Abstract
Diffuse pulmonary lymphangiomatosis (DPL) is a rare interstitial lung disease characterized by intrathoracic lymphatic system abnormalities often with involvement of both lungs. Here, we report a 24-year-old male patient with DPL initially located in one lung, presenting only with transient fever. Resection of the right middle and lower lobes was performed for diagnosis and complete removal of the lesions. The pathologic features shown by diffuse smooth thickening of the interlobular septa, bronchovascular bundles, infiltration of patchy ground glass opacities and specific immunohistologic D2-40 and CD34 positive staining confirmed the diagnosis of DPL. The patient did not show signs of relapse during the 2-year follow-up period, which suggests that surgery is an effective and reasonable method for treating DPL with relatively localized lesions.
INTRODUCTION
Diffuse pulmonary lymphangiomatosis (DPL) is an extremely rare disease and is characterized by diffuse proliferation of abnormal pulmonary lymphatic channels. It can occur in adults of different ages without gender predilection [1, 2]. Onset of the disease is insidious with unknown pathogenesis. Most patients present with non-specific symptoms such as cough, shortness of breath and hemoptysis with or without chylous pleuro-pericardial effusions, and pneumothorax. It is a slow progressive lung disease that causes respiratory failure. It is also often difficult to determine the correct diagnosis based on clinical features and radiologic manifestations. Pathologic examination is the diagnostic gold standard and is characterized by diffuse proliferation of anastomosing lymphatic spaces in the pulmonary lymphatic system involving the pleura, interlobular septa and peribronchovascular bundles. Bilateral pulmonary involvement has been noted in all previously published reports [3]. Here, we present an adult patient with DPL lesions located in a unilateral pulmonary area, diagnosed and treated by video-assisted thoracoscopy.
CASE REPORT
A 24-year-old man with abnormal chest radiographs was referred to our hospital. He developed fever 8 months previously, attended a local hospital and underwent chest radiographic and chest computed tomography (CT) examinations for suspected interstitial lung disease. He denied having cough, wheezing, chest pain and hemoptysis. His fever was relieved following an antibiotic infusion (amoxicillin/clavulanate potassium plus levofloxacin) for 2 weeks. The abnormality was unchanged after antibiotic therapy, and the patient was then transferred to our hospital. Although he was asymptomatic, the abnormal imaging findings persisted on follow-up CT scan at 6 months. He had a 5 pack/year smoking history and no risk of occupational or environmental exposure to inhaled toxins or materials. His family history and past medical history were unremarkable. The findings on physical examination including pulmonary function tests were normal. Laboratory examination revealed a hemoglobin level of 11.2 g/dL, a white blood cell (WBC) count of 7120/μL (79.1% neutrophils, 14% lymphocytes, 2.0% eosinophils, 4.9% monocytes and 0.0% basophils) and a platelet count of 181 000/μL. Blood chemistry findings and erythrocyte sedimentation rate were normal. Chest CT showed high-density infiltration in the right middle and lower lobes and diffuse interlobular septal thickening (Fig. 1a) without pleural effusion or hilar/mediastinal lymph node enlargement. Our primary radiologic impression was that the patient had lymphangitic carcinomatosis or lymphoma. Extra-thoracic involvement was not observed. The patient underwent bronchoscopy, and no endobronchial lesions were found. Bronchoalveolar lavage results were negative for malignant cells. The patient subsequently underwent resection of the right middle and lower lobes by video-assisted thoracoscopic surgery. In the surgical field, an abnormal honeycomb appearance was noted on the lung surface. Microscopic examination showed that proliferative lymphatic channels were diffusely infiltrative along the interlobular septum without cytological atypia (Fig. 1b) and were highlighted by immunopositivity for D2–40 (1:50; Dako, Glostrup, Denmark) and CD34 (1:500; Dako) (Fig. 1c). The final diagnosis of DPL was confirmed on the basis of these findings. The patient is currently alive and well without symptoms 2 years later and is under observation without specific treatment such as low-fat medium-chain triglyceride diets.
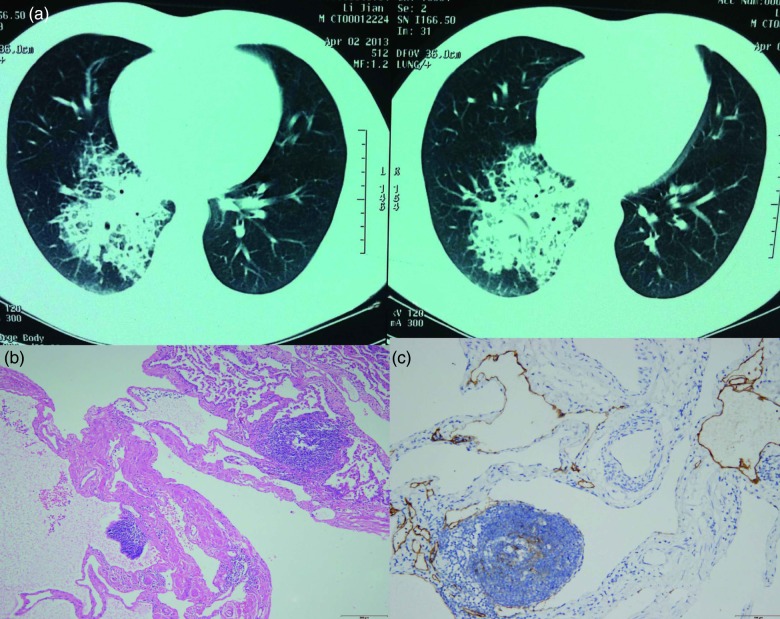
Figure 1:
(a) High-resolution CT scan demonstrating high-density opacity and interlobular septal thickening especially in the right lower lobe of the lung. (b) Microscopic examination showing diffuse pulmonary lymphangiomatosis located in the right middle and lower lobe. Hematoxylin and eosin-stained section of the lesion showing that proliferative lymphatic channels were diffusely infiltrative along the intralobular septum without cytological atypia (×200). (c) Immunohistochemical staining with D2-40 revealing proliferative lymphatic channels (×200).
DISCUSSION
Lymphangiomatosis is a rare disease characterized by diffuse infiltration of lymphangiomas in the lung, bone, kidney and other organs. Within the thorax, lymphangiomas may be found in the mediastinum, heart, thoracic duct, lung and pleura. DPL commonly presents with chylous effusions and other non-specific respiratory symptoms such as cough, chest pain, chest tightness, shortness of breath, dyspnea and wheezing. The patient in this report lacked the above symptoms and presented with only transient fever. Fever is seldom reported in DPL patients and is possibly due to the concurrent infection. This was supported by episodes of fever, increased WBC count and slightly decreased density of opacity after the antibiotic infusion in this case. To the best of our knowledge, all reported patients have had involvement of the pulmonary lymphatic vessels bilaterally or mediastinal structures including lymph nodes, thoracic duct and occasionally the pericardium [4]. There are rare reports of diffuse lymphangiomatosis involving one organ such as the kidney [5] and breast [6]. The unique features in the present case were that the lesion invaded two different lobes but was located in one area, and mediastinal involvement was not found. Hamartomata may result from the proliferation of abnormal smooth muscle-like spindle cells and have been postulated as being involved in the pathogenesis of DPL, which may be an explanation for limitation of the disorder seen in this case, similar to hamartomata that usually present with a localized lesion.
The common characteristics of DPL on high-resolution CT include the following: (i) bilateral smooth thickening of the interlobular septa and bronchovascular bundles; (ii) patchy ground glass opacities; (iii) diffuse liquid-like infiltration in the mediastinal and hilar soft tissue, resulting from diffuse proliferation of lymphatic channels and accumulation of lymphatic fluid; and (iv) bilateral pleura effusion [7]. Our case showed two of these characteristic changes. A large lesion such as in this case is rare compared with previously reported patients.
DPL has a poor prognosis and is characterized by slow progressive growth of the lymphatics. Current therapies are essentially palliative and used to alleviate clinical symptoms. We found no signs of relapse in our patient 2 years after surgery, which suggests that surgical resection is a reasonable treatment option for localized DPL lesions. The curative effect of surgery needs to be evaluated continuously in this case for at least 5 years. Successful surgical cases have been reported [8]. Other treatments for DPL include albumin transfusion, low-fat medium-chain triglyceride diets, interferon-alpha, radiation, corticosteroids, tamoxifen, medroxyprogesterone acetate, vincristine and thoracic duct ligation for chylothorax. More recently, treatment with propranolol, sirolimus and bevacizumab and lung transplantation have been shown to have an effect in the treatment of DPL [9, 10]. Although disease progression revealed on serial follow-up CT required differentiation from lymphangitic relapse and metastasis, this patient remained asymptomatic and did not require further treatment.
CONSENT
Written informed consent was obtained from the patient.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Satria MN, Pacheco-Rodriguez G, Moss J. Pulmonary lymphangiomatosis. Lymphat Res Biol 2011;9:191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadakia KC, Patel SM, Yi ES, Limper AH. Diffuse pulmonary lymphangiomatosis. Can Respir J 2013;20:52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim HJ, Han J, Kim HK, Kim TS. A rare case of diffuse pulmonary lymphangiomatosis in a middle-aged woman. Korean J Radiol 2014;15:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero Y, Pérez D, Cano JR. Diffuse pulmonary lymphangiomatosis with mediastinal affectation. Arch Bronconeumol 2011;47:474–475. [DOI] [PubMed] [Google Scholar]
- 5.Jeon TG, Kong do H, Park HJ, Kim S, Park WY, Kim SD et al. . Perirenal lymphangiomatosis. World J Mens Health 2014; 32:116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes SO, McLaughlin R, Kerin M, Rowaiye B, Connolly CE. A unique cause of a rare disorder, unilateral macromastia due to lymphangiomatosis of the breast: a case report. Breast J 2012;18:367–370. [DOI] [PubMed] [Google Scholar]
- 7.DU MH, Ye RJ, Sun KK, Li JF, Shen DH, Wang J et al. . Diffuse pulmonary lymphangiomatosis: a case report with literature review. Chin Med J (Engl) 2011;124:797–800. [PubMed] [Google Scholar]
- 8.Takemura T, Watanabe M, Takagi K, Tanaka S, Aida S. Thoracoscopic resection of a solitary pulmonary lymphangioma: report of a case. Surg Today 1995;25:651–653. [DOI] [PubMed] [Google Scholar]
- 9.Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med 2011;364:1380–1382. [DOI] [PubMed] [Google Scholar]
- 10.Kinnier CV, Eu JP, Davis RD, Howell DN, Sheets J, Palmer SM. Successful bilateral lung transplantation for lymphangiomatosis. Am J Transplant 2008;8:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]