Abstract
In recent years prescription of opioids has increased significantly. Although effective in pain management, bothersome gastrointestinal adverse effects are experienced by a substantial proportion of opioid-treated patients. This can lead to difficulties with therapy and subsequently inadequate pain relief. Collectively referred to as opioid-induced bowel dysfunction, these adverse effects are the result of binding of exogenous opioids to opioid receptors in the gastrointestinal tract. This leads to disturbance of three important gastrointestinal functions: motility, coordination of sphincter function and secretion. In the clinic this manifests in a wide range of symptoms such as reflux, bloating, abdominal cramping, hard, dry stools, and incomplete evacuation, although the most known adverse effect is opioid-induced constipation. Traditional treatment with laxatives is often insufficient, but in recent years a number of novel pharmacological approaches have been introduced. In this review the pathophysiology, symptomatology and prevalence of opioid-induced bowel dysfunction is presented along with the benefits and caveats of a suggested consensus definition for opioid-induced constipation. Finally, traditional treatment is appraised and compared with the latest pharmacological developments. In conclusion, opioid antagonists restricted to the periphery show promising results, but use of different definitions and outcome measures complicate comparison. However, an international working group has recently suggested a consensus definition for opioid-induced constipation and relevant outcome measures have also been proposed. If investigators within this field adapt the suggested consensus and include symptoms related to dysfunction of the upper gut, it will ease comparison and be a step forward in future research.
Keywords: antagonists, constipation, dysfunction, gut, opioids
Introduction
The use of opioids to alleviate severe acute and chronic pain has increased several fold in Europe and the USA in recent years [Casati et al. 2012]. Accordingly, opioids are the most commonly prescribed treatment for severe pain and it has been estimated that up to 90% of American patients treated at specialized pain centers receive opioids [Benyamin et al. 2008]. Despite the increasing use, the British National Institute for Health and Care Excellence (NICE) notes that pain resulting from advanced disease often remains undertreated, due to fear of addiction and concerns related to adverse effects [NICE, 2012].
The most common adverse effects to opioid treatment include nausea, headache, confusion and gastrointestinal (GI)-related symptoms, the latter collectively referred to as opioid-induced bowel dysfunction (OIBD) [Benyamin et al. 2008; De Schepper et al. 2004; Pappagallo, 2001]. OIBD occurs when exogenous opioids bind to opioid receptors of the enteric nervous system, and consequently disturb normal GI function [Camilleri, 2011; De Schepper et al. 2004; Holzer, 2014; Pappagallo, 2001; Wood and Galligan, 2004]. The adverse effects manifest as gastroesophageal reflux, vomiting, bloating, abdominal pain, anorexia, hard stools, constipation and incomplete evacuation. These symptoms can be severe and it is not uncommon for patients to discontinue treatment as a result, which naturally results in inadequate pain management [Looström et al. 2011; Pappagallo, 2001]. Opioid-induced constipation (OIC) is the most well described GI adverse effect, but in recent years the more universal expression OIBD has gained footing in the scientific community along with the acknowledgement that OIBD is the result of a combination of intricate pathophysiological processes of the entire GI tract of which OIC is an important piece [Pappagallo, 2001].
The typical treatment strategy to alleviate OIBD is based on combinations of pharmacological and nonpharmacological approaches, including laxatives coupled with increased dietary fiber and fluid intake, encouraging exercise, biofeedback, among others [Brock et al. 2012; Dorn et al. 2014]. However, these strategies do not address the underlying pathophysiological mechanisms, and therefore are likely to fall short of adequate relief [Poulsen et al. 2014].
Recently, a number of novel pharmacological approaches have been marketed for both constipation and OIC, such as the chloride channel activator lubiprostone and the selective 5-HT4 hydroxytryptamine receptor 4 (5-HT4) serotonin agonist prucalopride, as well as a number of competitive opioid antagonists that target the underlying pathophysiology through antagonism of the µ-opioid receptors in the gut.
In this review the pathophysiology, symptomatology and prevalence of OIBD are presented as background information. Recent approaches towards the development of a consensus definition for OIC suggested by an international multidisciplinary working group is reviewed [Camilleri et al. 2014]. Finally, traditional recommended treatment strategies are appraised and compared with the latest pharmacological developments.
Pathophysiology: opioid receptors and the gut
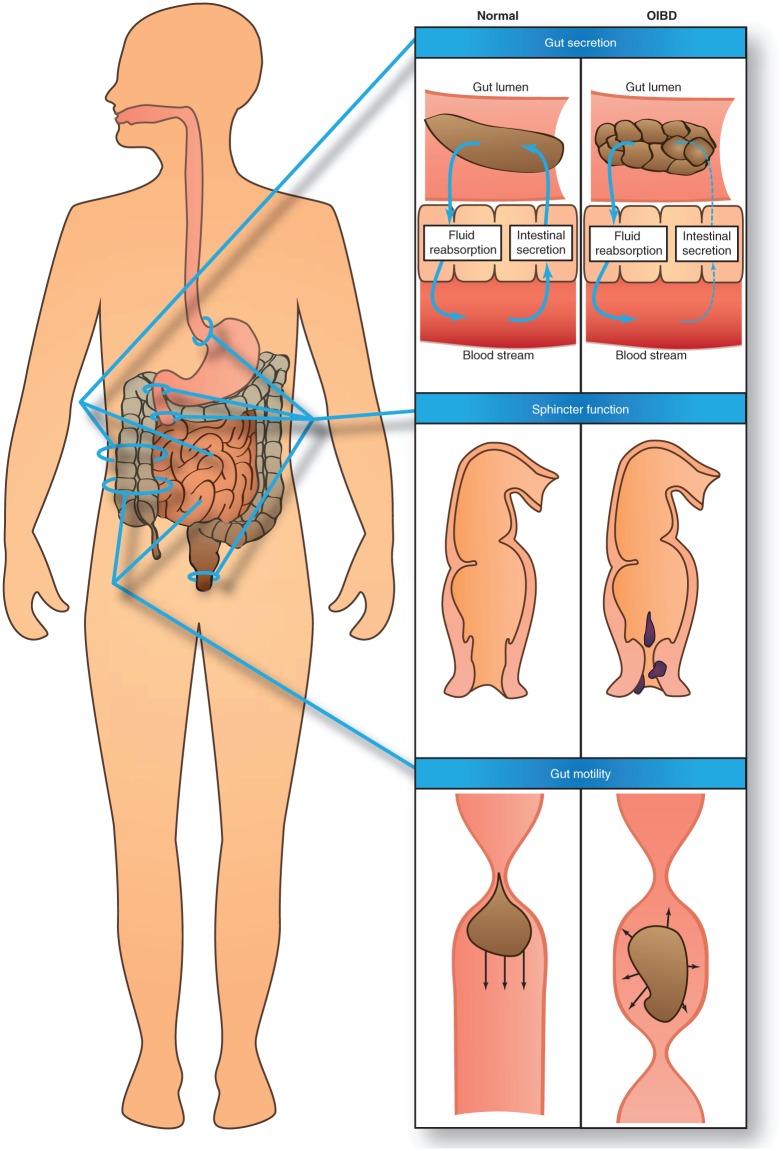
A detailed description of the underlying pathophysiology of OIBD is beyond the scope of this review (for a comprehensive review, the reader is referred to Kurz and Sessler) [Kurz and Sessler, 2003]. However, in order to understand the diverse clinical presentations of OIBD, an overview of pathophysiology is presented below and illustrated in Figure 1.
Figure 1.
Pathophysiology of opioid-induced bowel dysfunction. First row: decreased gut secretion of electrolytes and water to the intestinal lumen results in a dryer, harder stool. Second row: increased sphincter resting tone and decreased rectal sensitivity results in straining, which can result in hemorrhoids as illustrated, and the sensation of incomplete evacuation. Third row: increased contractile tone in the circular muscle layer and decreased tonic inhibition of the muscle tone along with occurrence of high-amplitude nonpropulsive phasic contractions in the small and large intestine results in stasis and reduced propulsive peristalsis.
Three types of opioid receptors are involved in controlling normal GI function: µ-, δ- and κ-receptors [Galligan and Akbarali, 2014; Holzer, 2014]. In animal studies δ- and κ-subtype receptors are expressed primarily in the stomach and proximal colon [Camilleri, 2011; Holzer, 2004; Sternini et al. 2004]. µ-receptors are the most widely expressed throughout the GI tract and predominantly localized on myenteric and submucosal neurons and on immune cells in the lamina propria [Galligan and Akbarali, 2014; Kurz and Sessler, 2003; Sternini et al. 2004].
Endogenous ligands and most exogenous opioids activate µ-receptors [Greenwood-Van Meerveld et al. 2004]. This triggers a comprehensive intracellular signaling pathway, which ultimately results in inhibition of the enzymatic conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP) through adenylate cyclase. Consequently, opioids decrease the formation of cAMP, which otherwise would have activated several target molecules to regulate cellular functions [Galligan and Akbarali, 2014; Sharma et al. 1975]. This is likely the main effect, but opioids are also involved in direct activation of K+-channels (membrane hyperpolarization) and inhibition of Ca++-channels (decreased neurotransmitter release) [Sharma et al. 1975]. Overall the result is reduced release of neurotransmitters and decreased neuronal activity.
Activation of µ-receptors in the GI tract by exogenous opioid results in disturbance of three essential GI functions: motility, coordination of sphincter function and secretion. In the esophagus, opioids induce nonpropulsive peristaltic contractions [Kraichely et al. 2010]. In the small and large intestine the contractile tone in the circular muscle layer is increased and in parallel there is a decreased tonic inhibition of the muscle tone [Frantzides et al. 1992; Sarna and Otterson, 1990; Telford et al. 1989]. This is accompanied by enhanced rhythmic contractions and occurrence of high-amplitude nonpropulsive phasic contractions. The net result is increased segmental spastic tone and less propulsive peristalsis [De Schepper et al. 2004; Kraichely et al. 2010; Sarna and Otterson, 1990; Telford et al. 1989; Thomas, 2008].
In terms of sphincter function, opioid-induced sphincter of Oddi dysfunction with biliary-like type of pain attacks is a well known side effect [Helm et al. 1988; Sharma, 2002; Torres et al. n.d.]. Similar results have been found for the lower esophageal sphincter where opioid treatment mainly induced inhibition of sphincter relaxation [Dowlatshahi et al. 1985]. In the anal canal, the tone of the internal sphincter has been shown to be increased and there is an accompanying decreased rectal sensitivity, which has been associated with straining and the sensation of incomplete evacuation [Göke et al. 1992; Musial et al. 1992]. Gut secretion is reduced as a direct result of inhibited cAMP and vasoactive intestinal peptide production [Furness and Costa, 1987; Huizinga and Lammers, 2009]. Moreover, stool water content is indirectly affected due to stasis and increased passive absorption of water. In concert, this results in dryer and harder stools [Thomas, 2008].
Prevalence of opioid-induced bowel dysfunction
Symptoms in patients treated with opioids have been examined in numerous studies, although different definitions have been used. A prospective survey of incidence, prevalence and severity of adverse effects during repeated individualized dosing of morphine for chronic cancer pain found that 95% of all patients reported dry mouth and 88% reported sedation and constipation [Glare et al. 2006]. GI symptoms were reported in an observational study in patients taking opioids for chronic noncancer-related pain and it was found that 47% experienced constipation. In the same study gastroesophageal reflux related symptoms were reported by 33% of all patients, nausea by 27% and vomiting by 9%. Furthermore, chronic abdominal pain was reported by 58% [Tuteja et al. 2010]. Similar results have been found in a population-based survey along with increased frequency of constipation-related symptoms (including straining, hard stools, bloating and infrequent bowel movements) [Choung et al. 2009].
OIC is probably the most well characterized adverse effect in opioid-treated patients. Nonetheless, the prevalence varies substantially between different studies ranging from 15% to 81% in patients without cancer [Allan et al. 2001; Bell et al. 2009; Cook et al. 2008; Kalso et al. 2004; Moore and McQuay, 2005]. One of the highest prevalence rates reported derives from a multinational, internet-based survey of 322 chronic opioid users: 81% reported constipation, despite concomitant use of laxatives! [Bell et al. 2009]. A larger population-based survey of 2055 patients treated with opioids and laxatives for chronic noncancer pain also found a high prevalence of constipation as 57% reported this [Cook et al. 2008]. In comparison, the prevalence of chronic constipation in the general population has been estimated to affect between 2% and 27% of the adult population with an average around 15%, depending on definition used [Higgins and Johanson, 2004; Sanchez and Bercik, 2011; Wirz et al. 2012]. Constipation can result in overflow diarrhea where liquid stool passes around the obstruction. Thus, diarrhea-related symptoms (including urgency, loose bowel movements and frequent bowel movements) have also frequently been reported in opioid-treated patients [Bril et al. 2011; Wojciech, 2012].
Despite the elaborate focus on OIC, which is often (too) simply defined as a reduction in the number of spontaneous bowel movements (SBMs), infrequent bowel movement ranks only number 5 in self-assessed constipation symptoms, whereas symptoms such as gas, straining and abdominal discomfort are far more prevalent [Johanson and Kralstein, 2007]. This strongly accentuates the limitations of such studies which potentially overlook symptoms regarded more bothersome for the patient.
Narcotic bowel syndrome (NBS) is another subset of OIBD characterized by chronic or frequently recurring abdominal pain that worsens during escalating doses of opioids [Choung et al. 2009; Drossman and Szigethy, 2014; Grunkemeier et al. 2007]. The paradoxical development of increased pain despite continued or escalating doses of opioids can result in an unfortunate downward spiral with serious consequences for the patient [Grunkemeier et al. 2007].
Other risk factors such as high age, low-fiber diet, sex, reduced mobility and different drugs may also contribute to the development of constipation or other GI symptoms in patients with pain [Wirz et al. 2012].
Collectively, these findings emphasize the multiplicity of GI-related adverse effects associated with chronic (often defined as > 90 days) and short-term opioid use. While constipation may be the dominant symptom in many cases, it is important to keep in mind the multifaceted presentation of OIBD, as illustrated in Figure 2.
Figure 2.
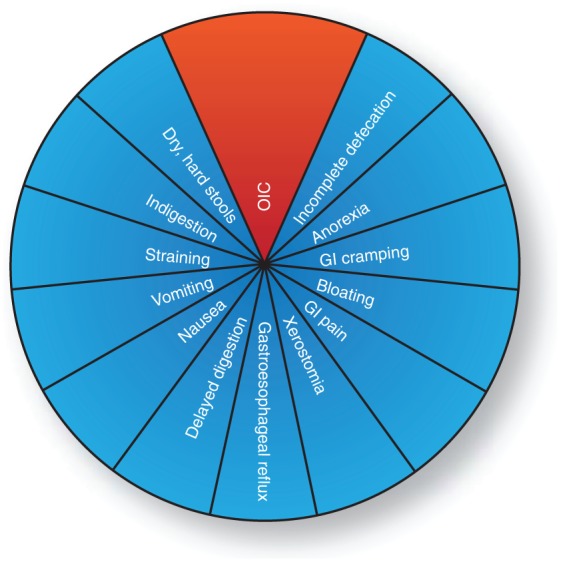
Schematic representation of the relation between opioid-induced constipation (OIC) and opioid-induced bowel dysfunction (OIBD). OIC is the most well known gastrointestinal (GI) adverse effect to opioid treatment, but only a part of the multiplicity of GI-related adverse effects associated with opioid treatment known as OIBD.
Towards a consensus definition for opioid-induced constipation?
Numerous subjective criteria and objective outcome measures for assessing OIBD and OIC exist [Gaertner et al. 2015; Olesen and Drewes, 2011]. The Patient Assessment of Constipation Symptoms questionnaire is one of the most widely used subjective instruments and has proven reliable in the assessment of treatment for OIC [Frank et al. 1999; Slappendel et al. 2006]. Other mentionable questionnaires are the Bowel Function Index specifically designed for OIC [Rentz et al. 2009] and the Bristol Stool Chart (BSC), which registers stool frequency and consistency on a seven-point scale [Lewis and Heaton, 1997]. Although these tools are valuable both clinically and in research, the lack of a consensus definition for OIC hampers comparison between OIC trials. A recent systematic review of 47 clinical trials evaluating OIC found that a clear definition for OIC was only provided in one-third (34%) of the trials [Gaertner et al. 2015]. Among the publications that provided a definition for OIC, it most frequently relied on history of present or recent opioid therapy; defecation frequency (most often, fewer than three SBMs per week); and at least two of the following symptoms at least 25% of the time: straining, hard or lumpy stool, incomplete evacuation and infrequent stools [Camilleri et al. 2014; Gaertner et al. 2015].
Consequently, due to the multifaceted presentation of OIBD and OIC, where a reduction in number of SBMs is not necessarily the most bothersome symptom for the patient, these definitions do not necessarily identify all patients who suffer from GI adverse effects following opioid administration [Clark and Currow, 2013]. Hence, OIBD and OIC is more likely underdiagnosed than overdiagnosed, supported by the fact that many opioid-treated patients report normal stool frequency, but still experience symptoms of OIBD [Bell et al. 2009].
This clearly demonstrates the need for a consensus definition in order to better encompass the clinical presentation of OIBD and OIC, but also in order to adequately compare studies evaluating efficacy of different treatments strategies. However, only recently a consensus definition for OIC has been suggested by an international multidisciplinary working group [Camilleri et al. 2014]. The authors suggest the following definition for OIC: ‘A change when initiating opioid therapy from baseline bowel habits that is characterized by any of the following: reduced bowel movement frequency, development or worsening of straining to pass bowel movements, a sense of incomplete rectal evacuation, or harder stool consistency’.
The essential improvement in this definition is the deviation from a specific number of bowel movements per week, often being a mandatory criterion, to a definition that encompasses individual changes in bowel habits. Furthermore, this definition considers not only the normal ‘baseline’ bowel habits of each individual patient and the fact that patients may have pre-existing constipation, but also the fact that patients’ response to opioid treatment is known to vary immensely, based on for example, genetic factors [Lötsch et al. 2004; Stamer et al. 2005].
However, a substantial drawback with the focus on change in bowel habits is that many patients have been treated with opioids for years and do not recall their ‘normal’ bowel habits, that is, before opioid therapy. Even though psychometric validation is warranted, this improvement of the definition is an important step towards a more covering definition, especially in patients who report ‘normal’ stool frequency, but still experience other symptoms of OIC. Nonetheless, as stated above, OIC is only a part of the OIBD complex (Figure 2). Thus, a definition that covers the whole spectrum of symptoms would be more suitable in clinical studies, as it reflects the clinical presentation even better. Such a definition will be more complex as many adverse effects should be included. However, as many symptoms will be new for the patient (in contrast to constipation which is very prevalent in the typical older patient) the sensitivity and validity may be better.
Existing and emerging paradigms
Satisfactory management of OIBD remains a challenge [Bell et al. 2009; Dorn et al. 2014]. The current recommendation of combining laxatives with dietary changes and lifestyle changes is often insufficient as it does not target the underlying problem and because the majority of patients receiving chronic pain treatment suffer from comorbidities resulting in, for example, less mobility [Diego et al. 2011; Dorn et al. 2014].
As the main focus often has been to increase the number of SBMs, other OIBD symptoms may persist. In the following an overview of the current treatment possibilities and pharmacological approaches is presented, and a comparison of the drug class with most alternatives, opioid antagonists, is summarized in Table 1.
Table 1.
Current pharmaceutical approaches for opioid-induced bowel dysfunction targeted at the peripheral opioid receptors.
| Drug | Pharmacological mechanism | Efficacy | Disadvantages |
|---|---|---|---|
| Combined prolonged release naloxone and oxycodone | Nonselective, competitive opioid receptor antagonist [Meissner et al. 2000] | Significant improvement in bowel function compared to oxycodone as assessed by BFI, number of complete SBMs, and PAC-SYM score. Laxative use reduced [Burness and Keating, 2014; Löwenstein et al. 2009; Simpson et al. 2008] | Only marketed in oral formulation in combination with oxycodone |
| Does not allow for opioid rotation or as add on to existing therapy | |||
| Methylnaltrexone | Peripherally acting, competitive µ-opioid receptor antagonist [Herndon et al. 2002] | Effective in inducing laxation in opioid treated patients within four hours of administration compared to placebo [Thomas et al. 2008] | Only available in subcutaneous formulation and only approved in palliative care in patients with advanced illness |
| Alvimopan | Peripherally acting, competitive µ-opioid receptor antagonist [Camilleri, 2005; Schmidt, 2001] | Significant increase in weekly SBMs compared to placebo. Improvement in a number of OIBD-related symptoms [Webster et al. 2008] | Cardiovascular safety concerns |
| Only approved in the USA following partial small or large bowel resection with primary anastomosis in hospitalized patients | |||
| Naloxegol | Peripherally acting, competitive µ-opioid receptor antagonist [Corsetti and Tack, 2015; Eldon et al. 2007] | Significantly higher response rates for a composite primary endpoint compared with placebo. Shorter time to first postdose SBM, and higher number of days per week with one or more SBMs [Chey et al. 2014] | Interaction with CYP3A4 inducers or inhibitors can affect plasma concentration |
BFI, Bowel Function Index; CYP3A4, cytochrome P450 3A4; OIBD, opioid-induced bowel dysfunction; PAC-SYM, Patient Assessment of Constipation Symptoms; SBM, spontaneous bowel movement.
Laxatives
Laxatives can be divided into different subgroups, including osmotic agents (magnesium, lactulose, polyethylene glycol), stimulants (bisacodyl, senna), bulking agents (methylcellulose, psyllium) and stool softeners (anionic surfactants). Studies comparing different laxative regimens in patients with OIC are very limited. Although traditional laxatives have proven useful in inducing bowel movements, there is no convincing evidence to suggest which, if any, laxative is optimal for OIC [Ahmedzai and Boland, 2010; Camilleri et al. 2014; Candy et al. 2011]. The few clinical trials comparing laxatives conclude that commonly used agents have comparable, suboptimal efficacy for OIC [Agra et al. 1998; Freedman et al. 1997; Ramesh et al. 1998; Ruston et al. 2013]. This consideration is also supported by a study in patients with chronic pain who reported their bowel habits before and after initiating treatment with oral opioids. All had laxatives prescribed together with opioids, and almost half were using two or more different types. Prior to opioid treatment, 70% reported at least three SBMs per week. After initiating oral opioid therapy, 55% reported having at least three bowel movements per week, but 81% still reported constipation as an opioid-induced adverse effect [Bell et al. 2009]. For further details about mechanisms and recommendations for laxatives, see studies by Lembo and Camilleri, and Rao [Lembo and Camilleri, 2003; Rao, 2007].
However, as stated earlier, the pronounced focus on bowel movements as outcome measure in older studies evaluating the effect of laxatives on OIC makes it difficult to assess their effect on other presentations of OIBD.
Chloride channel activator
Lubiprostone is derived from prostaglandin E1 and acts by specifically activating the CIC-2 chloride channels on the apical side of GI epithelial cells. It induces chloride secretion and thereby softens stool consistency [Lacy and Chey, 2009; Owen, 2008]. It was originally indicated for chronic constipation and irritable bowel syndrome with constipation, where effect of treatment was demonstrated by an increase in SBM frequency, but more importantly, stool consistency improved, and straining, bloating and severity of constipation decreased [Owen, 2008; Wong and Camilleri, 2011].
In 2013, lubiprostone was also approved in the USA for treatment of OIC in adult patients with noncancer pain [Camilleri et al. 2014]. Significant effect was demonstrated on the primary combined efficacy endpoint in a randomized, placebo-controlled phase III trial with an approximate number needed to treat of six. Furthermore, straining, stool consistency and constipation severity were also significantly improved in the lubiprostone group [Mazen Jamal et al. 2012]. One drawback is that methadone inhibits lubiprostone-induced chloride secretion in in vitro enterocytes, and although speculative it may have little or no effect in methadone-treated patients [Cuppoletti et al. 2013].
Selective 5-HT4 agonist
Prucalopride is a selective 5-HT4 agonist that alters colonic motility via serotonin 5-HT4 receptors in the gut. Primarily indicated and approved in many countries for chronic idiopathic constipation in women, but has demonstrated efficacy in patients with OIC in one randomized controlled trial from 2010 [Sloots et al. 2010]. However, the effect was only significant at 2 weeks of treatment but not after 4 weeks and the drug is not approved for OIC. However, it cannot be excluded to be effective in some patients when prescribed off label.
Tapentadol
Another approach to minimize the GI adverse effects of opioid treatment is to use opioids with additional effects, such as tapentadol. Besides µ-opioid receptor agonism, it has a noradrenergic reuptake inhibitory action that results in an additional analgesic effect [Tzschentke et al. 2009; Wade and Spruill, 2009]. Consequently for an equianalgesic dose, fewer opioid receptors (including those in the gut) are blocked, thereby improving the adverse effect profile [Afilalo and Morlion, 2013]. In an animal model it was demonstrated that nausea and vomiting were markedly reduced after tapentadol administration compared with equianalgesic doses of morphine [Tzschentke et al. 2009]. These results have been confirmed in a number of clinical studies in which tapentadol has demonstrated a superior GI tolerability profile and fewer treatment discontinuations compared with oxycodon [Buynak et al. 2010; Steigerwald et al. 2013; Wade and Spruill, 2009; Wild et al. 2010]. However, it is a relatively new drug for which more clinical experience is needed, and comparison to other opioids and the new peripheral-acting opioid antagonists is still lacking.
Opioid antagonists
In contrast to other treatment strategies for OIBD, competitive opioid antagonists target the underlying pathophysiology: blockage of the µ-opioid receptors in the gut. This drug class possesses the majority of alternatives, mainly separated by their pharmacokinetic properties.
Naloxone is a pure competitive antagonist. As a potent antidote, it is often administered intravenously or as an intramuscular injection to treat opioid overdose. Hereby, naloxone antagonizes both the centrally and peripherally mediated effects of opioids. However, when given orally a substantial amount of the drug reaches the systemic circulation, and has the capacity to cross the blood–brain barrier and cause reversal of the centrally mediated analgesia and opioid withdrawal symptoms. This is the main reason why orally formulated naloxone is not marketed as a standalone product to treat OIBD, despite improvement of GI symptoms [Meissner et al. 2000; Vondrackova et al. 2008].
Consequently, peripheral restriction is a crucial property for an opioid antagonist to be a successful candidate in the treatment of OIBD. One approach has been a combined oral prolonged release formulation of oxycodone and prolonged release naloxone in a 2:1 ratio. The aim of this formulation has been to counteract OIBD through the local antagonistic effect of naloxone in the gut, while maintaining peripheral and central analgesia due to the low bioavailability (<2%) of oral, prolonged release, low-dose naloxone [Smith et al. 2012]. Studies have shown promising analgesic efficacy as well as improvement in OIBD-related symptoms [Burness and Keating, 2014; Leppert, 2013a; Sykes, 1996]. However, as naloxone is primarily metabolized in the liver, there is a risk of increased bioavailability in patients with severe hepatic impairment [Kraft, 2008; Leppert, 2013b]. Furthermore, the maximum recommended daily dose may not be sufficient to relieve the pain. Additionally, the fixed combination to oxycodone necessitates opioid rotation in patients who are treated with other opioids, and although recommendations exist this may be difficult outside specialist centers [Drewes et al. 2013].
Methylnaltrexone bromide, a drug originally designed to shorten the length of postoperative ileus, is another approach [Portenoy et al. 2008]. It is a peripherally acting µ-opioid receptor antagonist and a derivative of the opioid antagonist naltrexone with an ammonium group that restricts it to the periphery [Herndon et al. 2002]. Methylnaltrexone bromide has been shown to relieve OIC and induce laxation [Schmidt, 2001; Thomas et al. 2008], and was the first peripherally acting µ-opioid receptor antagonist to be approved for the treatment of OIBD. However, it is only available in a subcutaneous formulation and only approved in palliative care in patients with advanced illness, and therefore it is of limited benefit for the general population with OIBD.
Alvimopan is another oral peripherally acting µ-opioid receptor antagonist that has been shown to increase the number of SBMs in opioid-treated patients [Camilleri, 2005; Paulson et al. 2005; Roberts et al. 2002]. However, cardiovascular safety concerns (increased risk of myocardial infarction) halted further development. Yet, the US Food and Drug Administration approved alvimopan for postoperative ileus following partial small or large bowel resection with primary anastomosis in hospitalized patients. It is only registered in the USA and hence it is of little benefit to the general OIBD population.
Naloxegol is a pegylated naloxone molecule. Pegylation is a process where a polyethylene glycol (PEG) moiety is attached to a therapeutically useful molecule in order to alter functionality and structural properties [Roberts et al. 2002]. Due to the pegylation the molecule is too large to pass the blood–brain barrier and is peripherally restricted [Webster et al. 2013]. It is administered orally once a day, but the key advantage is that it can be added to existing opioid therapy and thereby also allows for opioid rotation. It has proven efficacious compared with placebo on a number of different outcome measures [Chey et al. 2014] and has an acceptable safety profile [Bui et al. 2014a, 2014b; Webster et al. 2014, n.d.].
Other peripherally acting µ-opioid receptor antagonists in earlier stages of development are ADL-5945 and ADL-7445 (Cubist, Lexington, Massachusetts, USA) and TD-1211 (Theravance Biopharma Inc., San Francisco, California, USA). ADL-5945 and ADL-7445 have proven tolerable and effective in producing SBMs in phase I trials, but to the authors’ knowledge no phase II data have been published, even though a phase II study was announced in 2010 [Herndon et al. 2002]. The TD-1211 has been shown to be well tolerated and has a linear pharmacokinetic profile, but is still being evaluated in phase II trials [Belsey et al. 2010; Herndon et al. 2002].
Conclusion
Opioid consumption is increasing. Despite this, pain resulting from advanced disease remains undertreated due to fear of addiction and concerns related to adverse effects. OIC is the most well recognized GI adverse effect to opioid treatment, but other potentially more bothersome GI symptoms, collectively referred to as OIBD, are just as common and frequently overlooked by clinicians.
Traditional treatment, combining laxatives with dietary and lifestyle changes, is often insufficient as it does not target the underlying problem. However, new approaches, such as prucalopride and lubiprostone, opioids with effects on the monaminergic systems and drugs targeting the underlying pathophysiology with peripheral restricted opioid antagonists are emerging. That said, a substantial limitation of prior studies is the considerable diversity of definitions and outcome measures used, making it difficult to compare studies evaluating different treatments. However, an international multidisciplinary working group has recently suggested a consensus definition for OIC and in case similar definitions can be made for OIBD, it would be an important step forward not only making it easier to compare traditional treatments with the new developments, but also to simplify clinical practice.
Footnotes
Author contributions: All authors contributed equally to this review.
Funding: The authors have received financial support from Innovation Fund Denmark.
Conflict of interest statement: Asbjørn Mohr Drewes has received unrestricted research grants from Mundipharma, AstraZeneca, Grünenthal, Lundbeck and Pfizer and served as a Consultant/Advisory Board member for Mundipharma, Grünenthal, AstraZeneca, Almirall and Shire. The authors report no other conflicts of interest in this work.
Contributor Information
Jakob Lykke Poulsen, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark.
Christina Brock, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark.
Anne Estrup Olesen, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark.
Matias Nilsson, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark.
Asbjørn Mohr Drewes, Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Mølleparkvej 4, DK-9000 Aalborg, Denmark.
References
- Afilalo M., Morlion B. (2013) Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician 16: 27–40. [PubMed] [Google Scholar]
- Agra Y., Sacristán A., González M., Ferrari M., Portugués A., Calvo M. (1998) Efficacy of senna versus lactulose in terminal cancer patients treated with opioids. J Pain Symptom Manage 15: 1–7. [DOI] [PubMed] [Google Scholar]
- Ahmedzai S., Boland J. (2010) Constipation in people prescribed opioids. Clin Evid 2010: 2407. [PMC free article] [PubMed] [Google Scholar]
- Allan L., Hays H., Jensen N., de Waroux B., Bolt M., Donald R., et al. (2001) Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ 322: 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T., Panchal S., Miaskowski C., Bolge S., Milanova T., Williamson R. (2009) The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 10: 35–42. [DOI] [PubMed] [Google Scholar]
- Belsey J., Geraint M., Dixon T. (2010) Systematic review and meta analysis: polyethylene glycol in adults with non-organic constipation. Int J Clin Pract 64: 944–955. [DOI] [PubMed] [Google Scholar]
- Benyamin R., Trescot A., Datta S., Buenaventura R., Adlaka R., Sehgal N., et al. (2008) Opioid complications and side effects. Pain Physician 11: S105–S120. [PubMed] [Google Scholar]
- Bril S., Shoham Y., Marcus J. (2011) The ‘mystery’ of opioid-induced diarrhea. Pain Res Manag 16: 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C., Olesen S., Olesen A., Frøkjaer J., Andresen T., Drewes A. (2012) Opioid-induced bowel dysfunction: Pathophysiology and management. Drugs 72: 1847–1865. [DOI] [PubMed] [Google Scholar]
- Bui K., She F., Sostek M. (2014a) The effects of renal impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J Clin Pharmacol 54: 1375–1382. [DOI] [PubMed] [Google Scholar]
- Bui K., She F., Sostek M. (2014b) The effects of mild or moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J Clin Pharmacol 54: 1368–1374. [DOI] [PubMed] [Google Scholar]
- Burness C., Keating G. (2014) Oxycodone/naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs 74: 353–375. [DOI] [PubMed] [Google Scholar]
- Buynak R., Shapiro D., Okamoto A., Van Hove I., Rauschkolb C., Steup A., et al. (2010) Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother 11: 1787–1804. [DOI] [PubMed] [Google Scholar]
- Camilleri M. (2005) Alvimopan, a selective peripherally acting mu-opioid antagonist. Neurogastroenterol Motil 17: 157–165. [DOI] [PubMed] [Google Scholar]
- Camilleri M. (2011) Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 106: 835–842; quiz 843. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Drossman D., Becker G., Webster L., Davies A., Mawe G. (2014) Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 26: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy B., Jones L., Goodman M., Drake R., Tookman A. (2011) Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Sao Paulo Med J 129: 277. [DOI] [PubMed] [Google Scholar]
- Casati A., Sedefov R., Pfeiffer-Gerschel T. (2012) Misuse of medicines in the European union: A systematic review of the literature. Eur Addict Res 18: 228–245. [DOI] [PubMed] [Google Scholar]
- Chey W., Webster L., Sostek M., Lappalainen J., Barker P., Tack J. (2014) Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 370: 2387–2396. [DOI] [PubMed] [Google Scholar]
- Choung R., Locke G., Zinsmeister A., Schleck C., Talley N. (2009) Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 104: 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Currow D. (2013) Constipation in palliative care: what do we use as definitions and outcome measures? J Pain Symptom Manage 45: 753–762. [DOI] [PubMed] [Google Scholar]
- Cook S., Lanza L., Zhou X., Sweeney C., Goss D., Hollis K., et al. (2008) Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 27: 1224–1232. [DOI] [PubMed] [Google Scholar]
- Corsetti M., Tack J. (2015) Naloxegol, a new drug for the treatment of opioid-induced constipation. Expert Opin Pharmacother 16: 399–406. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J., Chakrabarti J., Tewari K., Malinowska D. (2013) Methadone but not morphine inhibits lubiprostone-stimulated Cl- currents in T84 intestinal cells and recombinant human ClC-2, but not CFTR Cl- currents. Cell Biochem Biophys 66: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper H., Cremonini F., Park M., Camilleri M. (2004) Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil 16: 383–394. [DOI] [PubMed] [Google Scholar]
- Diego L., Atayee R., Helmons P., Hsiao G., von Gunten C.F. (2011) Novel opioid antagonists for opioid-induced bowel dysfunction. Expert Opin Investig Drugs 20: 1047–1056. [DOI] [PubMed] [Google Scholar]
- Dorn S., Lembo A., Cremonini F. (2014) Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol 2: 31–37. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi K., Evander A., Walther B., Skinner D. (1985) Influence of morphine on the distal oesophagus and the lower oesophageal sphincter – a manometric study. Gut 26: 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes A., Jensen R., Nielsen L., Droney J., Christrup L., Arendt-Nielsen L., et al. (2013) Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol 75: 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman D., Szigethy E. (2014) The narcotic bowel syndrome: a recent update. Am J Gastroenterol 2: 22–30. [DOI] [PubMed] [Google Scholar]
- Eldon M., Song D., Neumann T. (2007) Oral NKTR-118 (oral PEG-naloxol), a PEGylated derivative of naloxone; demonstration of selective peripheral opioid antagonism after oral administration. Poster session presented at: The American Academy of Pain Management 18th Annual Clinical Meeting, 27–30 September, Las Vegas, NV. [Google Scholar]
- Frank L., Kleinman L., Farup C., Taylor L., Miner P. (1999) Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 34: 870–877. [DOI] [PubMed] [Google Scholar]
- Frantzides C., Cowles V., Salaymeh B., Tekin E., Condon R. (1992) Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg 163: 144–148; discussion 148–149. [DOI] [PubMed] [Google Scholar]
- Freedman M., Schwartz H., Roby R., Fleisher S. (1997) Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. J Clin Pharmacol 37: 904–907. [DOI] [PubMed] [Google Scholar]
- Furness J., Costa M. (1987) The enteric nervous system. London, UK: Churchill Livingstone. [Google Scholar]
- Gaertner J., Siemens W., Camilleri M., Davies A., Drossman D., Webster L., et al. (2015) Definitions and outcome measures of clinical trials regarding opioid-induced constipation a systematic review. J Clin Gastroenterol 49: 9–16. [DOI] [PubMed] [Google Scholar]
- Galligan J., Akbarali H. (2014) Molecular physiology of enteric opioid receptors. Am J Gastroenterol 2: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glare P., Walsh D., Sheehan D. (2006) The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care 23: 229–235. [DOI] [PubMed] [Google Scholar]
- Göke M., Ewe K., Donner K., Meyer zum Büschenfelde K. (1992) Influence of loperamide and loperamide oxide on the anal sphincter. A manometric study. Dis Colon Rectum 35: 857–861. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B., Gardner C., Little P., Hicks G., Dehaven-Hudkins D. (2004) Preclinical studies of opioids and opioid antagonists on gastrointestinal function. Neurogastroenterol Motil 16(Suppl. 2): 46–53. [DOI] [PubMed] [Google Scholar]
- Grunkemeier D., Cassara J., Dalton C., Drossman D. (2007) The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 5: 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm J., Venu R., Geenen J., Hogan W., Dodds W., Toouli J., et al. (1988) Effects of morphine on the human sphincter of Oddi. Gut 29: 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon C., Jackson K., Hallin P. (2002) Management of opioid-induced gastrointestinal effects in patients receiving palliative care. Pharmacotherapy 22: 240–250. [DOI] [PubMed] [Google Scholar]
- Higgins P., Johanson J. (2004) Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 99: 750–759. [DOI] [PubMed] [Google Scholar]
- Holzer P. (2004) Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett 361: 192–195. [DOI] [PubMed] [Google Scholar]
- Holzer P. (2014) Pharmacology of opioids and their effects on gastrointestinal function. Am J Gastroenterol 2(1): 9–16. [Google Scholar]
- Huizinga J., Lammers W. (2009) Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol 296, G1–G8. [DOI] [PubMed] [Google Scholar]
- Johanson J., Kralstein J. (2007) Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 25: 599–608. [DOI] [PubMed] [Google Scholar]
- Kalso E., Edwards J., Moore R., McQuay H. (2004) Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 112: 372–380. [DOI] [PubMed] [Google Scholar]
- Kraft M. (2008) Methylnaltrexone, a new peripherally acting mu-opioid receptor antagonist being evaluated for the treatment of postoperative ileus. Expert Opin Investig Drugs 17: 1365–1377. [DOI] [PubMed] [Google Scholar]
- Kraichely R., Arora A., Murray J. (2010) Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 31: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz A., Sessler D. (2003) Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 63: 649–671. [DOI] [PubMed] [Google Scholar]
- Lacy B., Chey W. (2009) Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother 10:143–152. [DOI] [PubMed] [Google Scholar]
- Lembo A., Camilleri M. (2003) Chronic constipation. N Engl J Med 349: 1360–1368. [DOI] [PubMed] [Google Scholar]
- Leppert W. (2013a) The place of oxycodone/naloxone in chronic pain management. Contemp Oncol (Pozn) 17: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert W. (2013b) New treatment possibilities for opioid-induced bowel dysfunction. Pain 154: 1491–1492. [DOI] [PubMed] [Google Scholar]
- Lewis S., Heaton K. (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: 920–924. [DOI] [PubMed] [Google Scholar]
- Looström H., Akerman S., Ericson D., Tobin G., Götrick B. (2011) Tramadol-induced oral dryness and pilocarpine treatment: effects on total protein and IgA. Arch Oral Biol 56: 395–400. [DOI] [PubMed] [Google Scholar]
- Lötsch J., Skarke C., Liefhold J., Geisslinger G. (2004) Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet 43: 983–1013. [DOI] [PubMed] [Google Scholar]
- Löwenstein O., Leyendecker P., Hopp M., Schutter U., Rogers P., Uhl R., et al. (2009) Combined prolonged-release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate-to-severe non-malignant chronic pain: a randomised controlled trial. Expert Opin Pharmacother 10: 531–543. [DOI] [PubMed] [Google Scholar]
- Mazen Jamal M., Mareya S., Woldegeorgis F., Joswick T., Joswick R. (2012) Lubiprostone significantly improves treatment response in non-methadone opioid-induced bowel dysfunction patients with chronic, non-cancer pain: results from a phase 3, randomized, double-blind, placebo-controlled clinical trial. Gastroenterology 142: S-144–S-145. [Google Scholar]
- Meissner W., Schmidt U., Hartmann M., Kath R., Reinhart K. (2000) Oral naloxone reverses opioid-associated constipation. Pain 84: 105–109. [DOI] [PubMed] [Google Scholar]
- Moore R., McQuay H. (2005) Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 7: R1046–R1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial F., Enck P., Kalveram K., Erckenbrecht J. (1992) The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol 15: 321–324. [DOI] [PubMed] [Google Scholar]
- NICE (2012) Opioids in palliative care: safe and effective prescribing of strong opioids for pain in palliative care of adults. Clinical Guideline 140. London: NICE; Available at: http://www.nice.org.uk/guidance/cg140/evidence/cg140-opioids-in-palliative-care-full-guideline3 (accessed 20 May 2015). [Google Scholar]
- Olesen A., Drewes A. (2011) Validated tools for evaluating opioid-induced bowel dysfunction. Adv Ther 28: 279–294. [DOI] [PubMed] [Google Scholar]
- Owen R. (2008) Lubiprostone – a novel treatment for irritable bowel syndrome with constipation. Drugs Today (Barc) 44: 645–652. [DOI] [PubMed] [Google Scholar]
- Pappagallo M. (2001) Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 182: 11S–18S. [DOI] [PubMed] [Google Scholar]
- Paulson D., Kennedy D., Donovick R., Carpenter R., Cherubini M., Techner L., et al. (2005) Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction - A 21-day treatment-randomized clinical trial. J Pain 6: 184–192. [DOI] [PubMed] [Google Scholar]
- Portenoy R., Thomas J., Moehl Boatwright M., Tran D., Galasso F., Stambler N., et al. (2008) Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage 35: 458–468. [DOI] [PubMed] [Google Scholar]
- Poulsen J., Brock C., Olesen A., Nilsson M., Drewes A. (2014) Clinical potential of naloxegol in the management of opioid-induced bowel dysfunction. Clin Exp Gastroenterol 7: 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh P., Kumar K., Rajagopal M., Balachandran P., Warrier P. (1998) Managing morphine-induced constipation: a controlled comparison of an Ayurvedic formulation and senna. J Pain Symptom Manage 16: 240–244. [DOI] [PubMed] [Google Scholar]
- Rao S. (2007) Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am 36; 687–711. [DOI] [PubMed] [Google Scholar]
- Rentz A., Yu R., Müller-Lissner S., Leyendecker P. (2009) Validation of the bowel function index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ 12: 371–383. [DOI] [PubMed] [Google Scholar]
- Roberts M., Bentley M., Harris J. (2002) Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev 54: 459–476. [DOI] [PubMed] [Google Scholar]
- Ruston T., Hunter K., Cummings G., Lazarescu A. (2013) Efficacy and side-effect profiles of lactulose, docusate sodium, and sennosides compared to PEG in opioid-induced constipation: a systematic review. Can Oncol Nurs J 23: 236–246. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Bercik P. (2011) Epidemiology and burden of chronic constipation. Can J Gastroenterol 25(Suppl. B): 11B–15B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna S., Otterson M. (1990) Small intestinal amyogenesia and dysmyogenesia induced by morphine and loperamide. Am J Physiol 258: G282–G289. [DOI] [PubMed] [Google Scholar]
- Schmidt W. (2001) Alvimopan* (ADL 8-2698) is a novel peripheral opioid antagonist. Am J Surg 182: 27S–38S. [DOI] [PubMed] [Google Scholar]
- Sharma S. (2002) Sphincter of Oddi dysfunction in patients addicted to opium: an unrecognized entity. Gastrointest Endosc 55: 427–430. [DOI] [PubMed] [Google Scholar]
- Sharma S., Nirenberg M., Klee W. (1975) Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A 72: 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson K., Leyendecker P., Hopp M., Müller-Lissner S., Löwenstein O., De Andrés J., et al. (2008) Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain. Curr Med Res Opin 24: 3503–3512. [DOI] [PubMed] [Google Scholar]
- Slappendel R., Simpson K., Dubois D., Keininger D. (2006) Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 10: 209–217. [DOI] [PubMed] [Google Scholar]
- Sloots C., Rykx A., Cools M., Kerstens R., De Pauw M. (2010) Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig Dis Sci 55: 2912–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Hopp M., Mundin G., Bond S., Bailey P., Woodward J., et al. (2012) Low absolute bioavailability of oral naloxone in healthy subjects. Int J Clin Pharmacol Ther 50: 360–367. [DOI] [PubMed] [Google Scholar]
- Stamer U., Bayerer B., Stüber F. (2005) Genetics and variability in opioid response. Eur J Pain 9: 101–114. [DOI] [PubMed] [Google Scholar]
- Steigerwald I., Schenk M., Lahne U., Gebuhr P., Falke D., Hoggart B. (2013) Effectiveness and tolerability of tapentadol prolonged release compared with prior opioid therapy for the management of severe, chronic osteoarthritis pain. Clin Drug Investig 33: 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C., Patierno S., Selmer I., Kirchgessner A. (2004) The opioid system in the gastrointestinal tract. Neurogastroenterol Motil 16(Suppl. 2): 3–16. [DOI] [PubMed] [Google Scholar]
- Sykes N. (1996) An investigation of the ability of oral naloxone to correct opioid-related constipation in patients with advanced cancer. Palliat Med 10: 135–144. [DOI] [PubMed] [Google Scholar]
- Telford G., Condon R., Szurszewski J. (1989) Opioid receptors and the initiation of migrating myoelectric complexes in dogs. Am J Physiol 256: G72–G77. [DOI] [PubMed] [Google Scholar]
- Thomas J. (2008) Opioid-induced bowel dysfunction. J Pain Symptom Manage 35: 103–113. [DOI] [PubMed] [Google Scholar]
- Thomas J., Karver S., Cooney G., Chamberlain B., Watt C., Slatkin N., et al. (2008) Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 358: 2332–2343. [DOI] [PubMed] [Google Scholar]
- Torres D., Parrinello G., Trapanese C., Licata G. (n.d.) Sudden severe abdominal pain after a single low dose of paracetamol/codein in a cholecystectomized patient: learning from a case report. Am J Ther 17: e133–e134. [DOI] [PubMed] [Google Scholar]
- Tuteja A., Biskupiak J., Stoddard G., Lipman A. (2010) Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 22: 424–430, e96. [DOI] [PubMed] [Google Scholar]
- Tzschentke T., Jahnel U., Kögel B., Christoph T., Englberger W., De Vry J., et al. (2009) Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today 45: 483–496. [DOI] [PubMed] [Google Scholar]
- Vondrackova D., Leyendecker P., Meissner W., Hopp M., Szombati I., Hermanns K., et al. (2008) Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J Pain 9: 1144–1154. [DOI] [PubMed] [Google Scholar]
- Wade W., Spruill W. (2009) Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther 31: 2804–2818. [DOI] [PubMed] [Google Scholar]
- Webster L., Chey W., Tack J., Lappalainen J., Barker P., Tummala R., et al. (n.d.) Efficacy and safety of naloxegol in patients with opioid-induced constipation: results from 2 identical phase 3, prospective, randomized, multicenter, double-blind, placebo-controlled trials. Poster session presented at: PAINWeek; 4–7 September 2013; Las Vegas, NV, USA. [Google Scholar]
- Webster L., Chey W., Tack J., Lappalainen J., Diva U., Sostek M. 2014. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther 40: 771–779. [DOI] [PubMed] [Google Scholar]
- Webster L., Dhar S., Eldon M., Masuoka L., Lappalainen J., Sostek M. 2013. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid-induced constipation. Pain 154: 1542–1550. [DOI] [PubMed] [Google Scholar]
- Webster L., Jansen J., Peppin J., Lasko B., Irving G., Morlion B., et al. (2008) Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain 137: 428–440. [DOI] [PubMed] [Google Scholar]
- Wild J., Grond S., Kuperwasser B., Gilbert J., McCann B., Lange B., et al. (2010) Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract 10: 416–427. [DOI] [PubMed] [Google Scholar]
- Wirz S., Nadstawek J., Elsen C., Junker U., Wartenberg H. (2012) Laxative management in ambulatory cancer patients on opioid therapy: a prospective, open-label investigation of polyethylene glycol, sodium picosulphate and lactulose. Eur J Cancer Care (Engl) 21: 131–140. [DOI] [PubMed] [Google Scholar]
- Wojciech L. (2012) Are we able to manage effectively opioid-induced bowel dysfunction? J Autacoids 1: 1–2. [Google Scholar]
- Wong B., Camilleri M. (2011) Lubiprostone for the treatment of opioid-induced bowel dysfunction. Expert Opin Pharmacother 12: 983–990. [DOI] [PubMed] [Google Scholar]
- Wood J., Galligan J. (2004) Function of opioids in the enteric nervous system. Neurogastroenterol Motil 16(Suppl. 2): 17–28. [DOI] [PubMed] [Google Scholar]