Abstract
Objectives:
Clinical evidence regarding radiological–endoscopic management of intrahepatic bile duct stones is currently lacking. Our aim is to report our 18-year experience in combined radiological–endoscopic management of intrahepatic difficult bile duct stones.
Methods:
From June 1994 to June 2012, 299 symptomatic patients with difficult bile duct stones were admitted to our institution. Percutaneous transhepatic cholangiography (PTC)/biliary drainage/s was performed, dilating the PTC track to 10 or 16 French within 3–7 days. Afterward we carried out percutaneous transhepatic cholangioscopy (PTCS) with electrohydraulic lithotripsy (EHL) and/or interventional radiology techniques. Follow up was made with clinical/laboratory tests and ultrasound (US). We retrospectively analyzed our radiological–endoscopic approach and reported our technical and clinical outcomes.
Results:
Complete stone clearance was achieved in 298 patients after a maximum of 4 consecutive sessions. Most patients (64.6%) were treated with PTCS/EHL alone, while the remaining with radiological techniques alone (26%) or a combination of both techniques (13.3%). Recurrence of stones occurred in 45 cases (15%, Tsunoda class III and class IV) within 2 years and were successfully retreated. Major adverse events were: 5 (1.6%) cases of massive bleeding that required embolisation, 2 (0.66%) perforations of the common bile duct and 31 cases (10.3%) of acute cholangitis managed with medical therapy or intervention.
Conclusion:
After 18 years of experience we demonstrated that our combined radiological–endoscopic approach to ‘difficult bile duct stones‘ may result in both immediate and long-term clearance of stones with a low rate of adverse events.
Keywords: bile ducts, biliary stones, cholangiography, endoscopy, lithotripsy
Introduction
Hepatolithiasis is more commonly found in East Asia (annual incidence 20–30%) than Western countries where it is most commonly related to an underlying disease or previous surgical intervention. It is noteworthy that hepatolithiasis may lead to recurrent cholangitis, biliary cirrhosis and cholangiocarcinoma [Yasuda and Itoi, 2013].
Over the past years, per-oral endoscopic procedures have replaced open surgery for treatment of hepatolithiasis. At present, endoscopic retrograde cholangiopancreatography (ERCP) associated with endoscopic sphincterotomy (EST) represents the benchmark treatment for the removal of biliary stones. However, some stones may be refractory to endoscopic removal under certain circumstances, needing additional or other therapeutic modalities.
The term ‘difficult bile duct stones’ was defined as a condition where due to several factors, complete stone clearance by means of ERCP fails. Possible factors leading to difficult bile duct stone are: periampullary diverticula, previous surgical operation, large size stones, presence of multiple stones, stones above a stricture, intrahepatic stones, impacted stones and clinical status of the patient [Yasuda and Itoi, 2013].
Several techniques have been described to cope with difficult bile duct stones such as per-oral cholangioscopy (POCS) with laser or electrohydraulic lithotripsy (EHL), extracorporeal shockwave lithotripsy, endoscopic papillary large balloon dilatation, enteroscopy, percutaneous transhepatic cholangioscopy (PTCS), endoscopic ultrasound guided antegrade technique and stenting [Yasuda and Itoi, 2013; Erim et al. 2013; Buxbaum, 2013; Kim et al. 2013; Trikudanathan et al. 2013]. Currently there are a few reports on PTCS with laser or EHL and they mainly describe treatment of common bile duct (CBD) stones. [Yasuda and Itoi, 2013; Buxbaum, 2013]. Due to instrument’s technical improvement, small calibre operative cholangioscopes (CSs) are now available either for endoscopic procedures or for percutaneous approach [Erim et al. 2013].
Our aim was to retrospectively analyze our 18-year experience in the management and treatment of difficult intrahepatic bile duct stones with PTCS/EHL and interventional radiology (IR) techniques.
Materials and methods
Between June 1994 and June 2012, 299 symptomatic patients with intrahepatic bile duct stones were managed by the Surgical Endoscopic Service and the Interventional Radiology Service. All patients were referred from the Emergency Department. Clinical presentation of all patients is reported in Table 1. This observational retrospective study was approved by our Institutional Review Board.
Table 1.
Clinical features of all patients at baseline.
| Clinical features | Value |
|---|---|
| Male:Female | 198:101 |
| Age, year mean (range) | 62.5 (40–85) |
| Mean follow up, months (range) | 66 (12–120) |
| Mean total bilirubin value at diagnosis (mean ± SD) | 16 ± 5.5 |
| Recurrent episodes of acute cholangitis, n (%) | 245 (81.9) |
| Recent right flank pain alone, n (%) | 54 (18.1) |
| Intrahepatic stone classification (Tsunoda) n (%): | |
| Class I | 66 (22.1) |
| Class II | 92 (30.7) |
| Class III | 91 (30.4) |
| Class IV | 50 (16.7) |
| Previous cholecystectomy, n (%) | 91 (31.4) |
| Previous liver transplant, n (%) | 42 (14.4) |
| Previous pancreatic or gastrointestinal surgical resection, n (%) | 86 (29.6) |
| Liver resection, n (%) | 71 (24.6) |
| Presence of strictures, n (%) | 141 (47.1) |
| Benign, n (%) | 76 (54) |
| Malignant, n (%) | 65 (46) |
| Associated disease at diagnosis: | |
| Biliary cirrhosis, n (%) | 30 (10) |
| Cholangiocarcinoma, n (%) | 29 (9.7) |
| Liver primary/secondary neoplasms, n (%) | 35 (11.7) |
SD, standard deviation.
All patients initially underwent an ultrasound (US) exam and then magnetic resonance cholangiopancreatography (MRCP) and/or computed tomography (CT) (according to the availability) to confirm the diagnosis, to locate the biliary stones and to study further anatomical abnormalities due to previous abdominal surgical operation.
CT and/or magnetic resonance (MR) findings were classified according to the Tsunoda classification [Tsunoda et al. 1985] (stone distribution – unilateral or bilateral – and the presence of associated intrahepatic duct stricture):
I) No marked dilatation or strictures of intrahepatic ducts;
II) Diffuse dilatation of intrahepatic ducts without strictures;
III) Unilateral solitary or multiple cystic dilatation of intrahepatic ducts with strictures;
IV) Bilateral.
According to the noninvasive imaging findings, ERCP was performed as first-line treatment by the same team of expert endoscopists (F.F., G.D. and F.C.) throughout the 18 years of the study for stones smaller than 20 mm and mainly located in the CBD. In these cases, percutaneous transhepatic cholangiography (PTC) was performed, only after ERCP failure. Alternatively, in the presence of larger stones (>20 mm), intrahepatic or impacted stones or associated with narrowed CBD, a direct percutaneous approach was performed. In case of surgical placement of T-tube it was used to perform PTCS with the smaller 2.7 mm CS. In particular we exchanged the T-tube with a 11 French (Fr) peelaway sheath, performed the procedure with the 2.7 mm CS and then left in place a 10 Fr biliary drainage for further control as per protocol.
PTC with biliary drainage was carried out to obtain an access for further manipulations and to perform a cholagiogram for a preliminary diagnosis. The PTC technique varied according to the Tsunoda class. In Tsunoda class I and II, US guided single puncture was made selecting the appropriate bile duct and then a coaxial system was used (Accustick, Boston Scientific, MA, USA); in extensive intrahepatic lithiasis (Tsunoda class III and IV), a double puncture technique was performed due to the many artefacts on US examination from the biliary stones. Treatment of intrahepatic stones was performed after 2–4 days if the previous procedure was uneventful.
In addition, a different approach was selected according to the Tsunoda class and stone size. In less complex cases (Tsunoda class I–II, stone size <25 mm), an initial approach was attempted with IR techniques alone. The radiological technique consisted of pushing the stones down into the duodenum with drainage catheters and with a saline flush using an introducer sheath (10 Fr); such technique was applied only if stones were <25 mm in size. Bile duct strictures were dilated with noncompliant balloons 4–10 mm in size (Mustang, Boston Scientific, MA, USA). When radiological techniques failed to obtain a complete clearance, PTCS was subsequently performed. If patients presented with complex and extensive stone disease (Tsunoda class III–IV or stone size >25 mm), PTCS was planned as the first-line approach.
All patients during the PTCS procedure underwent general anesthesia or deep sedation.
Stenting was used for strictures located in the CBD after clearing all stones. Permanent uncovered or covered metallic stent (Zilver™, Cook Medical, IN, USA; Viabil®, W.L. Gore & Associates, Flagstaff, AZ, USA) were used for malignant disease; removable covered stents or plastic stents were inserted in benign disease.
During the first 10 years of experience (1994–2004), two fiberoptic CS were used: the first introduced in our practice was 4.9 mm in size (15 Fr) with a 2.2 mm working channel (Olympus model CHF-P20, Olympus Optical Co. GmbH, Hamburg, Germany) (Figure 1), while the second scope was a 3.9 mm ureteroscope (12 Fr, Olympus, model URF-P), with a 1.2 mm (4 Fr) instrument channel. Over the last 8 years (2005–2012), we used a new 2.7 mm CS (Olympus model CHF-CB30S) (Figure 2). They were inserted via the orifice of the dilated fistula using an introducer sheath to approach the stones or the lesion.
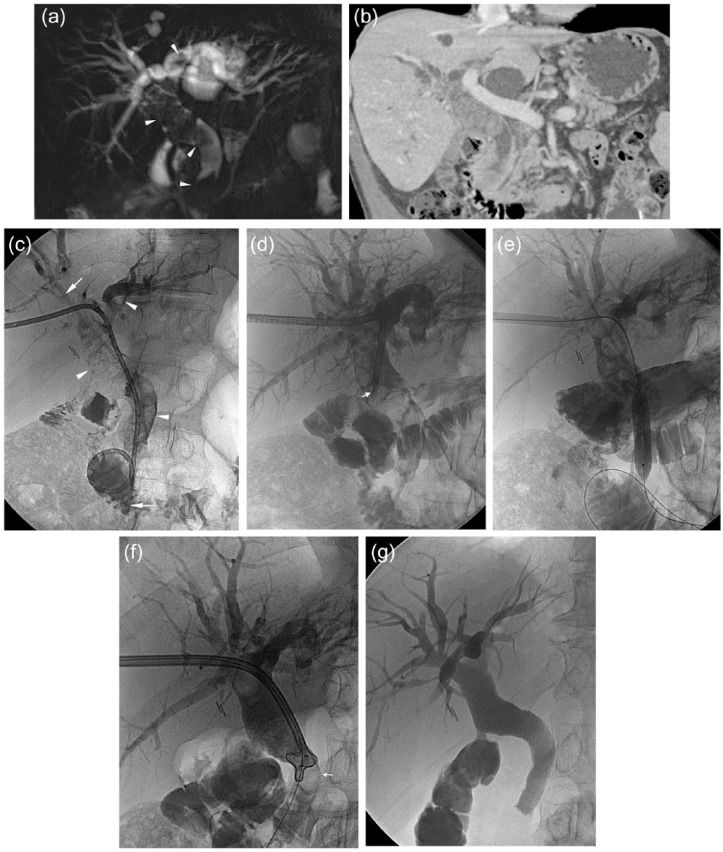
Figure 1.
A 73 year-old man with previous history of recurrent biliary lithiasis and pancreatitis underwent cholecystectomy and gastric-jejunal anastomosis with side-to-side choledochojejunostomy. He presented to our institution with recurrent cholangitis and ultrasound diagnosis of biliary stones in the common bile duct (CBD) (‘sump syndrome’). (a) Magnetic resonance (MR) cholangiography shows dilated CBD and intrahepatic ducts with the presence of multiple stones (arrowheads). (b) Computed tomography (CT) confirmed the MR findings: note the better visualization of the lateral choledochojejunal anastomosis (black arrow). (c) Using a right transhepatic approach (through a bile duct of the V segment) a 10 Fr biliary drainage was inserted. Cholangiography showed multiple bile stones (arrowheads) and the presence of a plastic prosthesis within the CBD (arrows). (d) After 3 days the first cholangioscopy was performed, introducing the large cholangioscope into a 16 Fr introducer sheath, by using electrohydraulic lithotripsy probes (arrowhead). Also the ampulla was dilated with a 9 mm balloon (e) and stones were pushed down with a basket-type catheter (arrow, f) after removing the plastic stent. (g) After three more sessions, final cholangiography showed optimal result: no stenosis and no stones were present in the biliary system.
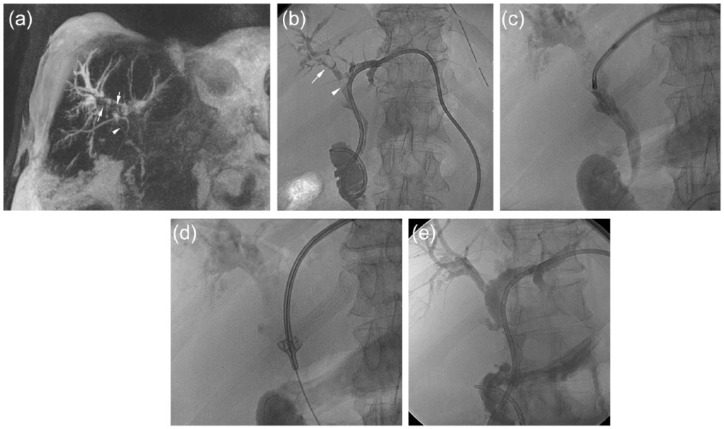
Figure 2.
A 64 year-old man with positive history for primitive sclerosant cholangitis, portal thrombosis and cholecystectomy presented with ascites, recurrent cholangitis and intrahepatic lithiasis. (a) Magnetic resonance (MR) T2w IR in coronal view shows bilateral stenosis of the bile ducts with presence of biliary stones in the right bile duct (arrows) and in the CBD (arrowhead) (Tsunoda class IV). Moreover abundant ascites is present around the liver. (b) A left transhepatic approach through the bile duct of the III segment was performed and a biliary drainage was placed. Cholangiography confirmed the MR findings: intrahepatic lithiasis (arrow) and lithiasis in the CBD (arrowhead). (c,d) Cholangioscopy was performed using a 11 Fr introducer with the small cholangioscope (size 2.7 mm) and also using a basket-type catheter. (e) After 3 days, cholangiography shows the optimal result without any sign of residual biliary stones.
Generally the choice of the larger CS was made according to the Tsunoda class (class III and especially class IV), the size of the bile ducts (size >15 mm) and to the stones’ size (size >25 mm). In the last 8 years of practice, both 2.5 mm CS and the 4.9 mm CS were used according to the characteristics of the stones and of the biliary system.
The endoscope was inserted over a guidewire (Radiofocus Standard type, Terumo, Japan) to get into the CBD or to the site to be explored. Then after removal of the guidewire, continuous flushing of saline was maintained to allow direct vision and to flush out small stones. In case of large stones (⩾20 mm), EHL was performed with 3 or 4.5 Fr probes. Bilateral biliary drainages were performed in case of angulated biliary branches difficult to reach from a single access. All manipulations were conducted under fluoroscopic guidance.
In each patient the number of sessions of EHL varied from 1 to 4; technical success was defined as immediate complete removal of stones. The assessment of complete removal of stones was made by cholangioscopy, cholangiography or US. After each session, a 10 Fr biliary drainage was inserted in order to manage any possible adverse event and removed 24–48 hours later if complete clearance was confirmed. Cholangioscopy also allowed to introduce biopsy forceps or to take off surgical stitches.
After discharge, patients were followed up with laboratory tests such as: aspartate transaminase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, albumin, International Normalized Ratio (INR) – and with US examination initially after 6 months then yearly thereafter. If there was suspicion of recurrence, CT or MRCP (since 1999 onwards) have been performed.
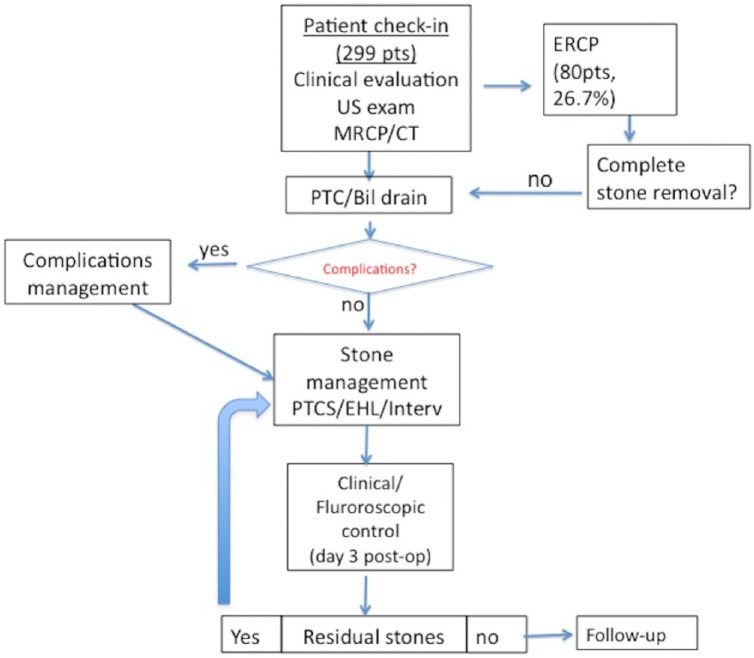
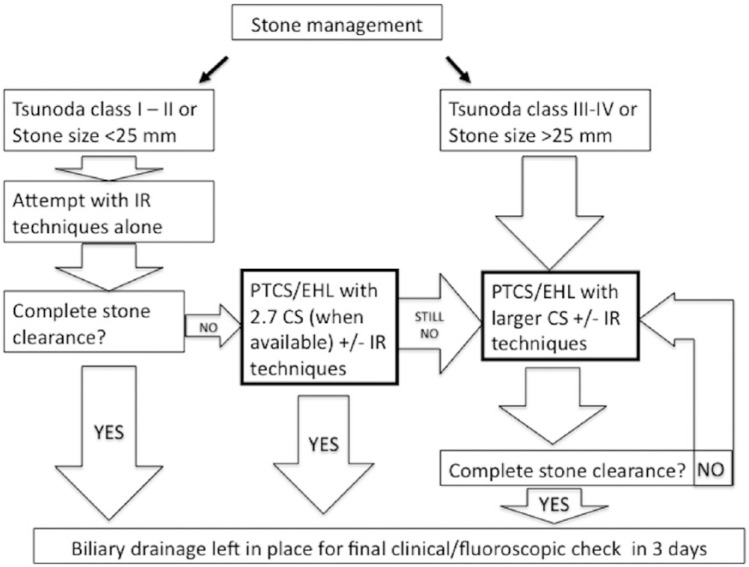
Flow charts of our general and technical management are represented in Figure 3 and Figure 4, respectively.
Figure 3.
Flow diagram representing our management of patients with difficult bile duct stones.
Bil, biliary; CT, computed tomography; EHL, electrohydraulic lithotripsy; ERCP, endoscopic retrograde cholangiopancreatography; Interv, intervention; MRCP, magnetic resonance cholangiopancreatography; PTC, percutaneous transhepatic cholangiography; pts, patients; US, ultrasound.
Figure 4.
Flow diagram showing our technical approach to the treatment of difficult bile duct stones.
CS, cholangioscope; EHL, electrohydraulic lithotripsy; IR, interventional radiology; PTCS, percutaneous transhepatic cholangioscopy.
Statistical analysis
All analyses were performed using SPSS for Windows (SPSS, Chicago, IL, USA). Continuous data are presented as the mean ± standard deviation (SD) or, if adequate, as the median and the range. The Kaplan–Meier method was used for survival analysis. Comparisons between two or more groups were analyzed for significant using the t-test or Mann–Whitney when appropriate. Significance was assumed at p < 0.05.
Results
Most patients (290, 97%) had previous history of surgical interventional abdominal procedures (duodeno-cephalo-pancreatectomy, cholecystectomy, liver transplant, biliary stenting). Clinical features of all patients are summarized in Table 1. In 80 (26.7%) patients, an endoscopic approach was unsuccessfully attempted with ERCP; 219 (73.3%) patients underwent directly biliary drainage/s due to evident anatomical alterations. Mild pancreatitis was the only ERCP related complication occuring in 3 out of 80 cases (3.7%) and was treated with fasting and intravenous (i.v.) gabexate 900 mg/day for 3 days; in all cases, blood test normalization occurred within 5 days. In these patients, PTC was postponed until pancreatitis was completely resolved.
According to Tsunoda classification, 66 patients (22.1%), 92 (30.7%), 91 (30.4%) and 50 (16.7%) were classified as type I, II, III and IV, respectively. Complete clearance of stones was achieved in 1–2 sessions of PTCS in Tsunoda type I and II. Patients with types III and IV needed 3–4 sessions (p < 0.05).
Different approaches were applied: 182 out of 299 patients (64.6%) underwent EHL; 40 out of 299 patients (13.3%) were treated only with radiological techniques; and 77 out of 299 patients (26%) were treated with both techniques (Figure 1). Of note, 256 (85.6%) PTCS were performed after biliary drainage access, while 43 (14.4%) using a T-tube previously surgically positioned. A comparison of these three approaches is illustrated in Table 2.
Table 2.
Follow up and procedural features.
| Feature | Overall | PTCS/EHL | PTCS/EHL and interventional techniques | Interventional techniques alone | p value |
|---|---|---|---|---|---|
| Patients, n (%) | 299 | 182 (64.6) | 77 (26) | 40 (13.3) | >0.05 |
| Procedure time, min (mean ± SD) | 54 ± 12 | 55 ± 15 | 60 ± 17 | 45 ± 10 | >0.05 |
| Mean patient dose mean ± SD (mGy) | 5.6 ± 0.7 | 6 ± 1.5 | 8 ± 1 | 3 ± 0.8 | <0.05 |
| Bilateral PTC access | 89 (29.7) | 34 (19.2) | 35 (45.4) | 10 (25) | 0.05 |
| Tsunoda class I, n (%) | 66 (22.1) | 15 (8.2) | 15 (19.4) | 36 (90) | <0.05 |
| Tsunoda class II, n (%) | 92 (30.7) | 73 (40.1) | 15 (19.4) | 4 (10) | <0.05 |
| Tsunoda class III, n (%) | 91 (30.4) | 69 (37.9) | 22 (28.5) | 0 | <0.05 |
| Tsunoda class IV, n (%) | 50 (16.7) | 25 (13.7) | 25 (32.4) | 0 | <0.05 |
| Sessions, mean (range) | 2 (1–4) | 3.5 (3–4) | 3 (2–4) | 2.5 (2–4) | >0.05 |
| Clearance rate, % | 99.8 | 100 | 99.6 | 100 | >0.05 |
| Recurrence rate (12 years), % | 15 | 24.7 | 13.4 | 9.1 | <0.05 |
| Major complications: | |||||
| Major bleeding, n (%) | 5 (1.7) | 3 (1.6) | 2 (2.5) | 0(0) | >0.05 |
| Perforations, n (%) | 2 (0.6) | 1 (0.5) | 1 (1.2) | 0 (0) | >0.05 |
| Cholangitis, n (%) | 31 (10.3) | 19 (10.4) | 10 (12.9) | 2 (5) | <0.05 |
| Minor complications, n (%) | 37 (12.3) | 13 (7.1) | 12 (15.5) | 12 (30) | >0.05 |
EHL, electrohydraulic lithotripsy; PTCS, percutaneous transhepatic cholangioscopy; SD, standard deviation.
Bilateral PTC access was necessary in 89 patients (29.7%) either to treat biliary stones located in difficult sites and/or for subsequent positioning of biliary plastic endoprosthesis using both transhepatic routes; such a technique was often applied in postoperative alterations of the CBD such as stenosis or leak. A total of 84 (28.1%) patients had biliary strictures which were dilated with a balloon catheter (4–10 mm diameter).
In almost all patients (298/299, 99.6%), stone clearance was achieved after a maximum of 4 sessions. In one patient with multiple biliary stones (Tsunoda class IV), PTCS with EHL (CS 4.9 mm) did not clear all the intrahepatic stones; after 4 attempts and the patient still have residual stones in the left liver lobe. During the fourth procedure, a bleeding from the transepatic tract was detected; an angiogram revealed an active bleeding from a segmental branch of the right hepatic artery, that was successfully embolised. Hence the patient underwent surgical resection of the left liver lobe.
All procedures performed using a T-tube did not show any differences in terms of stone clearance and complications when compared with the other procedures performed using a transhepatic access with the 2.7 mm CS. Therefore these have not been considered separately.
Technical success was achieved in 66 patients after the first session, in 121 patients in the second session, in 88 patients in the third session and in 25 patients in the fourth session.
During follow up, US examination and laboratory values remained in the normal range in patients without recurrence.
A total of 45 out of 299 (15%) patients returned for stone recurrences within 2 years after discharge referring episodes of cholangitis with increased total bilirubin (mean value 9 mg/dl), GGT and fever (in 40/45 cases, 88.9%) or biliary colic alone (5/45 cases, 10.1%) and were successfully retreated. A total of 20 patients (20/45, 44.4%) had recurrent intrahepatic stones within 12 months after discharge, 14 patients (31.1%) had recurrence within 18 months and 11 patients (24.4%) had recurrence within 24 months. Recurrence rates over time are reported in Figure 5.
Figure 5.
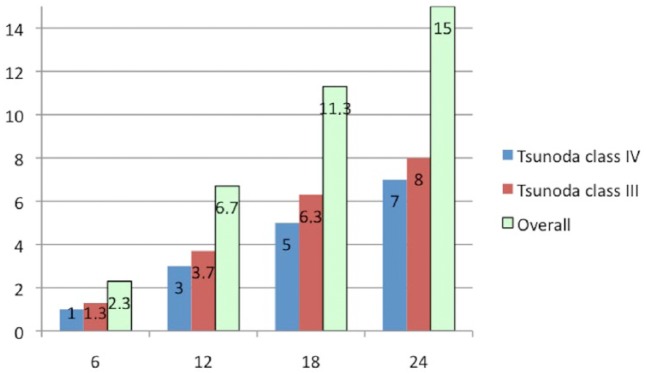
Recurrence rates of hepatolithiasis during the follow up (months).
In 141 out of 299 (47.1%) patients, PTCS was performed both to remove stones and to assess the nature of biliary strictures: 65 (46%) of them had malignant disease (cholangiocarcinoma, liver primary/secondary neoplasms) while 76 (54%) patients had benign fibrotic strictures.
In 40 malignant strictures and 50 benign strictures located in the CBD, a permanent metallic stent or a removable metallic stent/plastic prosthesis was placed (a metallic covered stent was placed in 30 patients and plastic stents in 20 patients of whom 10 had bilateral stenting). Stenting was usually inserted after all the stones were cleared.
Eighteen (6.1%) patients presented with recurrent cholangitis and bile duct stones due to obstruction/stenosis of a previously inserted biliary stent; all these cases were successfully treated with PTCS/EHL and IR techniques.
No cases of procedure-related death occurred.
Major adverse events were: 5 (1.6%) cases of major bleeding that required embolisation and 2 (0.66%) perforations of the CBD during the advancement of the guidewire, which were treated with repositioning of the transhepatic tube. No surgical treatment for such adverse events was required.
A total of 31 (10.3%) cases of acute cholangitis were treated with antibiotic therapy. Usual medical therapy was ceftriaxone, 2 g i.v., acetaminophen and fasting. In some cases we had to repeatedly flush with saline and change the biliary drainage/s. (Table 2).
Minor adverse events occurred in 37 cases (12.3%): 22 patients experienced severe pain or nausea (16 cases of acute mild pancreatitis resolved with medical therapy and 6 cases of fluid overload) and 15 (5%) presented self-limiting bleeding and did not require blood transfusions or urgent angiography (Table 2).
Learning curve analysis showed that most minor and major adverse events (5 major bleeding events and 1 perforation of the CBD) occurred during the first 9 years of experience and in particular when we used the larger CS (4.9 mm, 16 Fr introducer sheath). In the last 9 years we encountered fewer adverse events due to the predominant use of the smaller CS (3.9 mm and 2.7 mm in size). Moreover, the number of sessions to achieve complete clearance of stones significantly decreased proportionally with the size of CS.
Overall survival at 9 years was 88.3% (264/299): 33 patients died either of cholangiocarcinoma or primary/secondary neoplasms while two patients died following cardiovascular events.
After 18 years, survival was 76% (227/299): additional 32 patients died from cholangiocarcinoma or primary/secondary neoplasms, and three patients died from nonbiliary/liver related disease (one from lung cancer and the other two from hemorrhagic stroke).
The relationship between technical success, adverse events and different types of techniques is summarized in Table 2.
Discussion
When conventional endoscopic techniques fail to remove bile duct stones, PTCS may be considered a promising alternative tool to surgery as advised by previous studies [Kow et al. 2011; Cheon et al. 2009; Huang et al. 2003, Chen et al. 2005]. Surgical operation is mainly indicated in younger patients with good clinical condition/fair liver function with unilobar hepatolithiasis, particularly left-sided, atrophy, fibrosis and multiple abscesses secondary to cholangitis and/or suspicion of concomitant intrahepatic cholangiocarcinoma [Sakpal et al. 2009]. Surgery has well-known drawbacks such as previous abdominal surgery/intervention, bilateral intrahepatic lithiasis/strictures, elderly patients and/or poor clinical conditions.
Some authors have investigated the management of intrahepatic lithiasis (surgery, PTCS) in Asians and in a relative young population. Jarufe and colleagues reported a rate of postoperative clearance of 90.6% and a recurrence rate of 13.5% [Jarufe et al. 2012]. Long-term results reported by Cheon and colleagues showed no significant difference between recurrence rate of successful hepatectomy with postoperative cholangioscopy (18%), PTCS (21%) and ERCP (25%) [Cheon et al. 2009]. In Western countries, surgical treatment of hepatolithiasis was investigated by a recent single study, accounting for preoperative morbidity and mortality rates of 6.6% and 0%, respectively [Tabrizian et al. 2012].
Another alternative technique to classic endoscopy is the extracorporeal shock wave lithotripsy (ESWL) in combination with POCS, which has been attempted to improve the clearance of difficult bile duct stones [Yasuda and Itoi, 2013; Hassan et al. 2014; Muratori et al. 2010]. Allowing for some advantages such as the repeatability and no restriction in stone size, it has displayed several drawbacks as limited efficacy (i.e. variable stone clearance 73–90% [Yasuda and Itoi, 2013]), long treatment duration, number of sessions needed to complete stone clearance (even more than 5 [Muratori et al. 2010]) and complications (i.e. bleeding, arrhythmias) [Muratori et al. 2010]. In general, ESWL did not show superiority to other alternative techniques for stone clearance and available studies lack on reporting long-term stone recurrence rates [Yasuda and Itoi, 2013; Hassan et al. 2014; Muratori et al. 2010].
So far there is a shortfall of evidence regarding PTCS and interventional approaches in the treatment of difficult intrahepatic bile duct stones.
The leading aim of PTCS is to remove biliary stones in order to avoid several further adverse events such as cholangitis, biliary cirrhosis and cholangiocarcinoma. In particular, complete stone clearance represents the main objective since the incidence of recurrent cholangitis or cholangiocarcinoma is higher in patients with incomplete clearance or recurrent hepatolithiasis [Huang et al. 2003].
In our study, immediate stone clearance rate was higher (99.6%) than that reported in two previous studies by Cheon and colleagues (83%)[Cheon et al. 2009] and Huang and colleagues (85.3%)[Huang et al. 2003].
Factors that can be attributed to our success rate are: small calibre CS; the practice of multiple and sequential sessions; improvement of skills; and the combination of endoscopic and IR techniques. The advantages of small size CS are flexibility, steerability and easier introduction, allowing a smaller introducer sheath (10 Fr). Due to the small calibre these can be used through smaller tracts and tight strictures, thus extending the range of indications for PTCS. In addition, they have a smaller field of view but fair image resolution compared with larger CS. In our experience, these technical features resulted in less traumatism and damage to the bile ducts, thus causing less adverse events related to PTCS.
Major bleeding was related to the large dilatation (16 Fr) of the transhepatic route in order to insert the 4.9 mm CS. Perforations (only two cases reported) of the CBD were caused by the tip of the guidewire and thus were not strictly related to the PTCS/EHL procedure. When using smaller CS we observed only self-limiting bleeding. Of note there has been an increasing use of the smaller CS over time due to its technical advantages and low complication rates. Despite its narrow width of view, the smaller CS may be used also in cases of markedly dilated bile ducts (diameter >15 mm), obtaining satisfactory stone clearing results. A smaller CS was also useful to perform biopsy samples of suspect strictures by using a proper forceps device. Moreover, the last CS (2.7 mm) required a minor number of sessions and a reduction in additional IR to guarantee a complete clearance of stones (mean number of sessions: 2.5 versus 3 and 3.5 of ureteroscope and large CS, respectively).
Recently, a small case series and mid-term experience has been reported in using small calibre CS with EHL or YAG laser [Kow et al. 2011; Rimon et al. 2009; Schatloff et al. 2009; Ierardi et al. 2013; Lee et al. 2001]. Previous studies reported larger number of cases with long-term follow up, but using the large CS with a 16–18 Fr tract for hepatolithiasis evacuation by Dormia basket [Huang et al. 2003; Chen et al. 2005].
In a large cohort (245 patients) enrolled by Huang and colleagues, the major adverse events rate was 1.6% (4/245); however, there was a complete clearance rate in 85.3% of cases, with an high rate of recurrence (ranging from 44.4% to 56.2%) [Huang et al. 2003].
It is worth to outline that the large CS has been used in most of the Tsunoda IV cases due to the presence of larger stones and bile ducts. Due to the higher complexity of cases (Tsunoda class IV), large CS has been related to a higher rate of adverse events and the need of more sessions (also with the use of interventional techniques) and higher recurrence rate (Table 2). According to our results, large CS should only be used in extensive hepatolithiasis (Tsunoda class III and class IV). Moreover, at least three sessions are advised to obtain a higher clearance rate.
Of note some patients were treated only with IR techniques (i.e. without the use of CS). This is technically quicker and easier, but it is advised only in Tsunoda class I and II patients (Table 2). Otherwise either the use of PTCS alone or PTCS plus IR techniques can be effectively used in most complex cases (Tsunoda class III and class IV). When comparing the use of PTCS alone with PTCS plus IR techniques, despite the similar rates of technical success and complications, a lower recurrence rate over 12 months is noted in the latter (24.7% PTCS alone versus 13.4% PTCS + radiological techniques; p < 0.05) (Table 2). These results might be related to the capability of IR techniques to deal with biliary strictures which contribute to the higher risk of stone recurrence if left untreated.
In our case series, major adverse events were found in 9 cases (2.3%), with a clearance rate close to 100% and a low recurrence rate (15%) up to 10 years. A high immediate stone clearance rate is a primary aim; avoiding recurrence is another unsolved and fundamental issue. Long-term recurrence may be related to the immediate complete stones clearance, to Tsunoda class III and class IV and to presence of severe bile duct strictures [Lee et al. 2001].
Such differences with other studies are related to the different techniques used: Huang and colleagues dilated the transhepatic tract to extract stones with a Dormia basket. Thus their technique was less aggressive on the bile duct wall, but suffered from more limitations related to perform a larger transhepatic tract, presence of strictures and stone size [Huang et al. 2003]. Otherwise our technique with EHL caused some episodes of bleeding (major bleeding only with large CS), but showed a higher clearance rate and low recurrence rate due to the capability of reaching and bursting impacted and larger stones; also with the small calibre CS, it is possible to reach every small branch of the biliary system, having a complete visualization of the liver.
Different interventional techniques have been previously reported to perform percutaneous transhepatic removal of bile duct stones by using a Fogarty balloon catheter [Ozcan et al. 2012] or occlusion balloon [García-Vila et al. 2004]. Despite the large cohort studied by Ozcan and colleagues [Ozcan et al. 2012], the majority of stones (95% of cases) were located in the CBD; these are easier to remove with interventional techniques by pushing down and dilating the ampulla of Vater. In fact, the immediate clearance rate was higher when treating CBD stones (97.5%) than intrahepatic stones (61.5%).
In our study, interventional radiology collaboration is fundamental in treating tight strictures. We believe that planning the treatment with multiple sequential sessions, leaving in place 10 Fr biliary catheters and, in some cases, dilating bile duct strictures with balloon catheters, led to a sort of remodeling and thereby improved the outcomes of patients as well as reducing the risk of recurrence. Besides, the IR techniques alone are recommended only in easier cases to still get high clearance rates.
In case of malignant disease the recurrence of strictures/stones is often unavoidable (39/299 patients, 13%). Moreover, the use of basket type catheters with balloon dilatation of the ampulla of Vater was effective in performing the removal of bile duct stones.
Limitations of our study are: single center study; observational–retrospective setting; and a population only from Western countries.
From our 18 years’ experience we have learnt that our combined radiological–endoscopic approach to ‘difficult hepatolithiasis’ may result in both immediate and long-term clearance of stones, thus improving the clinical outcomes of patients with either malignant or benign biliary disease.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Alessandro Cannavale, Vascular and Interventional Radiology Unit, Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy.
Mario Bezzi, Vascular and Interventional Radiology Unit, Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy.
Fabrizio Cereatti, Department of General Surgery Paride Stefanini, Interventional Endoscopy Unit, Sapienza University of Rome, Rome, Italy.
Pierleone Lucatelli, Vascular and Interventional Radiology Unit, Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy.
Gianfranco Fanello, Department of General Surgery Paride Stefanini, Interventional Endoscopy Unit, Sapienza University of Rome, Rome, Italy.
Filippo Maria Salvatori, Vascular and Interventional Radiology Unit, Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy.
Fabrizio Fanelli, Vascular and Interventional Radiology Unit, Department of Radiological Sciences, Sapienza University of Rome, Rome, Italy.
Fausto Fiocca, Department of General Surgery Paride Stefanini, Interventional Endoscopy Unit, Sapienza University of Rome, Rome, Italy.
Gianfranco Donatelli, Department of General Surgery Paride Stefanini, Interventional Endoscopy Unit, Sapienza University of Rome, 324, Viale Regina Elena, 00161 Rome, Italy.
References
- Buxbaum J. (2013) Modern management of common bile duct stones. Gastrointest Endosc Clin N Am 23: 251–275. [DOI] [PubMed] [Google Scholar]
- Chen C., Huang M., Yang J., Yang C., Yeh Y., Wu H., et al. (2005) Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc 19: 505–509. [DOI] [PubMed] [Google Scholar]
- Cheon Y., Cho Y., Moon J., Lee J., Shim C. (2009) Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery 146: 843–853. [DOI] [PubMed] [Google Scholar]
- Erim T., Shiroky J., Pleskow D. (2013) Cholangioscopy: the biliary tree never looked so good! Curr Opin Gastroenterol 29: 501–508. [DOI] [PubMed] [Google Scholar]
- García-Vila J., Redondo-Ibáñez M., Díaz-Ramón C. (2004) Balloon sphincteroplasty and transpapillary elimination of bile duct stones: 10 years’ experience. AJR Am J Roentgenol 182: 1451–1458. [DOI] [PubMed] [Google Scholar]
- Hassan S., Butt M., Luck N., Abbas Z. (2014) Successful removal of intrahepatic bile duct stones by using a combination of extracorporeal shock wave lithotripsy and direct peroral cholangioscopy. Gastrointest Endosc 80: 1174. [DOI] [PubMed] [Google Scholar]
- Huang M., Chen C., Yang J., Yang C., Yeh Y., Chou D., et al. (2013) Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol 98: 2655–2662. [DOI] [PubMed] [Google Scholar]
- Ierardi A., Fontana F., Petrillo M., Floridi C., Cocozza E., Segato S., et al. (2013) Percutaneous transhepatic endoscopic holmium laser lithotripsy for intrahepatic and choledochal biliary stones. Int J Surg 11 (Suppl. 1): S36–S39. [DOI] [PubMed] [Google Scholar]
- Jarufe N., Figueroa E., Muñoz C., Moisan F., Varas J., Valbuena J., et al. (2012) Anatomic hepatectomy as a definitive treatment for hepatolithiasis: a cohort study. HPB (Oxford) 14: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yang M., Hwang J., Yoo B. (2013) Endoscopic papillary large balloon dilation for the removal of bile duct stones. World J Gastroenterol 19: 8580–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow A., Wang B., Wong D., Sundeep P., Chan C., Ho C., et al. (2011) Using percutaneous transhepatic cholangioscopic lithotripsy for intrahepatic calculus in hostile abdomen. Surgeon 9: 88–94. [DOI] [PubMed] [Google Scholar]
- Lee S., Seo D., Myung S., Park E., Lim B., Kim H., et al. (2001) Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: an evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc 53: 318–323. [DOI] [PubMed] [Google Scholar]
- Muratori R., Azzaroli F., Buonfiglioli F., Alessandrelli F., Cecinato P., Mazzella G., et al. (2010) ESWL for difficult bile duct stones: a 15-year single centre experience. World J Gastroenterol 16: 4159–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan N., Kahriman G., Mavili E. (2012) Percutaneous transhepatic removal of bile duct stones: results of 261 patients. Cardiovasc Intervent Radiol 35: 621–617. [DOI] [PubMed] [Google Scholar]
- Rimon U., Kleinmann N., Bensaid P., Golan G., Garniek A., Khaitovich B., et al. (2011) Percutaneous transhepatic endoscopic holmium laser lithotripsy for intrahepatic and choledochal biliary stones. Cardiovasc Intervent Radiol 34: 1262–1266. [DOI] [PubMed] [Google Scholar]
- Sakpal S., Babel N., Chamberlain R. (2009) Surgical management of hepatolithiasis. HPB (Oxford) 11: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatloff O., Rimon U., Garniek A., Lindner U., Morag R., Mor Y., et al. (2009) Percutaneous transhepatic lithotripsy with the holmium: YAG laser for the treatment of refractory biliary lithiasis. Surg Laparosc Endosc Percutan Tech 19: 106–109. [DOI] [PubMed] [Google Scholar]
- Tabrizian P., Jibara G., Shrager B., Schwartz M., Roayaie S. (2012) Hepatic resection for primary hepatolithiasis: a single-center Western experience. J Am Coll Surg 215: 622–626. [DOI] [PubMed] [Google Scholar]
- Trikudanathan G., Navaneethan U., Parsi M. (2013) Endoscopic management of difficult common bile duct stones. World J Gastroenterol 19: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda T., Tsuchiya R., Harada N., Yoshino R., Noda T., Izawa K., et al. (1985) Long-term results of surgical treatment for intrahepatic stones. Jpn J Surg 15: 455–462. [DOI] [PubMed] [Google Scholar]
- Yasuda I., Itoi T. (2013) Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc 25: 376–385. [DOI] [PubMed] [Google Scholar]