Abstract
Objective
This study was conducted to expand the sunitinib safety database in Japanese imatinib-resistant/-intolerant gastrointestinal stromal tumor patients. Retrospective analyses investigated common adverse events as potential prognostic markers.
Methods
Four hundred and seventy patients who received sunitinib between June 2008 and November 2009 were analyzed for safety, progression-free survival and overall survival; 386 for objective response rate; 88% received sunitinib on Schedule 4/2 starting at 50 mg/day.
Results
No unexpected safety issues occurred. Grade ≥ 3 adverse events occurred in 70%, most commonly thrombocytopenia (33%), neutropenia (22%) and leukopenia (15%). Objective response rate was 20% (95% confidence interval 16–24). Median progression-free survival was 22.4 weeks (95% confidence interval, 21.7–24.0). The overall survival rate at 24 weeks was 91% (95% confidence interval, 88–94). Higher relative dose intensity (≥70 vs. <70%) during the first 6 weeks and better Eastern Cooperative Oncology Group performance status (0 vs. ≥1) were associated with longer progression-free survival (24.0 vs. 20.1 weeks; P = 0.011; and 24.1 vs. 16.9 weeks; P < 0.001) and higher 24-week overall survival rate (94 vs. 83%; P < 0.001; and 96 vs. 83%; P < 0.001). Increased progression-free survival and overall survival rates were associated with specific adverse events. Cox proportional hazard modeling adjusted for relative dose intensity and performance status established hand–foot syndrome (hazard ratio = 0.636; 95% confidence interval, 0.456–0.888) and leukopenia (hazard ratio = 0.683; 95% confidence interval, 0.492–0.948) occurring within 12 weeks were significantly correlated with increased progression-free survival.
Conclusion
Sunitinib showed good efficacy and tolerable safety. Factors associated with greater efficacy were relative dose intensity, performance status and specific early adverse events.
Keywords: sunitinib, gastrointestinal stromal tumors, post-marketing, Japan, prognostic marker
Introduction
Sunitinib, an oral multi-targeted receptor tyrosine kinase (RTK) inhibitor, was approved in Japan in April 2008 for the treatment of imatinib-resistant/-intolerant gastrointestinal stromal tumor (GIST), unresectable or metastatic renal cell carcinoma (RCC) and, in 2012, for pancreatic neuroendocrine tumor (pNET). Approval in GIST was based primarily on a multicenter, international Phase III study in imatinib-resistant/-intolerant patients that demonstrated that sunitinib is an effective therapy for this patient population (1,2). Compared with placebo, sunitinib significantly improved both time to tumor progression (median: 26.6 vs. 6.4 weeks; P < 0.001) and objective response rate (ORR; 7 vs. 0%; P = 0.004), while being associated with a tolerable safety profile. Sunitinib has also demonstrated efficacy and a manageable safety profile in Japanese patients with GIST (3,4). In a Phase I/II study of 40 Japanese patients, pharmacokinetics and clinical outcomes with sunitinib were similar to those observed in non-Asian patients with GIST (4). Similarly, a small retrospective study of 18 Japanese patients showed that efficacy and safety data obtained with sunitinib in clinical practice were similar to those of the Phase III trial (3).
However, the number of Asian patients who have participated in clinical trials of sunitinib in GIST is relatively small. The final analysis of the pivotal Phase III study included a total of 243 patients treated with sunitinib (1); 97 patients were treated in a Phase I/II study (5); and a Phase II study treated 60 patients using a continuous daily dosing schedule (6). Details of the ethnicity of these 400 patients have not been reported. Sunitinib has also been evaluated for the treatment of patients with GIST in a worldwide treatment-use study in which 1124 patients received sunitinib: of these, only 201 were Asian (7). Given that there is some evidence from existing data in RCC that the safety profile of sunitinib may differ between Asians and non-Asians (8–11) and, as the safety and efficacy of sunitinib in daily clinical practice is largely unknown for Japanese patients with GIST, there is a need for further evaluation of sunitinib for the treatment of GIST in patients of Asian ethnicity.
The post-marketing study reported here was a regulatory requirement of the Pharmaceuticals and Medical Devices Agency at the time of Japanese approval of sunitinib, and was conducted to expand the safety database for the Japanese population and ensure appropriate use of sunitinib. The overall objectives of the study were to collect and report safety and efficacy data in Japanese patients with imatinib-resistant/-intolerant GIST treated with sunitinib in Japan. In addition, a retrospective exploratory analysis was performed to investigate whether the occurrence of common adverse events (AEs) correlated with efficacy in Japanese GIST patients treated with sunitinib, after adjusting for covariates possibly affecting the prognosis. In previous preliminary investigations, sunitinib was associated with significantly longer progression-free survival (PFS) in patients who developed several sunitinib-induced AEs, including neutropenia (12), hypertension (13), hand–foot syndrome (HFS) (14), asthenia and fatigue (15). Hence these sunitinib-induced AEs are potential factors associated with the efficacy of sunitinib in GIST patients. We report the results of the post-marketing study in Japanese patients with GIST, which includes data for safety, efficacy and potential prognostic markers of sunitinib efficacy.
Patients and methods
Study design and treatment
In this prospective, post-marketing study, all patients treated with sunitinib in Japan after 13 June 2008 (the release date for sunitinib in Japan) were registered in a central system until a pre-specified number of cases accumulated. It was recommended that all patients begin treatment with sunitinib at a starting dose of 50 mg once-daily orally on Schedule 4/2 (4 weeks on treatment followed by 2 weeks off) in repeating 6-week cycles, although lower starting doses were used in some patients according to physician's judgment. Registered patients were observed for 24 weeks from the start of treatment or until treatment was discontinued if sooner. Patients treated for 24 weeks were followed for up to 2 years, although only safety information was collected during follow-up. Physicians were required to complete an investigation form for each pre-specified portion of the 24-weeks observation period. Safety was assessed by monitoring AEs of Grade ≥3 in severity using Common Terminology Criteria for Adverse Events version 3.0, in addition to recording any serious AEs and unknown AEs (i.e. unexpected AEs or AEs that have previously been reported but that occurred with unexpected severity). Clinical efficacy was evaluated by investigator assessment using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0; central review of investigator assessments was not performed. The study conformed to Good Post-Marketing Study Practice guidelines.
Analytical and statistical methods
The incidences of treatment-related AEs (percentage of patients affected) were reported along with the median time to the onset of AEs (time from start of drug administration to the onset of an AE). Efficacy was mainly assessed by ORR at 24 weeks. Time-to-events of PFS and rates of PFS and overall survival (OS) at 24 weeks were secondarily evaluated under the limited observation period of 24 weeks. Median PFS was estimated using the Kaplan–Meier method. The log-rank test was used to compare PFS and OS in subpopulations based on selected factors, including relative dose intensity (RDI; ≥70 vs. <70%) during the first course (6 weeks) and Eastern Cooperative Oncology Group performance status (ECOG PS; 0 vs. ≥1). Although RDI was a post-treatment factor, RDI during the first 6 weeks was treated as a baseline factor due to its early measurement. The RDI was defined as the ratio of the actual total dosage administered in the 6 weeks to the planned dose [Schedule 4/2 (4 weeks on treatment followed by 2 weeks off treatment) at 50 mg/day; i.e. 50 mg × 28 days = 1400 mg; for the determination of RDI during the first 6 weeks of sunitinib therapy, sunitinib at 50 mg/day on Schedule 4/2 was considered the planned dosing schedule based on the regulatory-approved dose/schedule in GIST)] In order to further investigate the correlation between the early occurrence of common AEs (the 10 most common any-grade AEs) and the efficacy of sunitinib, hazard ratios (HRs) for PFS were estimated using a Cox proportional hazard model with covariates of first-course RDI and other factors. For the covariates, factors that had a highly significant effect on PFS in an initial model were selected from sex, age, ECOG PS, prior treatment for GIST metastases, chemotherapy other than imatinib, and comorbidities. The early occurrence of an AE was defined as its occurrence within the first 12 weeks (approximately two courses), because the first course might be too short to capture all of the early events.
Results
Baseline patient characteristics
Of 471 patients with imatinib-resistant/-intolerant GIST registered for treatment with sunitinib in Japan between June 2008 and November 2009, 470 were analyzed for safety and efficacy (referred to hereafter as the full analysis set). One patient was excluded from the full analysis set due to a protocol violation, because the patient was registered by a doctor whose participation was not approved by the site's Institutional Review Board. In addition to analysis using the full analysis set, ORR was evaluated in the evaluable analysis set of 386 patients in which 80 patients were excluded because of no determinable efficacy evaluation and 4 patients were excluded because of a non-GIST diagnosis [breast cancer (n = 1), soft part sarcoma (n = 1) and unavailable diagnosis (n = 2)].
Baseline demographics for the 470 patients included in the safety analysis are summarized in Table 1. The median age was 64 years, and there was a higher proportion of males (63%) than females (37%). Most patients (97%) had an ECOG PS of 0 or 1 and metastatic disease (94%). Approximately 85% of patients had undergone surgery for their primary lesion and 48% for a metastatic lesion. Overall, 14% of patients had received prior drug therapy (not including prior treatment with imatinib) and 3% prior radiotherapy. All patients had received prior imatinib (although 4 patients received the agent off-label); 458 patients who received imatinib on-label developed imatinib-resistant disease or became imatinib-intolerant.
Table 1.
Baseline patient characteristics
| Sunitinib (n = 470) | |
|---|---|
| Male/female, n (%) | 296/174 (63/37) |
| Median age (range), years | 64 (17–88) |
| Age < 55 years/≥55 years, n (%)a | 100/363 (21/77) |
| ECOG PS, n (%) | |
| 0 | 286 (61) |
| 1 | 169 (36) |
| ≥2 | 15 (3) |
| Metastatic disease, n (%) | 442 (94) |
| Disease duration (months), n (%) | |
| 0–12 | 45 (10) |
| 13–24 | 71 (15) |
| 25–48 | 113 (24) |
| 49–72 | 109 (23) |
| 73–96 | 53 (11) |
| ≥97 | 52 (11) |
| Prior treatment for GIST | |
| Surgery | |
| Primary tumor, n (%) | 398 (85) |
| Metastases, n (%) | 227 (48) |
| Radiation therapy, n (%) | 14 (3) |
| Imatinib, n (%)b | 466 (99) |
| Chemotherapy other than imatinib, n (%) | 64 (14) |
| Imatinib resistance or intolerance, n (%) | 458 (97) |
| Imatinib resistance, n (%) | 392 (83) |
| Imatinib intolerance, n (%) | 53 (11) |
| Imatinib resistance and intolerance | 13 (3) |
| RDI by observation period, %c | |
| Start to Week 6 (n = 448) | 75 |
| Comorbidities, n (%) | |
| Yes | 312 (66) |
| No | 158 (34) |
ECOG PS, Eastern Cooperative Oncology Group performance status; GIST, gastrointestinal stromal tumor; RDI, relative dose intensity.
Data are rounded to the nearest integer.
aData missing for seven patients (1%).
bImatinib use per label. Four patients received off-label imatinib.
cRDI was not calculated in 22 patients due to unknown dosage.
Treatment and disposition
The 470 patients in the safety analysis set received oral sunitinib administered on Schedule 4/2 at starting doses of 50 mg/day (n = 413; 88%), 37.5 mg/day (n = 41; 9%), 25 mg/day (n = 14; 3%) or other doses (n = 2; <1%; Supplementary Table S1), with 212 patients (53%) continuing treatment from the start of administration until the end of the 24-week observation period. A total of 262 patients required dose reductions and 283 patients discontinued treatment. Dose reduction was required in 246 of 413 patients (60%) who received a starting dose of 50 mg, 15 of 41 patients (37%) at 37.5 mg and in 1 of 14 patients (7%) at a starting dose of 25 mg (Supplementary Table S1).
Data during the 24-week observation period were analyzed for this study. The median follow-up duration (range) was 23.6 weeks (1.1–105.1). Data past 24 weeks were also reported in some patients (n = 146) and were therefore included in the results.
Safety analysis
The most common treatment-related AEs of any grade or of Grade ≥ 3 are summarized in Table 2. The most common AE was thrombocytopenia (reported in 66% of patients), followed by leukopenia (49%) and HFS (45%). Other common AEs included hypertension (35%), neutropenia (34%), anemia (29%), stomatitis (23%) and hypothyroidism (22%). Grade ≥ 3 AEs were reported in 329 patients (70%); the most commonly reported included thrombocytopenia (33%), neutropenia (22%), leukopenia (15%), anemia (12%), hypertension (11%) and HFS (9%). The median onset time of the common AEs were in the first 6 weeks of treatment except for hypothyroidism (median onset time: 52.0 days). The median onset time of neutropenia (29.0 days) and anemia (42.0 days) were relatively late following hypothyroidism. In contrast, pyrexia (13.0 days) and hypertension (14.0 days) occurred early. Notably, many Grade ≥3 AEs occurred within the first 6 weeks of treatment (Supplementary Fig. S1). Serious AEs were reported in 196 patients (42%), with cytopenias as the most frequently reported [decreased platelet count (19%), decreased white blood cell count (8%), neutrophil count decreased (7%); Supplementary Table S2].
Table 2.
Most commonly reported treatment-related AEs in 470 Japanese patients with gastrointestinal stromal tumor sorted by median time to onset
| AE | Median onset time (days) | Incidence, n (%) |
|
|---|---|---|---|
| All grades | Grade ≥3 | ||
| Any AE | 12.0 | 447 (95.11) | 329 (70.00) |
| Pyrexia | 13.0 | 70 (14.89) | 9 (1.91) |
| Hypertension | 14.0 | 163 (34.68) | 49 (10.43) |
| Nausea | 16.0 | 60 (12.77) | 7 (1.49) |
| Stomatitis | 17.0 | 108 (22.98) | 4 (0.85) |
| Diarrhea | 18.0 | 85 (18.09) | 3 (0.64) |
| Thrombocytopenia | 19.0 | 309 (65.74) | 153 (32.55) |
| Decreased appetite | 19.5 | 98 (20.85) | 19 (4.04) |
| Fatigue | 20.0 | 53 (11.28) | 6 (1.28) |
| Malaise | 20.5 | 52 (11.06) | 8 (1.70) |
| Hand–foot syndrome | 21.0 | 212 (45.11) | 40 (8.51) |
| Leukopenia | 25.0 | 229 (48.72) | 68 (14.47) |
| Neutropenia | 29.0 | 158 (33.62) | 101 (21.49) |
| Anemia | 42.0 | 137 (29.15) | 55 (11.70) |
| Hypothyroidism | 52.0 | 103 (21.91) | 6 (1.28) |
Events occurring in >10% of patients are listed.
Several demographic and baseline characteristics were found to be significantly associated with the incidence of AEs (Table 3). Female patients, patients ≥65 years, and those who had received prior radiotherapy were at an increased risk of developing Grade ≥3 AEs.
Table 3.
Demographic and baseline characteristics significantly associated with the incidence of Grade ≥3 AEs
| Variable (category) | AE incidence ratea by category | Adjusted HRb (95% CI) | P valueb |
|---|---|---|---|
| Sex (female vs. male) | 14.4 vs. 8.4 | 1.44 (1.05–1.99) | 0.026 |
| Age (≥65 vs. <65 years) | 12.6 vs. 8.2 | 1.49 (1.14–1.94) | 0.003 |
| Prior radiotherapy (yes vs. no) | 29.0 vs. 9.8 | 2.05 (1.04–4.07) | 0.039 |
| Renal impairment (yes vs. no) | 12.8 vs. 9.9 | 0.59 (0.36–0.94) | 0.025 |
AE, adverse event; CI, confidence interval; HR, hazard ratio.
aNumber of patients/1000 person-days.
bAdjusted HR and P values were obtained using a multivariate Cox proportional hazards model.
Efficacy analysis
In the evaluable analysis set of 386 patients, excluding 84 patients who had no determinable efficacy evaluation and/or non-GIST diagnosis, the ORR was 20% (95% confidence interval [CI], 16–24; of the 77 patients with responses, 2 had complete responses and 75 partial responses). A further 167 patients (43%) had stable disease, 109 (28%) progressive disease and 33 patients (9%) were not evaluable. An ORR of 17% (95% CI, 13–20) was reported for the full analysis set of 470 patients in which 81 patients with no determinable efficacy evaluation were treated as non-responders (of the 78 patients with responses, 2 had complete responses and 76 partial responses).
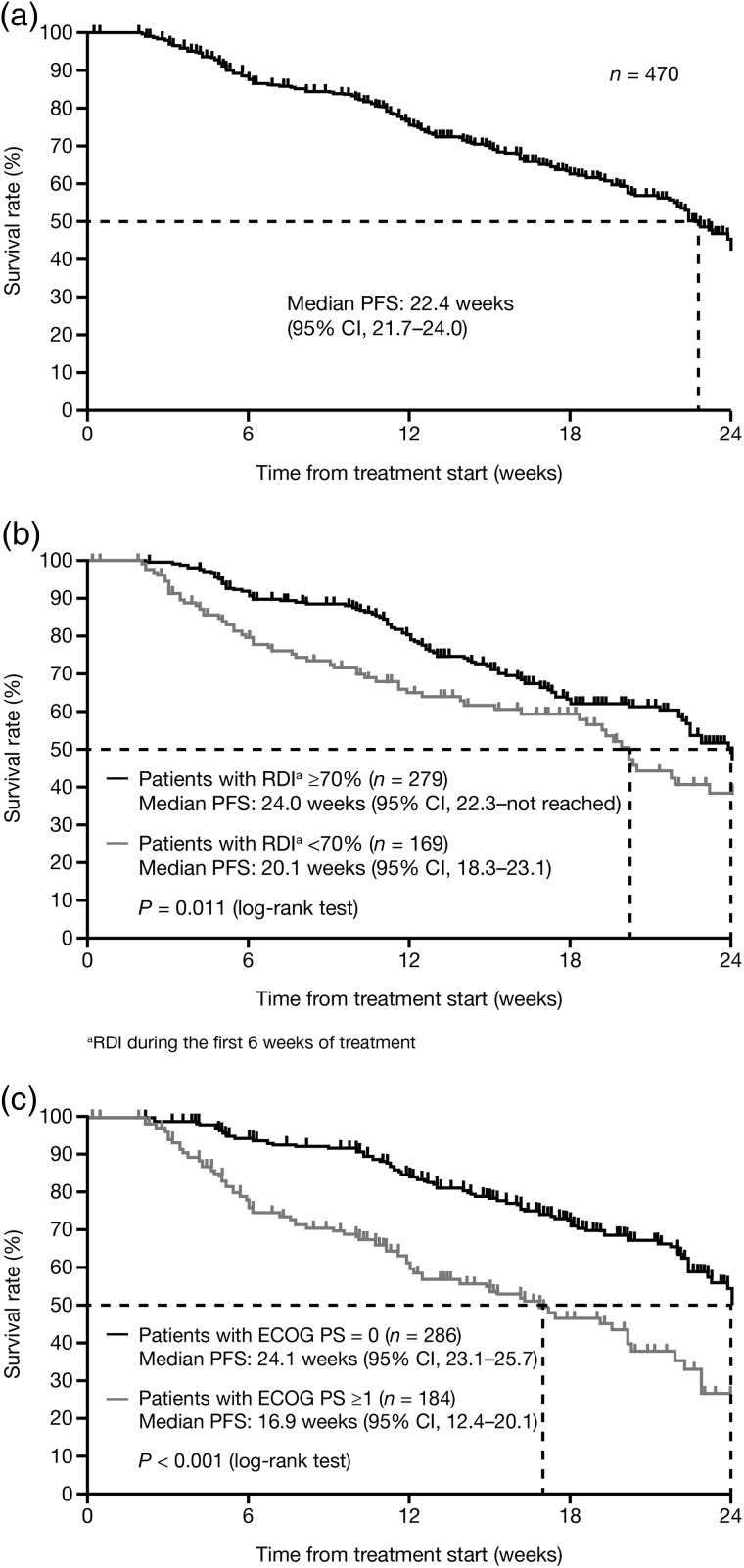
Median PFS in the full analysis set was 22.4 weeks (95% CI, 21.7−24.0; Fig. 1a). Subgroup analysis revealed that a higher RDI (≥70 vs. <70%) during the first 6 weeks was associated with a longer median PFS (24.0 vs. 20.1 weeks; P = 0.011; Fig. 1b) and an HR of 0.664 (95% CI, 0.453–0.970). In addition, a better baseline ECOG PS (0 vs. ≥1) was associated with a longer median PFS (24.1 vs. 16.9 weeks; P < 0.001; Fig. 1c) and an HR of 0.443 (95% CI, 0.322–0.609).
Figure 1.
Progression-free survival (a) in the overall population (full analysis set), (b) by relative dose intensity (RDI; ≥70 vs. <70% during the first 6 weeks of treatment) and (c) by Eastern Cooperative Oncology Group performance status (ECOG PS; 0 vs. ≥1).
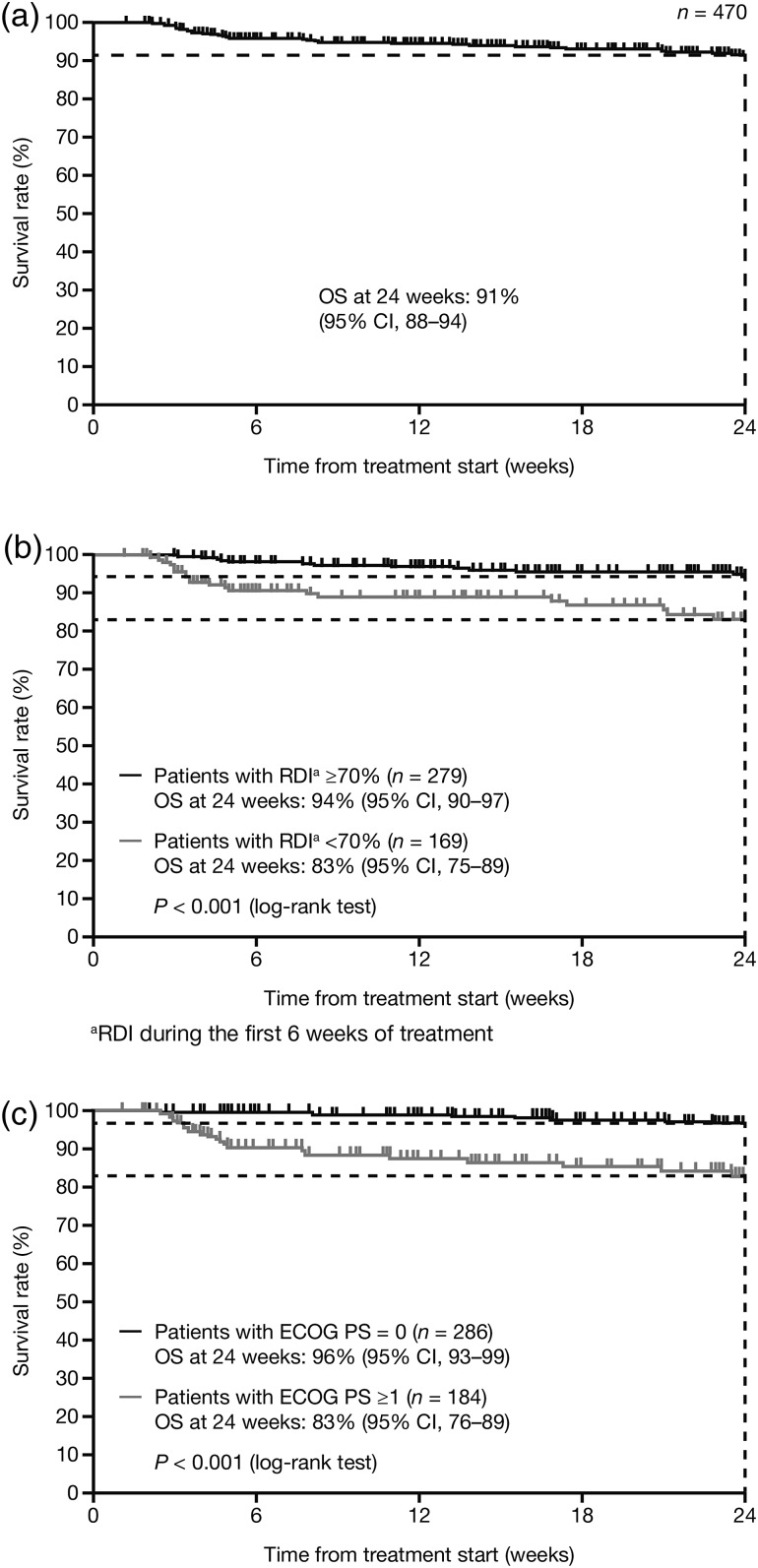
The OS rate at 24 weeks was 91% in the overall population (95% CI, 88−94; Fig. 2a). In subgroup analysis, a higher RDI (≥70 vs. <70%) during the first 6 weeks was associated with a higher OS rate (94 vs. 83%; P < 0.001; Fig. 2b). In addition, a better baseline ECOG PS (0 vs. ≥1) was associated with a higher OS rate (96 vs. 83%; P < 0.001; Fig. 2c).
Figure 2.
Overall survival (a) in the overall population (full analysis set), (b) by RDI (≥70 vs. <70% during the first 6 weeks of treatment) and (c) by ECOG PS (0 vs. ≥1).
Association of common AEs with antitumor efficacy of sunitinib
As AEs are considered to be correlated with RDI, next we examined whether the early occurrence of several AEs within the first 12 weeks of sunitinib therapy was predictive of significantly improved PFS even after adjustment for RDI. The first 12 weeks (approximately two courses) well covered the onset of the 10 most common any-grade AEs (median onset time, 14–52 days). HFS (HR = 0.595; 95% CI, 0.427–0.830), neutropenia (HR = 0.643; 95% CI, 0.448–0.922), leukopenia (HR = 0.657; 95% CI, 0.474–0.910), hypertension (HR = 0.663; 95% CI, 0.453–0.970) and thrombocytopenia (HR = 0.671; 95% CI, 0.483–0.931) were all significantly correlated with improved PFS (Table 4). Interestingly, further analysis of severe (Grade ≥ 3) compared with mild (Grade < 3) thrombocytopenia revealed that increasing severity of symptoms did not significantly predict improved clinical outcome over occurrence of any-grade thrombocytopenia (Grade < 3, adjusted HR = 1.00; Grade ≥3 adjusted HR = 0.845; 95% CI, 0.592–1.205; P = 0.351). Hypothyroidism and anemia, in which the onset time was relatively late, did not show a significant effect on PFS.
Table 4.
Effect of common early AEs (the 10 most common any-grade AEs) on progression-free survival (PFS), adjusted for RDI alone and for RDI plus ECOG PS, sorted by median onset time
| Factor | Median onset time (days) | Median PFS (weeks) |
Covariate: RDI alone |
Covariates: RDI plus ECOG PS |
|||||
|---|---|---|---|---|---|---|---|---|---|
| AE onset | AE not onset | HR | 95% CI | HR | 95% CI | ||||
| RDI | − | 24.0a | 20.1b | 0.664c | 0.478 | 0.920 | 0.723d | 0.520 | 1.005 |
| ECOG PS | − | 24.1e | 16.9f | − | − | − | 0.443d | 0.322 | 0.609 |
| Hypertension | 14.0 | 24.1 | 22.1 | 0.663g | 0.453 | 0.970 | 0.726 | 0.495 | 1.064 |
| Stomatitis | 17.0 | 24.1 | 22.3 | 0.677 | 0.449 | 1.019 | 0.739 | 0.49 | 1.114 |
| Diarrhea | 18.0 | 24.0 | 22.4 | 0.808 | 0.504 | 1.295 | 0.839 | 0.524 | 1.344 |
| Thrombocytopenia | 19.0 | 23.1 | 22.4 | 0.671g | 0.483 | 0.931 | 0.744 | 0.535 | 1.035 |
| Decreased appetite | 19.5 | 24.1 | 22.4 | 0.743 | 0.480 | 1.153 | 0.731 | 0.471 | 1.136 |
| Hand–foot syndrome | 21.0 | 23.3 | 22.3 | 0.595g | 0.427 | 0.830 | 0.636g | 0.456 | 0.888 |
| Leukopenia | 25.0 | 23.3 | 22.3 | 0.657g | 0.474 | 0.910 | 0.683g | 0.492 | 0.948 |
| Neutropenia | 29.0 | 24.1 | 22.4 | 0.643g | 0.448 | 0.922 | 0.744 | 0.517 | 1.071 |
| Anemia | 42.0 | 24.0 | 22.4 | 0.892 | 0.612 | 1.302 | 0.809 | 0.553 | 1.183 |
| Hypothyroidism | 52.0 | 24.0 | 22.4 | 0.729 | 0.434 | 1.225 | 0.768 | 0.457 | 1.291 |
aMedian PFS for the subgroup with RDI ≥ 70%.
bMedian PFS for the subgroup with RDI < 70%.
cBased on a model that includes RDI alone.
dBased on the model that includes RDI plus ECOG PS.
eMedian PFS for the subgroup with ECOG PS = 0.
fMedian PFS for the subgroup with ECOG PS ≥ 1.
gSignificant reduction in HR at α = 0.05 (with significant AEs by either model in bold font).
The HR for the covariate of baseline ECOG PS, in addition to RDI, was also estimated. Because ECOG PS showed a highly significant effect on PFS (P < 0.001; Supplementary Table S3), further evaluation was performed to determine whether the early occurrence of these AEs were still significant predictive markers for improved PFS, even after additional adjustment for ECOG PS. As shown in Table 4, occurrence of HFS and leukopenia still showed significant correration with improved PFS (HFS: HR = 0.636; 95% CI, 0.456–0.888; leukopenia: HR = 0.683; 95% CI, 0.492–0.948) even after adjusting for the effect of ECOG PS.
Discussion
Sunitinib was associated with good clinical efficacy in Japanese patients with imatinib-resistant/-intolerant GIST in this post-marketing study, as reported in previous clinical studies. This finding is consistent with data indicating that treatment effects reported in well-controlled, large observational studies may not be qualitatively different from those obtained in randomized controlled trials (16). No new AEs were reported, and the safety profile was acceptable and similar to that previously reported in Japanese patients (3,4). Many Grade ≥3 AEs occurred early (within the first 6 weeks of treatment) and were relatively infrequent afterwards, possibly as a result of dosing modification, although the number of patients remaining on study tended to fall after 6 weeks. The incidence of AEs was affected by several demographic and baseline characteristics, including gender, age, prior radiotherapy and renal impairment. A higher proportion of patients discontinued treatment due to AEs in this study (31%) compared with either the Phase III study (20%) (1) or the earlier Japanese studies (6 and 11%) (3,4). One of the reasons is that this surveillance study is comprised of patients given treatment within the first 16 months of sunitinib approval and, at the time, appropriate management of AEs was not sufficiently shared among doctors. As a result, there were many patients in whom individual dosage optimization was not fully established.
Many commonly reported AEs tended to be reported at a higher incidence in this study than in the international Phase III trial (1). In particular, hematologic AEs and HFS occurred more frequently and with greater severity than in the Phase III trial, consistent with the earlier Japanese studies (3,4). Comparable differences between the safety profiles of Asian and non-Asian patients have been reported in sunitinib trials in metastatic RCC (8–11).
Mechanisms underlying the likely difference in safety profiles between Asian and non-Asian patients have yet to be determined. One possible explanation could be differences in body weight, leading to higher drug exposure in Asian patients. However, total drug exposure was only found to be nominally higher in the earlier Phase I/II study in Japanese patients (15%) and, in that study, sunitinib pharmacokinetics were found to be comparable to that in non-Asian patients (4). Likewise, a population pharmacokinetic meta-analysis of 14 sunitinib trials involving healthy volunteers and patients with different tumor types found only a limited impact of Asian race, body weight or gender on sunitinib pharmacokinetic parameters (17). Differential drug metabolism in Asian patients could be another possible mechanism for the population-based safety-profile differences. Several reports have linked specific genetic polymorphisms with sunitinib toxicity in patients with RCC (18–20), although prevalence rates in specific geographic populations have not been reported. In conclusion, individual dosage optimization (for lasting long treatment) is recommended based on the latest evidence, as we do not have a solid reason for differentiation in treatment.
The ORR reported in the present study (20%) was higher than that previously reported, either in the international Phase III trial (7% ORR in the sunitinib arm) (1) or in Japanese studies (11 and 5.6% ORR) (3,4). There are several possible reasons for this. Methodological differences between the clinical trials and this post-marketing study, such as differences in inclusion criteria and differences in the observation period (i.e. 24-week maximum observation period in this post-marketing study compared with an unspecified observation period in clinical trials where the efficacy evaluation may therefore continue for >24 weeks), may underlie this disparity. Additionally, response was evaluated by independent radiologic review in the clinical trials, whereas it was assessed by investigators in the present study. The timing of the studies may have also played a role: the clinical studies were performed at a time when there was considerable unmet medical need among patients with metastatic GIST and, consequently, a higher proportion of patients with more advanced disease may have participated in the earlier trials. For example, 45% of patients in the Phase III study had a baseline ECOG PS of 0, whereas 61% did in the current study. Additionally, physicians were more likely to have become accustomed to prescribing sunitinib in the more recent trial, which took place after regulatory approval of the drug. On the other hand, median PFS in the present study (22.4 weeks), while similar to that of the Phase III study (22.9 weeks), was shorter than those of the earlier Japanese studies [28.3 weeks (4) and 29.6 weeks (time to treatment failure defined as the total time on treatment) (3)].
Good ECOG PS and higher RDI were associated with significantly improved PFS. The association of RDI and efficacy is consistent with the results of an earlier pharmacokinetic–pharmacodynamic meta-analysis of six sunitinib studies involving predominantly non-Asian patients with a variety of tumors, which indicated that increased sunitinib exposure was associated with improved clinical outcomes (21). We also conducted detailed retrospective exploratory analyses to investigate whether the early occurrence of common AEs within the first 12 weeks correlated with the efficacy of sunitinib in Japanese patients with GIST. After adjusting for the effect of RDI and ECOG PS, the early occurrence of two AEs (HFS and leukopenia) appeared to be associated with greater sunitinib efficacy. This correlation between the early occurrence of these AEs and sunitinib efficacy is similar to that seen in previous studies (12–15). However, these studies considered specific AEs and most of them had no adjustment for prognostic covariates; as such, no conclusion could be drawn as to which AE (if any) was the best predictor of clinical outcome. In this study, we have demonstrated that HFS and leukopenia were associated with improved PFS even after adjusting for the prognostic effects of RDI and ECOG PS. Given that specific AEs were associated with improved sunitinib efficacy among multiple AEs, there may be potential common pathways between sunitinib efficacy and these specific AEs.
Sunitinib is a multi-targeted RTK inhibitor of vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors (22), inhibition of which has been reported to play a vital role in the treatment of RCC. Hypertension and HFS, which are induced by VEGFR inhibition, for example, have been shown to be good predictors of efficacy in RCC. In this study and previous reports (13,14), it has been shown that sunitinib-induced AEs, such as HFS, had relatively strong associations with the efficacy of sunitinib in GIST (HR = 0.35–0.86). Considering these outcomes, not only the inhibition of c-KIT signaling pathways, but several other inhibitory pathways may also potentially restrain disease progression, which means multiple concurrent AEs are perhaps indicative of inhibition of multiple receptor types, including VEGF, and it is tempting to speculate that the consequent inhibition of multiple signaling pathways results in more effective targeting of GIST. With this surveillance, we revealed that the patients who experienced common AEs, such as HFS and leukopenia, within the first 12 weeks of sunitinib therapy without discontinuation were expected to achieve good outcomes in efficacy. As such, we believe these findings provide valuable insight into sunitinib efficacy in GIST (23). Also, AE management and establishment of individual dose optimization early during treatment with sunitinib would be very important to continue treatment.
In summary, sunitinib was associated with good clinical efficacy and tolerable safety in this post-marketing study of Japanese patients with imatinib-resistant/-intolerant GIST. The data presented here also confirm that the clinical outcome is positively correlated to RDI and ECOG PS, and support the hypothesis that some sunitinib-associated, high-incidence AEs may be viable early markers of sunitinib antitumor efficacy in GIST, although these findings require confirmation in prospective studies. Based on this analysis, careful monitoring and management of sunitinib-related AEs is necessary to maximize treatment outcomes.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Funding
This phase IV study was sponsored by Pfizer. The study is ongoing. Funding to pay the Open Access publication charges for this article was provided by Pfizer.
Conflict of interest statement
Y.K. has received speaker's fees from Pfizer, Novartis and Bayer, and a research grant from Novartis and Bayer. N.U., Y.T., K.T. and E.O. are Pfizer employees and own Pfizer stock. H.H. is a Pfizer employee. A.Y. is a former Pfizer employee. E.U. is a former Pfizer employee and owns Pfizer stock. T.N. has received speaker's fees from Pfizer, Novartis and Bayer, and a research grant from Novartis.
Supplementary Material
Acknowledgements
We would like to thank all of the participating patients and their families, as well as the investigators, research nurses, study coordinators and operations staff. We also thank Yinhua Li of Pfizer Japan (Tokyo, Japan) for her contributions to the pharmacological analyses. This phase IV study was sponsored by Pfizer. Medical writing support was provided by Rachel Mason and Wendy Sacks at ACUMED®, an Ashfield company, part of UDG Healthcare plc (Tytherington, UK, and New York, NY, USA), with funding from Pfizer.
References
- 1.Demetri GD, Garrett CR, Schöffski P et al. . Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 2012;18:3170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demetri GD, van Oosterom AT, Garrett CR et al. . Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–38. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Sawaki A, Mizuno N et al. . Clinical efficacy and safety of sunitinib after imatinib failure in Japanese patients with gastrointestinal stromal tumor. Jpn J Clin Oncol 2011;41:57–62. [DOI] [PubMed] [Google Scholar]
- 4.Shirao K, Nishida T, Doi T et al. . Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs 2010;28:866–75. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, Heinrich MC, Fletcher JA et al. . Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res 2009;15:5902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George S, Blay JY, Casali PG et al. . Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959–68. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt P, Kang YK, Rutkowski P et al. . Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer 2015;121:1405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrios CH, Hernandez-Barajas D, Brown MP et al. . Clinical outcomes of Asian vs. non-Asian patients with metastatic renal cell carcinoma with continuous once-daily dosing of sunitinib as first-line therapy. Ann Oncol 2010;21(Suppl. 8):viii286 (abstract 913P). [Google Scholar]
- 9.Lee S, Mainwaring P, Ng C et al. . An Asian subpopulation analysis of the safety and efficacy of sunitinib in metastatic renal cell carcinoma. Poster presented at the joint 15th Congress of the European Cancer Organisation and 34th Congress of the European Society for Medical Oncology, Berlin, Germany, September 20–24, 2009 (abstract 7118). [Google Scholar]
- 10.Tomita Y, Shinohara N, Yuasa T et al. . Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010;40:1166–72. [DOI] [PubMed] [Google Scholar]
- 11.Uemura H, Shinohara N, Yuasa T et al. . A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 2010;40:194–202. [DOI] [PubMed] [Google Scholar]
- 12.Donskov F, von Mehren M, Caru A et al. . Neutropenia as a biomarker of sunitinib efficacy in patients (pts) with gastrointestinal stromal tumour (GIST). Eur J Cancer 2011;47(Suppl. 1):S135 (abstract 1138). [Google Scholar]
- 13.George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol 2012;23:3180–7. [DOI] [PubMed] [Google Scholar]
- 14.Puzanov I, Michaelson MD, Cohen DP et al. . Evaluation of hand-foot syndrome (HFS) as a potential biomarker of sunitinib (SU) efficacy in patients (pts) with metastatic renal cell carcinoma (mRCC) and gastrointestinal stromal tumour (GIST). J Clin Oncol 2011;47(Suppl.) (abstract e21113). http://meetinglibrary.asco.org/content/83198-102. [Google Scholar]
- 15.Davis MP, Goldstein D, George S et al. . Asthenia and fatigue as predictors of sunitinib efficacy in gastrointestinal stromal tumour (GIST). Eur J Cancer 2011;47(Suppl. 1):S135 (abstract 1140). [Google Scholar]
- 16.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878–86. [DOI] [PubMed] [Google Scholar]
- 17.Houk BE, Bello CL, Kang D, Amantea M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009;15:2497–506. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Donas J, Esteban E, Leandro-García LJ et al. . Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 2011;12:1143–50. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Vaziri SA, Rini BI et al. . Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer 2012;118:1946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno T, Fukudo M, Terada T et al. . Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet 2012;27:631–9. [DOI] [PubMed] [Google Scholar]
- 21.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357–71. [DOI] [PubMed] [Google Scholar]
- 22.Chow QM, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007;25:884–96. [DOI] [PubMed] [Google Scholar]
- 23.Michel MC. Post-marketing studies can make important contributions to medical knowledge. BMJ 2012;345:e4740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.