Abstract
Background
Tumor protein (P53) and heat shock protein 70 (HSP70) play key roles in chronic liver diseases. This study aimed to characterize P53 and HSP70 expression in chronic hepatitis (CH), liver cirrhosis (LC), early and advanced HCC, and to analyze their diagnostic value in hepatocellular carcinoma (HCC).
Material/Methods
Immunohistochemical staining was conducted to evaluate the expression of P53 and HSP70 in 200 human liver tissue specimens, with advanced HCC (n=80), early HCC (n=30), CH (n=30), LC (n=30), and Controls (n=30).
Results
P53 expression levels were lower in LC than those of HCC, but remained on par with those of CH and Controls. HSP70 expression levels were higher in HCC than those of LC, CH, and Controls. The sensitivity and specificity for HCC diagnosis were: 50.9% and 98.9% for P53, and 78.2 and 77.8% for HSP70, respectively. The sensitivity and specificity of different combinations were: 95.5% and 85.5% with either P53 or HSP70 being positive, and 33.6% and 98.9% if both were positive. Among the differentiation stages marked low, intermediate, and high in HCC, the P53 positive rate was higher in the low than in the intermediate, which was higher than that in the high. HSP70 positive rate was higher in the low and the intermediate than in the high, but no obvious changes were found between the low and the intermediate.
Conclusions
P53 and HSP70 could be potential biomarkers for HCC diagnosis, and proper combinations of these 2 markers could improve diagnostic accuracy.
MeSH Keywords: Carcinoma, Hepatocellular; Hepatitis, Chronic; HSP70 Heat-Shock Proteins; Liver Cirrhosis; Tumor Suppressor Protein p53
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world [1]. Multiple etiologies are related to HCC occurrence and development, including chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol consumption, and cirrhosis [2,3]. It has been reported that half of HCC in china occurs because of the high prevalence of HBV infection [4]. Persistent HBV-derived inflammations contribute to hepatic fibrosis/cirrhosis, which results in increased risks of HCC. The high morbidity and mortality of HCC are mainly attributable to late diagnosis; early detection of HCC is therefore the only way to reduce the effect of HCC [5]. The criterion standard for HCC diagnosis has been liver biopsy or surgical specimens. Currently, the conventional pathological diagnosis is confined to the morphological changes and limited histochemical stains. More specific and sensitive immunochemical biomarkers are thus urgently needed to bring about more alternatives [6].
Persistent chronic liver diseases evolve into liver cirrhosis and eventually HCC via a sustained and long process called the ‘trilogy’ of HCC development. Patients with chronic liver diseases, especially liver fibrosis or cirrhosis, are at high risk of HCC; therefore, it is very important to closely monitor the progression from liver diseases to malignant neoplasm [7].
HCC features a series of molecular changes, such as cyclase-associated protein 2 (CAP2), glypican-3 (GPC3) and IGF-1R, as well as heat-shock protein (HSP) family and P53. HSP70 and P53 are frequently reported molecules that are over-expressed in HCC biopsy, especially in advanced HCC [5]. Both of them are believed to be putative biomarkers of HCC, but the relationship between HSP70 and P53 expression in the process of chronic hepatitis (CH), LC, and early and advanced HCC has not been reported [8,9].
HSP70, a stress-induced gene in the tumor genesis that modulates cell apoptosis and proliferation, demonstrates an anti-apoptosis effect, which ensures the survival of cells and promotes tumor cell proliferation [10]. It has been reported that both the mRNA and protein levels of HSP70 increase markedly more in advanced HCC than in early HCC [11]. Serum HSP70 is up-regulated in both liver cirrhotic and HCC patients [10]. Moreover, HSP70 is seen in both overt and early HCC and is less likely to be positive in the regenerative nodule in hepatic cirrhotic liver, as determined by immunochemistry staining [12].
P53 is the most studied tumor suppressor in a handful of cancers, including HCC [13]. Most human cancers are related to the abnormal expression and inactivation of P53 [14]. As a tumor-suppressor protein, P53 executes the function of DNA reparation, pro-apoptosis, and cell cycle arrest in tumor cells [15,16]. Mutation of P53 frequently occurs in HCC. Farazi et al. showed that reduced levels of P53 play an important role in the progression of chronic hepatic diseases to HCC by cooperating with telomere dysfunction [14]. Over-expression of P53 in liver, including both wild-type and its mutations, shows an increased predictability in HCC [16]. Due to the role of P53 in progression of CH to HCC, the expression level of P53 in the process of chronic liver diseases can be of help for monitoring and identification of HCC at an early stage [17,18].
Herein, we simultaneously detected the expression of HSP70 and P53 in CH, LC, and HCC in both early and advance stages. Furthermore, we analyzed the relationship between both predictors and their clinicopathologic features in advanced HCC.
Material and Methods
Patients and tissure samples
Patients pathologically diagnosed with advanced HCC (n=80), early HCC (n=30), LC (n=30), and CH (n=30), and patients with only liver hemangioma (Controls, n=30, distal tissues), in accordance with WHO criteria, were obtained from the First Affiliated Hospital of Xinjiang Medical University between 2009 and 2014. Clinical characteristics were collected by reviewing medical records and pathologic dates. All patients enrolled in the study signed consent forms and the study was approved by the Medical Ethics Committee of Xinjiang Medical University (No. 20140929-02). All resected surgical specimens were immediately fixed in formalin (10%) and embedded in paraffin; 4-μm-thick sections were cut serially and stained for P53 and HSP70.
Immunohistochemistry
Tissue sections were processed with xylene (10 min, 3 times) and graded alcohol for deparaffinization and hydration, after which sections were heated in 0.01 M citrate buffer solution (PH9.0) in microwave for 20 min for antigen retrieval. The sections were naturally cooled to room temperature (RT) before being treated with 3% H2O2 in methanol 15 min to block endogenous peroxidase activities. Sections were incubated overnight at 4°C with the primary antibodies against P53 (1: 100, mouse monoclonal antibody, Fuzhou Maixin Biotech, CN) and HSP70 (1: 200, mouse monoclonal antibody, SANTA CRUZ, USA), then they went through incubation 1 more time for 30 min with the secondary antibodies (ZSGB, China) at 37°C and stained with DAB solution. After counterstaining with hematoxylin, the sections were passed through graded ethanol and sealed.
The results of immunohistochemical processing for P53 (nucleic staining) and HSP70 (nucleocytoplasmic staining) were analyzed in blind fashion by 2 independent, experienced pathologists. Ten areas were selected randomly and the rating was done according to the methods reported by previous studies [19–21]. Staining density was graded: 1 (≤25% staining), 2 (26–75% staining), and 3 (≥76% staining). Staining intensity was evaluated: 1 for no positive staining, 2 for mild staining, and 3 for intense staining. The 2 grades of each specimen were then multiplied and categorized into negative (−) for scores <3 and positive (+) for scores ≥3.
Statistical analysis
Statistical analysis was performed with 1-way ANOVA using SPSS version 17.0 (SPSS, Chicago, USA). For the comparisons of the data of 5 groups and data between clinicopathologic parameters and factors, the significance of differences was evaluated by Pearson’s χ² test or Fisher’s exact test. Differences were considered to be statistically significant when P value <0.05 and all P values are 2-tailed in all analyses.
Results
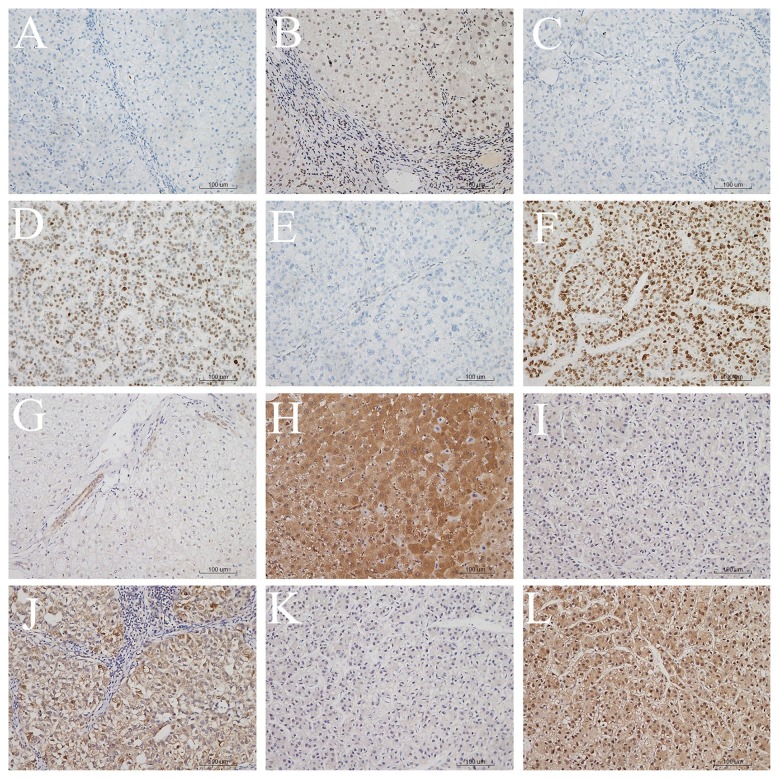
Immunoreactivity of P53 and HSP70 of the Controls, CH, LC, and early and advanced HCC was evaluated by immunohistochemical analysis. The expression of P53 appeared in the form of a nucleonic staining pattern, while the expression of HSP70 was localized in the nucleus and/or cytoplasm (Figure 1). Clinicopathologic characteristics of the groups are listed in Table 1.
Figure 1.
Immunohistochemical staining of P53 or HSP70 in LC, early HCC or advanced HCC (×200). (A) P53-negative staining in LC tissue, (B) P53-positive staining in LC tissue, (C) P53-negative staining in early HCC tissue, (D) P53-positive staining in early HCC tissue, (E) P53-negative staining in advanced HCC tissue, (F) HSP70-positive staining in advanced HCC tissue, (G) HSP70-negative staining in LC tissue, (H) HSP70-positive staining in LC tissue, (I) HSP70-negative staining in early HCC tissue, (J) HSP70-positive staining in early HCC tissue, (K) HSP70-negative staining in advanced HCC tissue, (L) HSP70-positive staining in advanced HCC tissue.
Table 1.
The clinicopathologic characteristics of the Control, CH, LC, Early HCC and Advanced HCC.
| Characteristics | Control | CH | LC | Early HCC | Advanced HCC |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 11 (36.67%) | 18 (60.00%) | 23 (76.67%) | 21 (70.00%) | 63 (78.75%) |
| Female | 19 (63.33%) | 12 (40.00%) | 7 (23.33%) | 9 (30.00%) | 17 (21.25%) |
| Age | |||||
| <55 | 22 (73.33%) | 26 (86.67%) | 17 (56.67%) | 14 (46.67%) | 46 (57.50%) |
| ≥55 | 8 (26.67%) | 4 (13.33%) | 13 (43.33%) | 16 (53.33%) | 34 (42.50%) |
| Tumor size | |||||
| <3 cm | – | – | – | 30 (100.00%) | – |
| ≤3 and <6 cm | – | – | – | – | 47 (58.75%) |
| ≥6 cm | – | – | – | – | 33 (41.25%) |
| Cancer embolus | – | – | – | 6 (20.00%) | 28 (35.00%) |
| Differentiation | |||||
| High | – | – | – | 12 (40.00%) | 21 (26.25%) |
| Middle | – | – | – | 11 (36.67%) | 28 (35.00%) |
| Low | – | – | – | 7 (23.33%) | 31 (38.75%) |
| HBs Ag | – | 24 (80.00%) | 16 (53.33%) | 22 (73.33%) | 63 (78.75%) |
| HCV Ab | – | 2 (6.67%) | 3 (10.00%) | 4 (13.33%) | 5 (6.25%) |
| Alcoholism | – | 4 (13.33%) | 11 (36.67%) | 3 (10.00%) | 8 (10.00%) |
| Cryptogenic | – | 0 (0%) | 0 (0%) | 1 (3.33%) | 4 (5.00%) |
Control – patients with only liver hemangioma; CH – chronic hepatitis; LC – liver cirrhosis; HCC – hepatocellular carcinoma; HBs Ag – anti-hepatitis B surface antigen; HCV Ab – anti-hepatitis antibody.
As shown in Table 2, the positive expression rate of P53 in advanced HCC tissues was 60.0% (48/80), significantly higher than that in early HCC tissues (26.7%, 8/30, P=0.003), LC tissues (3.3%, 1/30, P<0.001), CH tissues, and tissues of Controls (0.0%, 0/30, P<0.001, both). The positive expression rate in early HCC tissues was 26.7% (8/30), significantly higher than that in LC tissues (3.3%, 1/30, P=0.026), CH tissues, and tissues of Controls (0.0%, 0/30, P=0.007, both).
Table 2.
Immunohistochemical for expressions of P53 and HSP70 in Control, CH, LC, early HCC and advanced HCC tissues.
| Tissues | P53 | HSP70 | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Control | 0 | 30 | 0 | 30 |
| CH | 0 | 30 | 7 | 23 |
| LC | 1 | 29 | 13 | 17 |
| Early HCC | 8 | 22 | 25 | 5 |
| Advanced HCC | 48 | 32 | 61 | 19 |
Control – patients with only liver hemangioma; CH – chronic hepatitis; LC – liver cirrhosis; HCC – hepatocellular carcinoma.
The positive expression rate of HSP70 in advanced HCC (76.3%, 61/80) was significantly higher than that in LC tissues (43.3%, 13/30, P=0.003), CH tissues (23.3%, 7/30, P<0.001) and tissues of Controls (0.0%, 0/30, P<0.001). Meanwhile, the positive rate in early HCC tissues (83.3%, 25/30) was also significantly higher than that in LC tissues (43.3%, 13/30, P<0.001), CH tissues (23.3%, 7/30, P<0.001) and tissues of Controls (0.0%, 0/30, P<0.001). However, there was no significant statistical difference in the positive expression rate of HSP70 in early HCC and advanced HCC (P>0.5). Furthermore, the expression patterns of P53 in HCC tissues were significantly correlated with that of HSP70(r=0.684; P<0.001).
The Diagnostic accuracy and the combinations of the 2 positive markers are summarized in Table 3. The sensitivity, the specificity, and the positive and negative predictive values for parallel combination were 95.5%, 85.5%, 89.0%, and 93.9%, respectively, with at least 2 markers being positive, and the sensitivity and negative predictive values of the parallel were comparatively higher. The 4 indicators for serial combination were 33.6%, 98.9%, 97.4%, and 54.9% respectively, with both markers being positive, and the specificity and positive predictive values of the serial combination were relatively higher. These results may indicate that proper combinations of the 2 predictors would help us reach a more reliable and accurate diagnosis of HCC, including early and advanced HCC, compared with use of only 1 marker.
Table 3.
Diagnostic accuracy for detection of hepatocellular carcinoma using one or two markers.
| Markers | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR− |
|---|---|---|---|---|---|---|
| One marker | ||||||
| P53 | 50.9 | 98.9 | 98.2 | 62.2 | 46.3 | 0.50 |
| HSP70 | 78.2 | 77.8 | 81.1 | 74.5 | 3.53 | 0.28 |
| Two markers | ||||||
| Parallel | 95.5 | 85.5 | 89.0 | 93.9 | 6.6 | 0.05 |
| Serial | 33.6 | 98.9 | 97.4 | 54.9 | 30.27 | 0.67 |
PPV – positive predictive value; NPV – negative predictive value; LR – likelihood ratio. Parallel – at least 1 positive. Serial – both 2 positive.
The correlations between clinicopathologic characteristics and expression of P53 and HSP70 were assessed in the patients with advanced HCC, with 5 clinicopathologic parameters – tumor thrombus, tumor size, differentiation, age, and sex – as revealed in the present study (Table 4). As listed in Table 4, Pearson’s χ² test or Fisher’s exact showed that the differences in differentiation for P53 and HSP70 were statistically significant (P<0.001, both). The positive expression rates of P53 and HSP70 were both higher in patients with lower differentiation. In advanced HCC tissues, the positive expression rate of P53 in low-differentiation tissues was 90.03% (28/31), significantly higher than in moderately differentiated tissues (53.6%, 15/28, P=0.002) and highly differentiated tissues (23.8%, 5/21, P<0.001), and the positive rate of P53 in moderately differentiated tissues was also higher than in highly differentiated tissues (P=0.036). In addition, the positive rate of HSP70 in advanced HCC tissues of low differentiation (96.8%, 30/31) was higher than in intermediate differentiation tissues (78.6%, 22/28, P>0.05), but the differences were not statistically significant. The rate of low differentiation was significantly higher than in high differentiation tissues (42.9%, 9/21, P<0.001). Similarly, the rate of intermediate differentiation was higher than that of high differentiation (P=0.01). There was no significant statistical association between the 2 factors in terms of tumor thrombus, tumor size, age, or sex, suggesting that these 4 variables do not affect the expression of P53 and HSP70 in advanced HCC.
Table 4.
The expression of P53 and HSP70 in human advanced hepatocellular carcinoma tissues with different clinicopathologic features.
| Features | N.O patients | P53 | HSP70 | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Gender | |||||
| Male | 63 | 40 | 23 | 50 | 13 |
| Female | 17 | 8 | 9 | 11 | 6 |
| Age | |||||
| <55 | 45 | 29 | 16 | 34 | 11 |
| ≥55 | 35 | 19 | 16 | 27 | 8 |
| Tumor size | |||||
| <6 cm | 47 | 28 | 19 | 38 | 9 |
| ≥6 cm | 33 | 20 | 13 | 23 | 10 |
| Tumor embolus | |||||
| Presence | 18 | 13 | 5 | 14 | 4 |
| Absence | 62 | 35 | 27 | 47 | 15 |
| Differentiation* | |||||
| High | 21 | 5 | 16 | 9 | 12 |
| Intermediate | 28 | 15 | 13 | 22 | 6 |
| Low | 31 | 28 | 3 | 30 | 1 |
Statistically significant differences among the high, moderate and low differentiation in expression of P53 (P<0.001) and HSP70 (P<0.001).
Discussion
HCC is a major cause of death in chronic liver diseases, especially in patients with liver cirrhosis. Preventing chronic hepatitis and/or liver cirrhosis from progressing to HCC is still an important challenge. Early diagnosis is an effective way to reduce the incidence of HCC. As HCC is closely related to liver cirrhosis, the monitoring of chronic liver diseases is of considerable importance. Morphological distinction of HCC among advanced chronic liver diseases and early neoplastic lesion still poses a number of problems. Therefore, more support is needed in the conventional pathological detection of HCC from chronic liver diseases, especially advanced hepatic cirrhosis.
The study used immunocytochemistry to measure the expression pattern of HSP70 and P53 in different stages of chronic hepatic diseases and their correlation with clinicopathological parameters. Findings derived are: (1) P53 obviously increased its presence in HCC more than in chronic hepatitis and cirrhosis, which can be used to detect early-stage HCC; (2) HSP70 could clearly distinguish HCC from chronic hepatitis/cirrhosis, but failed to distinguish between early and advanced HCC; (3) P53 and HSP70 are correlated with malignancy and are highly expressed in poorly differentiated tumors.
Furthermore, our study found that the parallel combination of P53 and HSP70 could increase the sensitivity at the expense of specificity loss, which was the opposite of the series combination. Therefore, proper combination of P53 and HSP70, in parallel or in series, could increase the specificity and sensitivity at the same time, which would help reach a more reliable and accurate diagnosis of HCC, including early and advanced HCC, rather than use of only 1 marker.
P53 is related to more than half of human cancers [22]. Mutation and sequential inactivation of P53 are characteristics of HCC [23]. Our study found the expression levels of P53 were consistent with previous reports, and specific expressions of P53 in tumors demonstrated no expression in chronic hepatitis, 3.3% (1/30) being positive in liver cirrhosis; and 26.7% and 60.0% in early and advanced HCC, respectively. There was a significant difference between early and advanced HCC, indicating that P53 is sensitive enough to distinguish early HCC from the other stages. Our data showed that P53 had no obvious relationship with sex, age, tumor size, or cancer embolus of patients with advanced HCC. Moreover, P53 was correlated with the differentiation levels of HCC, with well-differentiated HCC showing a lower P53 level.
HSP70, together with the other 2 biomarkers – GPC3 and glutamine synthetase (GS) – has demonstrated utility in detecting early and grade 1 HCC in the dysplastic nodules of cirrhosis [24,25]. Other investigations also showed highly expressed levels of HSP70 in HCC [12]. The positive rate of HSP70 in this study was found to gradually increase in the progression of chronic liver diseases: 23.3% (7/30) in hepatitis, 43.3% (13/30) in liver cirrhosis, and 83.3% (25/30) in early-stage HCC. There was no significant change of HSP70 between hepatitis and cirrhosis (P>0.05). The early and advanced HCC (76.3%, 61/80) both possessed a higher level of HSP70 (P>0.05). Similar to P53, there was no obvious relationship between HSP70 and patient sex, age, tumor size, and cancer embolus. However, it displayed a significant correlation with the differentiation, indicating HSP70 as a potential prognostic indicator for malignancy. We found HSP70 was more positively expressed in lower-differentiated tumors.
HCC diagnosis at an early stage is crucially important. A series of tumor biomarkers have recently emerged as important diagnostic indicators for HCC. Our results showed that P53 was highly expressed in neoplastic tissues and was almost only expressed in HCC, while HSP70 could be detected in both non-neoplastic (CH and LC) and neoplastic liver tissues. Kamal et al. reported that by combining 3 different biomarkers (CGP3, HSP70, and GS), the diagnostic accuracy could be increased [9]. Furthermore, our study found that the parallel combination of P53 and HSP70 could increase the sensitivity at the expense of specificity loss, which was the opposite of the series combination. Therefore, proper combination of P53 and HSP70, in parallel or in series, could increase the specificity and sensitivity at the same time, which would help reach a more reliable and accurate diagnosis of HCC, including early and advanced HCC, rather than use of only 1 marker.
The current study has a few limitations. Firstly, we only enrolled the patients in Xinjiang area of China in 1 center, which limits applicability of the conclusion that combination of P53 and HSP70 could improve the diagnosis of HCC. Secondly, the sample size is small, and for clinical application of P53 and HSP70 to diagnose HCC, a cohort with more cases from multiple centers should be included. Secondly, we only used immunohistochemistry staining in this study, but ELISA and Western blot should be used to detect the protein levels. More patients will be enrolled in our next study, and we will also follow up these patients to analyze the relationship between survival time and these 2 bio-markers.
Conclusions
In conclusion, this study shows that P53 could be used to distinguish early HCC from advanced HCC, but HSP70 cannot. The proper combination of these 2 well-known markers of HCC is a more sensitive and accurate alternative to monitor the progression of chronic liver diseases and diagnosis HCC at an early stage, through dynamic observation of P53 and HSP70 in different stages of chronic liver diseases.
Footnotes
Source of support: This study is supported by the grant of the Special Foundation for Young Scientists of Bureau of Health, Xinjiang Uygur Autonomous Region
References
- 1.Li H, Wang S, Wang G, et al. Yes-associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Digestive Surgery. 2014;31:468–78. doi: 10.1159/000370252. [DOI] [PubMed] [Google Scholar]
- 2.Ji X, Zhang Q, Du Y, et al. Somatic mutations, viral integration and epigenetic modification in the evolution of hepatitis B virus-induced hepatocellular carcinoma. Current Genomics. 2014;15:469–80. doi: 10.2174/1389202915666141114213833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahnassy AA, Zekri A-RN, El-Bastawisy A, et al. Circulating tumor and cancer stem cells in hepatitis C virus-associated liver disease. World J Gastroenterol. 2014;20:18240. doi: 10.3748/wjg.v20.i48.18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 5.Tawada A, Kanda T, Yokosuka O. Current and future directions for treating hepatitis B virus infection. World J Hepatol. 2015;7:1541–52. doi: 10.4254/wjh.v7.i11.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelfattah MR, Abaalkhail F, Al-Manea H. Misdiagnosed or incidentally detected hepatocellular carcinoma in explanted livers: lessons learned. Ann Transplant. 2015;20:366–72. doi: 10.12659/AOT.893782. [DOI] [PubMed] [Google Scholar]
- 7.Lun-Gen L. Antiviral therapy of liver cirrhosis related to hepatitis B virus infection. J Clin Transl Hepatol. 2014;2:197–201. doi: 10.14218/JCTH.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang GH, Lee BS, Lee ES, et al. Prognostic significance of p53, mTOR, c-Met, IGF-1R, and HSP70 overexpression after the resection of hepatocellular carcinoma. Gut Liver. 2014;8:79–87. doi: 10.5009/gnl.2014.8.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremosini S, Forner A, Boix L, et al. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut. 2012;61:1481–87. doi: 10.1136/gutjnl-2011-301862. [DOI] [PubMed] [Google Scholar]
- 10.Gehrmann M, Cervello M, Montalto G, et al. Heat shock protein 70 serum levels differ significantly in patients with chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Front Immunol. 2014;5:307. doi: 10.3389/fimmu.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin E, Ryu HS, Kim SH, et al. The clinicopathological significance of heat shock protein 70 and glutamine synthetase expression in hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18(4):544–50. doi: 10.1007/s00534-010-0367-0. [DOI] [PubMed] [Google Scholar]
- 12.Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci. 2005;20:829–34. doi: 10.3346/jkms.2005.20.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Din HG, Ghafar NA, Saad NE, et al. Relationship between codon 249 mutation in exon 7 of p53 gene and diagnosis of hepatocellular carcinoma. Arch Med Sci. 2010;6:348–55. doi: 10.5114/aoms.2010.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J-C, Qu K, Wang Z-X, et al. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin. 2015;36(2):241–51. doi: 10.1038/aps.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi X-L, Yang J, Mao N, et al. Nutlin-3-induced redistribution of chromatin-bound IFI16 in human hepatocellular carcinoma cells in vitro is associated with p53 activation. Acta Pharmacol Sin. 2015;36(2):252–58. doi: 10.1038/aps.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ei-Emshaty H, Saad E, Toson E, et al. Apoptosis and cell proliferation: correlation with BCL-2 and P53 oncoprotein expression in human hepatocellular carcinoma. Hepatogastroenterology. 2013;61:1393–401. [PubMed] [Google Scholar]
- 17.Farazi PA, Glickman J, Horner J, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66:4766–73. doi: 10.1158/0008-5472.CAN-05-4608. [DOI] [PubMed] [Google Scholar]
- 18.Guan Y-S, La Z, Yang L, et al. p53 gene in treatment of hepatic carcinoma: Status quo. World J Gastroenterol. 2007;13:985–92. doi: 10.3748/wjg.v13.i7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou C-X, Gao Y. Aberrant expression of β-catenin, Pin1 and cylin D1 in salivary adenoid cystic carcinoma: relation to tumor proliferation and metastasis. Oncol Rep. 2006;16:505–11. [PubMed] [Google Scholar]
- 20.Ma Y, Ma L, Guo Q, Zhang S. Research expression of bone morphogenetic protein-2 and its receptors in epithelial ovarian cancer and their influence on the prognosis of ovarian cancer patients. J Exp Clin Cancer Res. 2010;29:85. doi: 10.1186/1756-9966-29-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bani-Hani K, Martin IG, Hardie LJ, et al. Prospective study of cyclin D1 overexpression in Barrett’s esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92(16):1316–21. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 22.Marcel V, Catez F, Diaz J. p53, a translational regulator: contribution to its tumour-suppressor activity. Oncogene. 2015 doi: 10.1038/onc.2015.25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Meng X, Franklin DA, Dong J, Zhang Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014;74:7161–67. doi: 10.1158/0008-5472.CAN-14-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagana SM, Salomao M, Bao F, et al. Utility of an immunohistochemical panel consisting of glypican-3, heat-shock protein-70, and glutamine synthetase in the distinction of low-grade hepatocellular carcinoma from hepatocellular adenoma. Appl Immunohistochem Mol Morphol. 2013;21:170–76. doi: 10.1097/PAI.0b013e31825d527f. [DOI] [PubMed] [Google Scholar]
- 25.Reis H, Pütter C, Megger DA, et al. A structured proteomic approach identifies 14-3-3Sigma as a novel and reliable protein biomarker in panel based differential diagnostics of liver tumors. Biochim Biophys Acta. 2015;1854(6):641–50. doi: 10.1016/j.bbapap.2014.10.024. [DOI] [PubMed] [Google Scholar]