 | 10.2 | 7.7 | |
| Gastric emptying test 4 hour | 23.3 | 24.1 | 4.9 | 3.1 | 35.7 | 24.0 | 3.5 | 3.5 | |
| Gastric emptying test total time | 136 | 65.9 | 93.2 | 25.7 | 172.6 | 55.3 | 38.9 | 20.1 | |
GET, gastric emptying test.
Baseline gastric emptying results are shown in Table 2. For patients with normal GET, retention values at 1 hour were 59.6%, at 2 hours were 28.7%, and at 4 hours were 4.9%. The delayed emptying group had gastric emptying times at 1, 2, and 4 hour values of 77.8%, 59.1%, and 35.7% respectively. The rapid emptying group at 1, 2, and 4 hours had gastric emptying times at 25.2%, 10.2%, and 3.5% respectively. Total gastric emptying times for normal, delayed and rapid were 93.2, 172.6, and 38.9 minutes respectively.
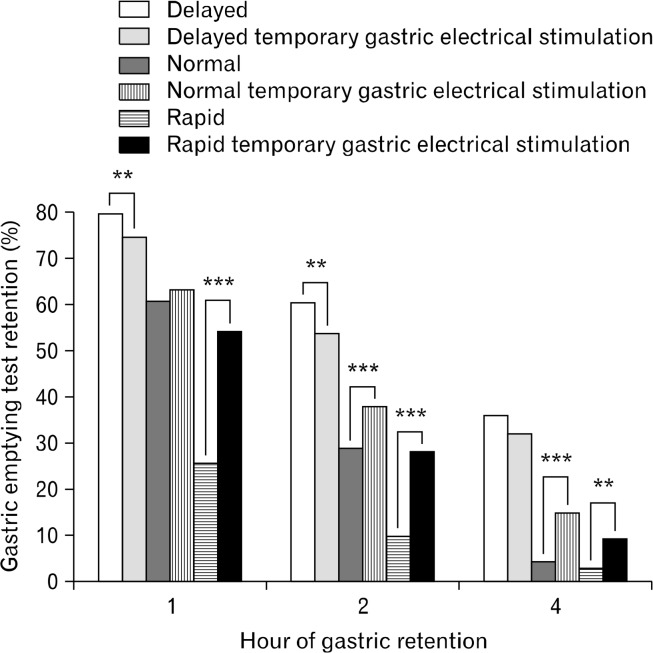
Of the 452 eligible patients, 73 did not wish to proceed with tGES implantation. A total of 379 patients underwent implantation with tGES. They subsequently had repeat GS and TSS at the end of 5 days. The results of pre- and post- GET, AUC, TGE, and TSS can be seen in Figures 2 and 3. We noted the following: (1) Patients with delayed GET had decreased gastric retention (1 and 2 hours), TGE, and AUC after tGES implantation (P < 0.01 level from Student’s t test); (2) Patients with normal GET had increased TGE and gastric retention at 2 and 4 hours after implantation (P < 0.001 level); they had increased AUC after implantation (P < 0.05). (3) Patients with rapid GET had increased gastric retention (at 1 and 2 hours), TGE, and AUC after implantation (P < 0.001). The patients with rapid GET had increased gastric retention at 4 hours after implantation as well (P < 0.01). Thus, in the rapid emptying patients, treatment with tGES resulted in normalization of gastric emptying to all parameters. Changes after tGES can be seen in Figure 2 (GET) and in Figure 3 (TSS).
Figure 2.
Gastric retention rates, after insertion of temporary gastric electrical stimulation in delayed, normal, and rapid patients. GS, gastric scintigraphy; TSS, total symptom score; tGES, temporary gastric electrical stimulation. **P < 0.01, ***P < 0.001.
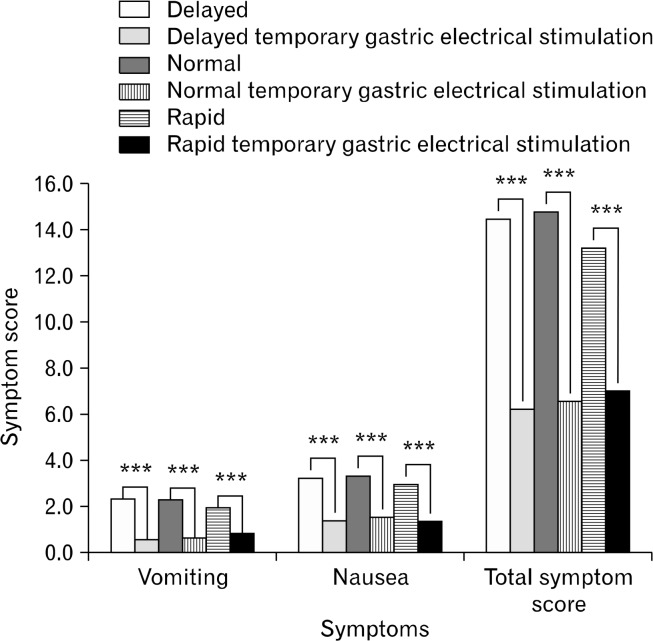
Figure 3.
Symptoms after insertion of temporary gastric electrical stimulation in delayed, normal, and rapid patients. ***P < 0.001
Symptom scores for nausea, vomiting, and TSS improved after tGES for normal, delayed, and rapid populations at the P < 0.001 level. Patients with delayed emptying accelerated and those with rapid emptying slowed. Patients with “normal” GET demonstrated decreased emptying times and improvement of symptoms as well.
Discussion
In our prospective study that found statistical difference between groups, application of gastric electrical stimulation improves symptoms of gastroparesis regardless of gastric scinitigraphy. The advantage of using temporary GES is that many patients, who might not otherwise be considered candidates for GES can be studied to see if they might respond. Patients diagnosed with gastroparesis (via gastric scintigraphy) and intolerant to pro-kinetic and anti-nausea medications demonstrated improved quality of life and decreased GI symptomatology with temporary gastric electrical stimulation.14 In those patients without gastroparesis, who have been relatively un-studied with electrical stimulation, our study demonstrated that temporary gastric stimulation improved symptomatology. This population may be diagnosed as functional dyspepsia in Rome III as they have symptoms of Gp but normal GS results. A recent review of the controversies regarding aspects of dyspepsia vs gastroparesis/ gastroparesis-like syndrome has outlined many possible diagnostic, therapeutic and terminologic options.15 The patients in this report, may represent a previously unstudied population for tGES, and as studied here, may benefit from GES.15
Our study further defined the epidemiology of GLS. We demonstrated that 39.6% of patients had gastroparesis-like symptoms with non-delayed GET in a patient population of refractory nausea and vomiting. Of these patients, Appendix demonstrates classification of non-delayed GET into normal gastric emptying and rapid gastric emptying (RGE) by re-analysis of the original Tougas data and application towards our patient population. This combined patient group has recently been described by the National Institutes of Health (NIH) Gastroparesis Clinical Research Consortium (GpCRC), and others, as gastroparesis-like syndrome, or GLS.6 The prospective NIH data set was designed to include approximately 25% of their patients as non-delayed emptiers.6 However, the true percentage of patients with GLS is unknown. Based on this sample of 551 consecutive patients, and 452 analyzable GETs, the percentage of patients with GLS is likely much closer to the 40% of patients in this series. Mechanisms for GLS may include rapid movement of food to the small bowel resulting in small bowel distention and accompanied nausea, pain, and diarrhea, ie, similar symptoms to that of gastroparesis.16,17 Other mechanisms may relate to proximal gastric activity, which might be better demonstrated by liquid gastric emptying, something not evaluated in this study. Indeed, patients with normal gastric emptying may be more similar to RGE than delayed gastroparesis as a mechanism for their symptomatology. After all, it remains unclear how tGES improves symptomatology in patients with normal gastric emptying as these patients demonstrated slowing of gastric emptying after implantation with tGES. Regarding how tGES slows gastric emptying, the exact mechanism remains to be unknown.
The differentiation between patients with symptoms of gastroparesis, whether they have delayed or accelerated gastric emptying, is not distinct. In a retrospective cohort of 642 patients with presumed gastroparesis and RGE, the correlation between symptoms and GET were only 62% and 29%, respectively.18 Some studies even report a preponderance of RGE over gastroparesis in non-specific symptoms. In a small 57 patient trial exploring gastric emptying in patients with functional dyspepsia, RGE was more common (21%) than delayed gastric emptying of solids (13%).19 Our study supports prior studies reporting the poor correlation between clinical symptomatology and the diagnosis of gastroparesis.17,18
This study did not look at response of tGES to pain, except as part of a larger symptoms spectrum. However, a recent report did examine pain, in relation to neuropathic changes in the GI tract, and found that some patients with abdominal pain do respond well to tGES.20 We also did not measure use of pro-kinetic or anti-emetic drug before and after tGES implantation, which may be a surrogate marker, along with TSS, to discern patient symptomatology.
There are 3 main limitations to our study. First, the gastric emptying studies performed previously in healthy volunteers as well our patients were limited to solid gastric emptying. Diagnostic criteria based on liquid or liquid-plus-solid gastric emptying may have influenced the proportion of patients classified as rapid. Secondly, the response to tGES in our patients was determined on the basis of an un-blinded study design. A sham controlled study may have yielded different results in any of the sub-groups (delayed, rapid, or normal) that were studied. A significant benefit our patients experienced may have been from a placebo effect. Our patients may have had a significant psychiatric history contributing to their perceived symptoms; however no psychiatric history was obtained nor were any psychotropic medicine screened. Lastly, this study looked at only temporary GES and not permanent. In prior trials temporary GES predicted improvement with permanent GES and tGES has been validated using a double- masked crossover study.10,21
Notwithstanding these limitations, our study demonstrated that temporary endoscopic GES is an effective therapy for gastroparesis and GLS. We also demonstrated a new possible entity of GLS categorized by RGE. Our research in a diverse patient population has shown that GES may be effective in normalizing gastric emptying, as well as significantly decreasing symptoms, for patients with gastroparesis and GLS who have failed traditional medical therapy. Larger controlled trials are now needed to test these findings.
Acknowledgments
The authors would like to thank An Li, Yana Nikitina, and Roland Maude-Griffin for their help in analyzing the healthy volunteers’ data; the staff of Gastroenterology Division and the Nuclear Medicine department at the University of Mississippi Medical Center; and Ed Miller and Catherine McBride with the University of Louisville for help with manuscript formatting and submission. We would also like to thank Gervais Tougas and colleagues11 for help with the 123 original volunteers for the gastric emptying study. This study was performed without any additional funding. Patients paid for testing as part of their care. Any costs for analysis and manuscript preparation were covered by internal research.
Appendix.
Re-analysis of the Tougas et al11 data was undertaken to examine the top 5th and 10th percentiles of gastric retention to define rapid emptying. The data involved 123 patients with the following characteristics: age 19–73 years, n = 60 for women, and n= 63 for men. The patients were healthy volunteers with no gastrointestinal symptomatology during the time of the gastric emptying procedure. For the normal control data, the original data from 123 healthy volunteers in the Tougas report was analyzed at the 5th and 10th percentiles using gastric emptying test (GET) at 1 and 2 hours, total gastric emptying (TGE), and total area under the curve (TAUC).
A re-analysis of the normal control data is shown in Appendix Table 1. GET values observed in the 10th percentile of normal volunteers at 1-, 2-, and 4-hour retention were values less than 37%, 6.4%, and 0% respectively. At the 5th percentile of normal volunteers, the 1, 2, and 4 hour values were 32%, 3.8%, and 0%. The 5th and 10th percentiles of TGE (summation of 1, 2, and 4 hours) were 38.2% and 47.3%. Additionally, the 5th and 10th percentile AUC was computed for 1, 2, and 4 hours. The 5th percentile and 10th percentile for AUC (%-hours) were 66.1 and 68.5 at 1 hour; 86.3 and 91.1 at 2 hours; and 90.8 and 102.7 at 4 hours, respectively. The parameter that included the most number of patients as rapid emptiers without classifying any delayed or normal patients as rapid was GET at 1 hour (<37% gastric retention).
Comparison of AUC, TGE, and GET at 1, 2, and 4 hours can be seen in Appendix Table 2. The modality that classified the most number of patients as rapid emptiers was 1 hr GET < 37% encompassing 42 patients. AUC < 68.5 also identified 42 patients (100%) as rapid emptiers whereas TGE identified 23 patients (55%) as rapid emptiers. GET < 6.4% at 2 hours identified 8 additional patients (19%) that were classified as normal by GET < 37% at 1 hour. None of the patients classified as rapid emptiers by 1 hour GET or 1 hour AUC were classified as Normal or Delayed by standard (Tougas et al11) criteria. We defined rapid gastric emptying as the modality that encompassed the largest number of non-delayed patients; thus, our principal criteria for rapid gastric emptying was 1 hour GET < 37%.
Column A lists the number of patients classified as rapid emptiers with each respective criteria. Column B lists the number of additional subjects classified as rapid emptiers that were not included by the 1 hour GET < 37%. GET < 6.4% at 2 hours classifies the most (8) additional patients as rapid emptiers.
Appendix Table 1.
Re-analysis of Normal Control Data Including Area Under the Curve, Total Gastric Emptying Test
| Time (hr) | No. | 5th percentile AUC (%-hr) | 10th percentile AUC (%-hr) | Gastric retention | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | 5th percentile (%) | 10th percentile (%) | ||||
| 1 | 123 | 66.1 | 68.5 | 64.9 | 32.0 | 37.0 |
| 2 | 123 | 86.3 | 91.1 | 26.9 | 3.8 | 6.4 |
| 4 | 123 | 90.8 | 102.7 | 2.9 | 0.0 | 0.0 |
| TGE | 38.2 | 47.3 | ||||
TGE, total gastric emptying.
Appendix Table 2.
Comparison of Gastric Emptying Test, Total Gastric Emptying, and Area Under the Curve for Rapid Gastric Emptying in Sample of 451 Patients
| Criteria | A | B |
|---|---|---|
| 1 hr GET < 32% (5th percentile) | 23 | 0 |
| 1 hr GET < 37% (10th percentile) | 42 | 0 |
| 2 hr GET < 3.8% (5th percentile) | 18 | 5 |
| 2 hr GET < 6.4% (10th percentile) | 24 | 8 |
| TGE, 4 hr, < 38.2% (5th percentile) | 16 | 0 |
| TGE, 4 hr, < 47.3% (10th percentile) | 25 | 2 |
| AUC, 1 hr, < 66.1 (5th percentile) | 25 | 0 |
| AUC, 1 hr, < 68.5 (10th percentile) | 42 | 0 |
| AUC, 2 hr, < 86.3 (5th percentile) | 20 | 0 |
| AUC, 2 hr, < 91.1 (10th percentile) | 33 | 1 |
| AUC, 4 hr, < 90.8 (5th percentile) | 15 | 0 |
| AUC, 4 hr, < 102.7 (10th percentile) | 30 | 6 |
GET, gastric emptying test; TGE, total gastric emptying; AUC, area under the curve.
Footnotes
Financial support: None.
Conflicts of interest: Thomas Abell is a consultant, licensor, and investigator with Medtronic. Certain aspects of the technology used are covered by intellectual property assigned from the University of Mississippi to ADEPT-GI.
Author contributions: Sanjeev Singh: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and statisical analysis; Jeff McCrary: drafting of the manuscript; Archana Kedar: analysis and interpretation of data, drafting of the manuscript, and statistical analysis; Stephen Weeks: administrative, technical, or material support; Brian Beauerle: critical revision of the manuscript; Andrew Weeks: administrative, technical, or material support; Chris Lahr: acquisition of data; Omer Endashaw: manuscript editing; Warren Starkebaum: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and statistical analysis; Thomas Abell: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and statistical analysis.
ORCID: Thomas Abell, http://orcid.org/0000-0002-3175-5161.
References
- 1.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/A:1026665728213. [DOI] [PubMed] [Google Scholar]
- 3.Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74–101. doi: 10.1016/j.disamonth.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Parkman HP, Schwartz SS. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch Intern Med. 1987;147:1477–1480. doi: 10.1001/archinte.1987.00370080113021. [DOI] [PubMed] [Google Scholar]
- 5.Brady PG, Richardson R. Gastric bezoar formation secondary to gastroparesis diabeticorum. Arch Intern Med. 1977;137:1729. doi: 10.1001/archinte.1977.03630240061020. [DOI] [PubMed] [Google Scholar]
- 6.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567–576. e1–e4. doi: 10.1016/j.cgh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–319. e1. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Daram SR, Tang SJ, Abell TL. Video: temporary gastric electrical stimulation for gastroparesis: endoscopic placement of electrodes (ENDOstim) Surg Endosc. 2011;25:3444–3445. doi: 10.1007/s00464-011-1710-5. [DOI] [PubMed] [Google Scholar]
- 9.Jayanthi NV, Dexter SP, Sarela AI, Leeds Gastroparesis Multi-Disciplinary Team Gastric electrical stimulation for treatment of clinically severe gastroparesis. J Minim Access Surg. 2013;9:163–167. doi: 10.4103/0972-9941.118833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496–503. e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 12.Abell T, Starkebaum W, Abidi N, et al. How common is rapid gastric emptying in gastroparesis. Gastroenterology. 2006;130(suppl 2):A244–A245. [Google Scholar]
- 13.Hou Q, Lin Z, Dusing R, Gajewski BJ, McCallum RW, Mayo MS. Optimizing the diagnostic power with gastric emptying scintigraphy at multiple time points. BMC Med Res Methodol. 2011;11:84. doi: 10.1186/1471-2288-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/S0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 15.Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972–1978. doi: 10.1136/gutjnl-2013-306084. [DOI] [PubMed] [Google Scholar]
- 16.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 17.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am J Gastroenterol. 2007;102:618–623. doi: 10.1111/j.1572-0241.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 18.Balan K, Sonoda LI, Seshadri N, Solanki C, Middleton S. Clinical significance of scintigraphic rapid gastric emptying. Nucl Med Commun. 2011;32:1185–1189. doi: 10.1097/MNM.0b013e32834bf262. [DOI] [PubMed] [Google Scholar]
- 19.Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457–464. [PMC free article] [PubMed] [Google Scholar]
- 21.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/S0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]