Abstract
Microbubbles were initially invented as contrast agents for ultrasound imaging. However, lately more and more therapeutic applications of microbubbles are emerging, mostly related to drug and gene delivery. Ultrasound is a safe and noninvasive therapeutic modality which has the unique ability to interact with microbubbles and release their payload in situ in addition to permeabilizing the target tissues. The combination of drug-loaded microbubbles and ultrasound has been used in preclinical studies on blood–brain barrier opening, drug and gene delivery to solid tumors, and ablation of blood vessels. This review covers the basic principles of ultrasound–microbubble interaction, the types of microbubbles and the effect they have on tissue, and the preclinical and clinical experience with this approach to date in the field of gastrointestinal oncology.
Keywords: liver tumors, microbubbles, oncology, pancreatic cancer, review, ultrasound
Introduction
Diagnostic ultrasound is widely used today for imaging in just about any medical discipline, including oncology. Diagnostic ultrasound probes transmit plane or divergent ultrasound waves that get reflected or scattered by tissue inhomogeneities and are then detected by the same probe. It thus relies on the contrast in scattering between different types of tissue. Since tumors do not always provide enough contrast, injection of highly reflective contrast agents may enhance some of the structures of interest (e.g. neovascularization or blood flow).
The natural choice for a highly reflective ultrasound target is an air bubble, and the first publication of the contrast ultrasound technique described the application of bubbles produced by rapid intracardiac saline injections to enhance delineation of aortic blood flow [Gramiak and Shah, 1968]. However, such in situ generated microbubbles dissolved within a few seconds due to the high solubility of air in blood [Dayton et al. 1999]. Over two decades later, the first generation ultrasound contrast agent – Albunex – became commercially available [Keller et al. 1989]. These bubbles were approximately 4 µm in diameter and utilized a stabilizing albumin shell which improved circulation time substantially over unencapsulated microbubbles. Various compositions have since been used to produce an encapsulating shell, including proteins, lipids and polymers. The commercially produced contrast agents Optison™ (Mallinckrodt, San Diego, CA, USA) and Definity (DuPont Pharmaceuticals Co., North Billerica, MA, USA), which are currently the only two US Food and Drug Administration (FDA) approved agents in the US still in production, utilize albumin and phospholipid encapsulation, respectively [Goldberg et al. 2001]. The composition of the gas core is also critical in preventing osmotically driven size changes of the microbubbles under physiological conditions [Ibsen et al. 2013]. Commercial formulations of the inner gas core have included air (Albunex®), sulfur hexafluoride (SonoVue®, Bracco, SpA, Milan, Italy) or perfluorocarbons (Definity®, Lantheus Medical Imaging, North Billerica, MA, USA). During a contrast imaging exam, a solution of microbubbles is injected through a peripheral vein. Typically, the microbubbles remain in circulation on the order of several minutes before their gas content dissolves into the blood and is rapidly cleared through exhalation from the lungs.
Microbubble interaction with ultrasound can result in a rich diversity of responses dictated primarily by the amplitude of the ultrasound wave. At low acoustic pressures, microbubbles shrink and expand in response to the alternating cycles of compression and rarefaction in the incident wave: a phenomenon known as stable cavitation. Stably oscillating bubbles efficiently scatter ultrasound energy; detection of the scattered waves enables visualization of the bubble distribution in the vasculature [Martin and Dayton, 2013]. This regime is desirable for most diagnostic purposes. The other regime termed nonstable or inertial cavitation is realized at higher acoustic pressures, when the diameter of the microbubble increases by more than 2-fold during the expansion phase [Qin et al. 2009]. This growth is unstable and leads to a rapid and violent collapse during the compressional cycle, resulting in destruction and fragmentation of the bubble. The latter phenomenon is accompanied by microstreaming, jetting and ultrasound shock wave emission which have the ability to rupture the membranes of the adjacent cells and create pores in the capillary walls [Tzu-Yin et al. 2013]. This destructive type of bubble behavior defines the main therapeutic applications of ultrasound contrast agents – permeabilization of tissue and/or vasculature with the aim of targeted drug or gene delivery.
This review covers the physical mechanisms associated with microbubble–ultrasound interaction which facilitates the drug delivery, the available preclinical studies relevant to gastrointestinal oncology and the early clinical results.
Types of microbubbles and their effect on tissue
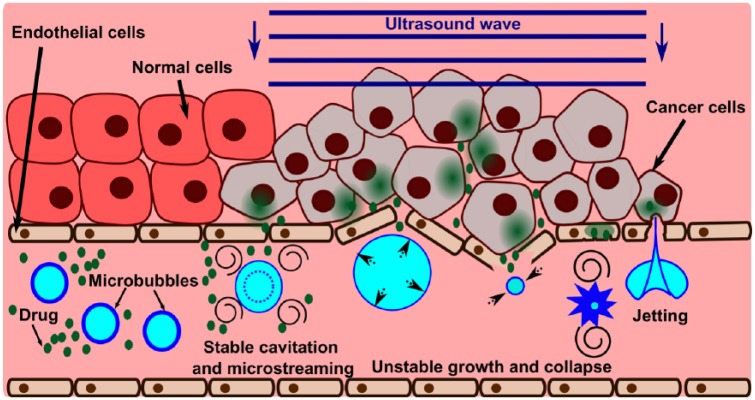
The basic principles of enhancing drug delivery through ultrasound-driven microbubble activity are shown in Figure 1. The microbubbles entering the ultrasound beam undergo oscillations, thus creating fluid flow around them. This microstreaming facilitates drug convection within the vasculature and enhances the extravasation of the drug into surrounding tissue. In the case of leaky tumor vasculature, microstreaming may augment the enhanced permeability and retention (EPR) effect. Furthermore, collapses of the bubbles undergoing inertial cavitation can rupture the capillary walls and endothelial cell membranes through a variety of mechanisms including: shear stress from enhanced microstreaming around the collapsing bubble; mechanical stresses on the vessel wall caused by the extension and invagination of the vessel by the expanding and collapsing bubble [Chen et al. 2011]; and endothelial disruption due to liquid jetting that occurs with asymmetric bubble collapse [Hwang et al. 2005]. The therapeutic agent can then extravasate through the permeabilized vasculature into the interstitium. Over time, with further ultrasound pulses the drug has a chance to spread further away from the vessel and into the targeted tissue. Although the drug that is not taken up by the targeted tissue remains in the circulation, the resulting systemic dose is smaller than that encountered by simple drug injection.
Figure 1.
Illustration of the physical mechanisms of microbubble-enhanced ultrasound drug delivery. At lower ultrasound intensities (e.g. at the edge of the beam), bubbles may undergo slight (up to about two-fold) oscillations in size – stable cavitation. This process causes fluid flow around the bubble (microstreaming) which may provide means for active convection in the vessel and increase the extravasation of the drug. At higher ultrasound intensities, the bubble undergoes an unstable growth followed by a rapid collapse that causes the distension and invagination of the vessel wall, respectively. These deformations are associated with high mechanical stress and damage to the endothelial lining of the vessel, thus allowing the drug to penetrate beyond the vessel wall. The bubble collapse is also accompanied by enhanced microstreaming and formation of liquid jets that may impinge on the vessel wall and disrupt cell membranes.
The above description implies co-administration of drug and microbubbles. Lately, several other configurations of microbubbles were proposed and tested in preclinical trials for applications in oncology: drugs or DNA loaded on top of or inside the microbubble shell. This allows further reduction of nonspecific accumulation of chemotherapeutics in healthy tissue, while achieving enhanced drug penetration into the tumor. Several drugs which have been evaluated for microbubble delivery to date include paclitaxel, doxorubicin, rapamycin and 10-hydroxycamptothecin [Martin and Dayton, 2013]. Several groups have explored echogenic liposomes (or acoustically-active liposomes) which contain small pockets of gas and therefore can provide image contrast and/or can be triggered to release therapeutic contents by the influence of ultrasound [Nahire et al. 2012; Radkhakarishnan et al. 2012]. Others have developed targeted perfluorocarbon nanodroplets that increase in echogenicity as they accumulate in sites of atherosclerosis and angiogenesis and can be made to carry therapeutic payloads [Lanza et al. 2010].
Many recent studies have focused on phase change contrast agents (PCCAs), which combine the functional similarity of microbubbles with unique delivery and activation possibilities. PCCAs are encapsulated droplets with liquid perfluorocarbon cores capable of being triggered to the gaseous phase when stimulated with acoustic energy from an ultrasound transducer [Kripfgans et al. 2000]. Once vaporized, PCCAs expand to form bubbles on the order of 5–6 times larger than the precursor droplets [Sheeran and Dayton, 2012]. As a result, the advantages of each state (i.e. stability and small size in liquid state, echogenicity and ultrasound interaction in gas state) can be utilized efficiently. PCCAs have been produced by several groups with mean diameters both in the microscale and nanoscale for a variety of unique approaches not possible with standard microbubble agents [Rapoport, 2012].
One other drug delivery vehicle that stands apart in its activation mechanism is temperature-sensitive liposomes (TSLs). Upon injection, these long circulating liposomal drug carriers are taken up in the tumor mediated by the EPR effect. The drug remains encapsulated in the aqueous lumen of the TSL at body temperature, but when heated up to temperatures in the range of 40–4 °C (hyperthermia), the drug is released. Local heating of the tumor can be established using a number of techniques (radiofrequency, light, water bath) including high intensity focused ultrasound (HIFU), which has an added advantage of being completely noninvasive. Thermodox® TSLs have reached the clinical trial phase for several applications in oncology, including radiofrequency (RF) ablation of hepatocellular carcinoma (HCC) primary liver tumors and liver metastasis. Detailed review of TSLs properties is beyond the scope of this article and can be found elsewhere [Hijnen et al. 2014].
Ultrasound devices for performing and monitoring drug and gene delivery treatments
Classes of ultrasound devices that have been used for drug and gene delivery, together with the relevant ultrasound exposure parameters, are illustrated in Figure 2. The most widely used device is the conventional ultrasound imaging probe, which combines the capability of both delivering and targeting the treatment. This approach utilizes the fact that microbubbles can undergo inertial cavitation at diagnostic-level pressures, usually described in this case in terms of mechanical index (MI): , where is the peak rarefactional pressure and is the ultrasound frequency [Qin et al. 2009]. The highest FDA-approved MI of 1.2 is a common setting for the color Doppler mode of an ultrasound imaging system. Therefore, during treatment the probe is positioned so that the Doppler region of interest coincides with the target and the Doppler pulses (usually 3–5 cycles) are delivered line by line, similarly to how an ultrasound image is formed (see Figure 2a). This approach is attractive due to the ease of clinical translation and wide availability of ultrasound imaging probes. The major disadvantage, however, is the lack of control over the in situ acoustic pressure in targeted tissue: deep tissues will receive lower amplitude pulses due to acoustic attenuation. This may lead to diminished bioeffects in deeper tissues. In addition, even the highest MI provided by conventional probes may not be sufficient for certain applications, in which more violent bubble activity is needed, such as gene delivery. In order to overcome these drawbacks, planar (Figure 2c) or spherically focused (Figure 2b) custom-built single-element transducers are used. Focused transducers allow concentration of ultrasound energy in a small elliptically shaped focal area (1–3 mm laterally, 5–10 mm axially) thus affecting only the tissue at the focus. Control over the transducer power allows tailoring of the in situ focal intensity depending on the depth of the target. This solution, however, may be impractical for treating large volume targets, where mechanical scanning of the focal area over the entire region of interest would take a longer time than the circulation time of the drug and/or the microbubbles. In that case, the use of planar or slightly focused transducers (Figure 2c) may be beneficial, if the tissues of interest are relatively superficial or in preclinical studies in small animals [Rapoport et al. 2010; Noble et al. 2013; Kotopoulis et al. 2014].
Figure 2.
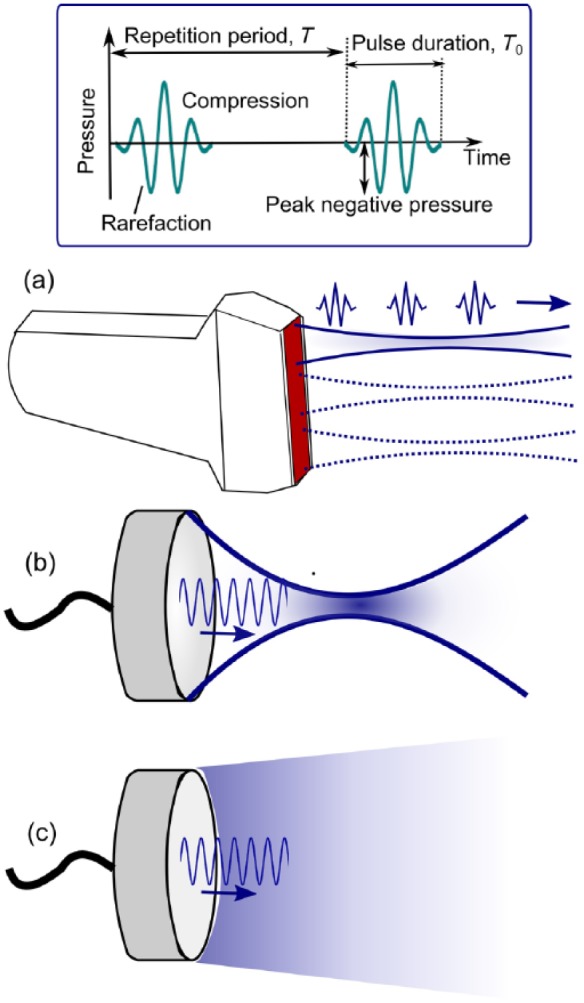
Ultrasound devices used for microbubble-enhanced drug and gene delivery. (a) Ultrasound imaging probe emits diagnostic level, short ultrasound pulses in a consecutive series of slightly focused beams to form an image. The ultrasound exposure parameters that influence the outcome of the treatment the most (top box) are peak negative (or peak rarefactional) pressure, ultrasound frequency, pulse duration T0, pulse repetition frequency, PRF=1/T and duty factor DF=(T0/T)·100%. (b) Spherically focused ultrasound transducers allow the achievement of the necessary pressure levels at large depth of tissue in a spatially localized focal area. (c) Plane ultrasound transducers are optimal for large, shallow targets.
Applications in gastrointestinal oncology
The two therapeutic applications of microbubble-enhanced ultrasound in gastrointestinal oncology which have received the most attention in the past years are pancreatic cancer and liver cancer. The former is one of the deadliest malignancies, with a dismal survival rate and substantial biological barriers to chemotherapy. The latter includes both primary liver cancer –HCC – and metastatic tumors from many other cancers, most notably, colorectal cancer (CRC); in both cases, surgical resection, ablation and chemotherapy are the main treatment options. The role of microbubble-enhanced ultrasound in the treatment of these two diseases is different and is therefore discussed separately.
Liver cancer: preclinical studies
HCC is the sixth most common neoplasm and the third most frequent cause of cancer death in the world. Surgical resection, transplantation and ablation (e.g. ethanol injection, RF, laser or microwave ablation) are treatments that offer a high rate of complete responses and, thus, potential for cure. In nonresectable disease, transarterial chemoembolization (TACE) and sorafenib were shown to improve survival. Systemic chemotherapy has marginal activity with frequent toxic effects, without survival benefit [Forner et al. 2012]. In contrast, secondary liver cancer, which is very common after CRC, can be treated with chemotherapy, whether resectable or not. Without treatment, surgical resection is not possible in 70–90% of patients with liver metastases from CRC. The prognosis of CRC patients is poor if metastases cannot be removed surgically; the aim of conversion chemotherapy is to achieve resectability rather than a complete response. In patients with initially unresectable disease, chemotherapy can convert 16% of patients to resectability [Adam et al. 2012].
Doxorubicin (Dox) has been successfully used in the past to treat liver tumors [Gerwirtz 1999]. However, the systemic delivery of Dox has been associated with cardiotoxic effects including cardiac arrhythmias and congestive heart failure which have limited its use [Singal and Iliskovic, 1998]. In order to reduce the cardiotoxic effects, a number of strategies has been proposed, including incapsulation within liposomes [Gabizon et al. 1994] resulting in the FDA approved formulation Doxil®, polymeric micelles [Nishiyama and Kataoka, 2006] and other formulations of nanoparticles that can accumulate within tumors via the EPR effect.
To further enhance the targeted delivery of Dox, Eisenbrey and colleagues developed microbubbles with an air core and polylactic acid (PLA) shell that incorporated Dox [Eisenbrey et al. 2010]. Although the microbubbles themselves were 1.8 µm in diameter, upon activation and destruction by ultrasound, they turned into much smaller 400 µm shards that were able to extravasate through EPR. The biodistribution of these microbubble shards were then compared with that of free Dox and Dox-loaded nanoparticles (also solid PLA nanoparticles 200 µm in diameter) in an orthotopic rat model of HCC (Morris hepatoma 3924a) [Cochran et al. 2011]. The ultrasound exposure was delivered using an ultrasound imaging probe operated in color Doppler regime at a mechanical index of 0.4–0.45 and a pulse repetition frequency of 1000 Hz for 20 minutes after the injection of the drug/nanoparticles/microbubbles. The authors observed a significantly greater (over 6-fold) plasma concentrations for free Dox compared with microbubbles in the short term (5–15 minutes after treatment). At the same time, tumor concentration of Dox, both long term (over 14 days) and short term was significantly greater in the microbubble group compared with free Dox [2.4% injected dose (ID) per g of tissue versus 0.37% ID/g]. The tumor growth rate was significantly lower in that group over 14 days of observation. However, the accumulation of Dox in other organs – spleen, myocardium and liver – was higher in the short term (4 hours), but generally went down after 14 days in the case of microbubbles. Autoradiography of tumor sections showed that the majority of Dox was restricted to the periphery of the tumor. The penetration of the microbubbles into the center of the tumor may have been restricted by high intratumoral pressure or by poor vascularization of necrotic regions within the tumor. Thus, although this approach is not anticipated to cause a complete tumor response, it is successful in reducing systemic toxicity yet allows maintenance of tumoricidal drug concentration over an extended period of time, which is suitable for the goal of tumor reduction for resectability.
In addition to TACE and ablation, gene delivery has emerged as an appealing treatment option for liver cancer in recent years. Phase I and II clinical trials have been conducted in patients with liver cancer using a variety of genes, and although the treatment has been well tolerated and toxicity has been low, the clinical benefit has so far been marginal [Havlik et al. 2002]. Similar to systemic chemotherapy, the major obstacle here is poor efficiency of delivery of genes to cancer cells due to the distribution-limiting nature of tumor vasculature and microenvironment. The feasibility of using microbubble-enhanced ultrasound to overcome this obstacle in the case of liver tumors was demonstrated in a number of preclinical studies in small animals [Zhou et al. 2010; Hauff et al. 2005; Sakakima et al. 2005].
In particular, the delivery of specific ‘suicide’ genes which potentiate the rapid activation of select chemotherapeutic agents has been widely used in liver cancer treatment. Zhou and colleagues investigated the ultrasound–microbubble mediated delivery of specific ‘suicide’ gene (herpes simplex virus thymus kinase, HSV-TK), which potentiates the rapid activation of a chemotherapeutic agent (ganciclovir, GCV) [Sangro et al. 2010] to mouse hepatomas (h22 subcutaneous tumors), and found a substantial reduction in tumor growth rate and increase in survival [Zhou et al. 2010]. In the study, the HSV-TK plasmid was attached to the shell of lipid, perfluoropropane-filled microbubbles, 2–4 µm in diameter. The plasmid and microbubbles were administered systemically via tail vein every 3 days, followed by 5-minute ultrasound treatment with a 1 MHz transducer operating at a sound intensity of 2 W/cm2 (peak negative pressure of approximately 0.25 MPa). GCV was administered intraperitoneally48 hours after the first treatment. Although the TK protein expression was evaluated only qualitatively by Western blotting, the combination of HSV-TK/GCV therapy with microbubble-enhanced ultrasound appeared to improve the efficiency in gene delivery to the hepatic tumor.
In the study by Sakakima and colleagues, phospholipid microbubbles filled with perfluoropentane were injected intratumorally into nude mice bearing human HCC xenografts (SK-Hep1) together with cDNA plasmid expressing IFN-b [Sakakima et al. 2005]. The ultrasound treatment was only performed once, for 10 minutes, using a 1 MHz planar transducer operating at 2 W/cm2 and 50% duty factor. Again, although the transfection efficiency was not directly quantified, the relative growths of tumors were significantly reduced during the 6-week observation period compared with the group that did not receive ultrasound treatment.
Ultrasound-mediated gene delivery was directly visualized by Hauff and colleagues in orthotopic CC531 liver tumors in rats [Hauff et al. 2005]. A model plasmid that contained Escherichia coli LacZ gene for β-galactosidase was incorporated into the shell of a gas-filled poly(D,L-lactide-co-glycolide) microparticles with a mean size of 5 µm. The microparticle injection via tail vein and the ultrasound treatment of the livers were performed simultaneously for 5 minutes. The sonication was delivered by a diagnostic ultrasound probe using color Doppler and the highest mechanical index available (0.9). β-Galactosidase blue staining of frozen tumor sections collected 3 days post treatment revealed a substantial increase in gene expression which was not observed when treated with naked plasmid + ultrasound or naked plasmid alone.
As seen in the above referenced studies, many of the ultrasound-mediated gene delivery methods were only moderately efficient largely due to the lack of ultrasound optimization and control over ultrasound parameters. Some of the most thorough methodological investigations of the efficacy of ultrasound-mediated gene delivery to the liver were undertaken by Miao and colleagues, Shen and colleagues, and Noble and colleagues, [Miao et al. 2005; Shen et al.2008; Noble et al. 2013]. Although in these studies the end goal was the treatment of hemophilia, the dependence of the transfection efficiency on the ultrasound parameters can be applied to other hepatic conditions, including liver cancer.
In the first studies in mice [Miao et al. 2005], Optison ultrasound microbubbles were co-administered with the human factor IX plasmid, pBS-HCRHP-FIXIA, by intrahepatic injection and then sonicated by a 1 MHz focused ultrasound transducer with varying pressure levels, pulse durations and repetition frequencies. Transfection efficiency was measured from the level of FIX antigen in the circulation [enzyme-linked immunosorbent assay (ELISA)] directly by sampling blood on days 1 and 4. It was shown that statistically significant levels of gene delivery were only observed after the peak negative pressure exceeded 2 MPa and the transfection rate approached a clinically relevant dose (85-fold versus plasmid alone) at 4 MPa pressure. The transfection efficiency was not dependent on the ultrasound pulse duration (10–10,000 us), but was enhanced at lower pulse repetition frequency (1–50 Hz versus 500 Hz). At the most successful transfection parameters, hemorrhagic liver damage was also observed, but it was reversible and was repaired within 10 to 30 days. In the subsequent study, it was demonstrated that intraportal injection was just as efficient or more efficient as intrahepatic [Shen et al. 2008]. The optimal peak negative pressure was also refined: a peak increase in transfection was observed at 3 MPa, whereas higher ultrasound amplitudes (4 MPa) led to the increase of hemorrhagic liver damage, but not transfection efficiency.
The next study undertaken in a larger animal model, in which pDNA carrying a reporter luciferase gene, pGL4 was delivered to canine liver, brought out a different set of challenges intrinsic to larger species [Noble et al. 2013]. The insonated volume had to be larger to affect the larger organ and so a large-aperture (5 cm) plane ultrasound transducer was used, which was powerful enough to maintain a 2.7 MPa peak negative pressure throughout a 5 cm3 insonated area. Furthermore, the microbubbles injected into the intraportal vein only persisted in the liver for 25 s; to retain the microbubbles in the liver for a longer time, transient occlusion of inferior vena cava was performed to allow for a 1 minute insonation time. With all of the parameters optimized, almost 700-fold increase in gene expression compared with sham treatment was obtained, with little to no damage to the liver lobes (mild edema, sinusoidal and vascular congestion that was attributed to the extrahepatic vascular outflow obstruction, minor focal subcapsular hemorrhage and acute inflammation).
One of the important differences in the above mentioned studies compared with ultrasound-mediated chemotherapeutic drug delivery is the substantially larger ultrasound amplitude or peak negative pressure required to significantly enhance transfection (3 MPa versus <1 MPa). One possible reason for this difference is that the plasmid constructs that are meant to be delivered in these applications are generally much larger than the chemotherapeutic drugs, and therefore the degree of tissue disruption generally has to be larger to achieve clinically meaningful levels of transfection.
One other notable application of ultrasound microbubbles in treatment of hepatic conditions is enhancement of ablation, in particular, ethanol ablation. As mentioned above, ethanol ablation is one of the first-line treatments for both primary and secondary liver cancer. However, ethanol ablation is limited by the tumor size (less than 3 cm); in addition, the ethanol is rapidly washed out by the tumor or liver circulation, making its accumulation at a high concentration at the injection site difficult [Koda et al. 2000]. Thus, patients with hypervascular small HCCs tend to have a worse long-term prognosis and a higher local recurrence rate than patients with nonhypervascular HCCs who are treated with percutaneous ethanol ablation (PEA). Microbubble-enhanced therapeutic ultrasound allows an increase in the tissue volume ablated by PEA, as demonstrated in the study in rabbit liver by Liu and colleagues [Liu et al. 2013]. In that study, ultrasound-driven microbubbles were shown to shut down regional liver perfusion in the sonicated area, most probably due to permeabilization of the vessel walls, as well as cell membranes. This led to swelling of hepatocytes due to enhanced calcium uptake which, in turn, led to the compression and consequently closing of the intervening sinusoids and space of Disse. To achieve this effect, the authors used a custom-built weakly focused ultrasound transducer (25 mm in diameter, 160 mm radius of curvature) operating at the frequency of 831 kHz, pulse repetition frequency of 9 Hz and 0.5 ms pulse duration, with 4.3 MPa peak negative pressure. This treatment was applied to surgically exposed rabbit liver for 5 minutes, with the simultaneous intravenous injection of microbubbles, immediately followed by ethanol injection. According to diagnostic contrast-enhanced ultrasound, the circulation was shut down for more than 60 minutes. This resulted in an increase in the ablated volume by 10-fold compared with ethanol injection alone. Although this was not demonstrated in an HCC animal model, only healthy liver, the authors had previously demonstrated that the same approach shuts down the circulation in a subcutaneous rat tumor and leads to tumor necrosis. Overall, this is a promising technique, for enhancing ablative therapies (RF, HIFU, ethanol injection) that suffer from the heat sink effect.
Pancreatic cancer: preclinical studies
Pancreatic cancer is one of the deadliest malignancies with very few effective therapeutic options. The median survival rate for resectable tumors is only 2 years, and in the case of unresectable tumors, the care is mostly palliative [Hidalgo, 2010]. Systemic chemotherapy with gemcitabine only offers a modest survival benefit, although it had been previously shown to be effective in xenograft mouse models. It is thought that the reason for this discrepancy is the nature of pancreatic tumors, which are very poorly vascularized, highly fibrotic and have a high interstitial pressure. All those morphological characteristics represent barriers to the penetration of chemotherapeutic agents and are not adequately represented in the xenograft models [Olive et al. 2009]. The use of ultrasound microbubbles to enhance drug delivery in this case may not be as efficient as in other tumors because they are confined to vasculature and need to penetrate both the vessel wall and the fibrotic stromal matrix. However, nanoscale microbubble precursors or nuclei, such as nanoemulsions can accumulate in the tumor via the EPR effect, and then get activated by ultrasound.
The use of perfluoropentane nanoemulsions that convert into bubbles upon ultrasound radiation and subsequently release their payload has been reported [Rapoport et al. 2010, 2011]. The remaining bubbles coalesce into larger bubbles that can be used for ultrasound imaging: a bright hyperecho is observed on B-mode ultrasound imaging when the droplets undergo droplet-to-bubble transition. This approach was successfully applied to the treatment of MiaPaCa-2 tumors implanted orthotopically into the pancreatic tail of nu/nu mice. An unfocused ultrasound device was used for the treatments after the administration of a nanoemulsion loaded with either gemcitabine or paclitaxel (718 nm in diameter). Ultrasound at the frequency of 1 MHz and peak rarefactional pressure of 0.61 MPa was applied continuously to the mouse abdomen for 30 s; the procedure was repeated twice a week for 2 weeks. Systemic chemotherapy by nanodroplet-encapsulated paclitaxel combined with gemcitabine and ultrasound resulted in dramatic tumor regression. The treatments that involved tumor sonication resulted in a significantly reduced number of metastatic foci and suppression of ascites formation. However, with any treatment protocol, local tumor recurrence was observed after completion of treatment, in the course of 6 weeks. Similarly to the case of liver cancer treatment, this was likely due to the nonuniform drug distribution in the tumor caused by the irregularity in tumor vascularization and distribution of interendothelial gaps. Ultrasound imaging manifested a highly nonuniform distribution of nanodroplets throughout the tumor volume. As a result, some tumor sites may be exposed to subtherapeutic concentrations of drug, which would favor development of drug resistance.
The same orthotopic mouse tumor model was used by Kotopoulis and colleagues to evaluate the effect of commercially available ultrasound contrast agent (SonoVue) combined with gemcitabine and diagnostic level ultrasound [Kotopoulis et al. 2014]. A focused ultrasound transducer (44 mm radius of curvature, 25 mm diameter) was operated at 40% duty factor and the peak negative pressure of 0.2 MPa to treat the abdomen of the mice for 10 minutes immediately after SonoVue injection. The treatments were repeated weekly for 8 weeks, starting from week 3 after tumor inoculation, and compared with administration of gemcitabine alone. The tumor growth was significantly retarded but not inhibited; inhibition of metastatic development was also noted. It is worth noting that the treatments started when the tumors were still small (~3 mm in size) compared with the starting tumor sizes used in the studies by Rapoport’s group (over 1 cm).
As noted in the studies described above, neither the microbubble vehicles nor the echogenic liposomes have the ability to deliver the drug in necessary concentrations to the entire tumor volume. Even after dramatic tumor regression, as observed by the Rapoport group, the poorly perfused areas of the tumor later become sites of recurrence. It may therefore be desirable to induce cavitation bubble activity in the interstitium throughout the tumor, outside of the vasculature. One way to pursue this effect is by operating the ultrasound transducers at much higher peak negative pressure (over 9 MPa) that can be achieved by focused transducers. Such pressures in the focal area are sufficient to ‘tear’ the tissue apart and generate transient microcavities in situ that undergo cavitation activity and disappear as soon as ultrasound is turned off. Li and colleagues used pulsed exposures (peak negative pressures of 2–19 MPa) by focused 1.5 MHz transducer to enhance the penetration of doxorubicin to pancreatic tumors in a KPC mouse model [Li et al. 2014b].
This genetically engineered mouse model of a spontaneously occurring pancreatic ductal adenocarcinoma closely recapitulates the genetic mutations, clinical symptoms and histopathology found in human pancreatic cancer [Olive et al. 2009]. The enhancement of drug uptake in the treated area of the tumor was evaluated by multispectral imaging, fluorescence microscopy and high-pressure liquid chromatography (HPLC) immediately after the treatment. The results demonstrated that reliable and intense cavitation correlated with enhanced Dox uptake by 2–4 fold beyond tumor vasculature. Further survival studies are ongoing to investigate whether this enhancement provides a survival benefit.
Clinical studies
The clinical studies in microbubble-enhanced ultrasound-mediated drug delivery are scarce, perhaps due to the fact that microbubbles, as well as other contrast agents, are only approved in the US for cardiac imaging. In Europe, one group was able to combine diagnostic ultrasound imaging with the administration of microbubbles (SonoVue) and gemcitabine with the intention to permeabilize tumors in pancreatic cancer patients [Kotopoulis et al. 2013]. A GE Logiq 9 scanner with a 4C curvilinear imaging probe were used to perform continuous power Doppler imaging of the tumor with the following parameters: frequency 1.9 MHz; peak negative acoustic pressure 0.41 MPa (0.27 MPa if de-rated to the appropriate depth in tissue); pulse duration 3 µs; and duty factor 1%. The imaging was performed for 30 minutes, after the 30-minute administration of gemcitabine, and was accompanied with multiple administrations of SonoVue every 3 minutes. This treatment was repeated with each administration of gemcitabine, which followed the standard protocol: once weekly for up to 7 weeks (or until toxicity necessitates reducing or holding a dose), followed by a week of rest from treatment. Subsequent cycles consisted of infusions once weekly for 3 consecutive weeks out of every 4 weeks. Only a limited cohort of patients has been treated to date (five patients) and tumor growth retardation was observed in one patient; however, it remains too early to draw conclusions from this study. The main goal of the study was to demonstrate that it is safe and feasible to combine chemotherapy administration with microbubbles and ultrasound, with minimal alterations to the clinical procedures that are already in place. A larger study at that institution is now ongoing.
Conclusion
The clinical application of microbubbles for therapeutic purposes in gastrointestinal oncology is clearly promising. As presented in this review, extensive preclinical studies have been performed using various preparations of microbubbles and nanobubbles for applications in liver and pancreatic tumors. The use of ultrasound activated microbubbles for enhancing drug and gene delivery is attractive since the procedure is completely noninvasive and has the potential to significantly enhance the delivery of drug and genes in a targeted fashion while minimizing systemic toxicity.
However, there are several hurdles that slow down the clinical translation that are discussed below. First, there are currently no dedicated clinical ultrasound systems for applications other than ablation and imaging. Although commercial ultrasound imaging systems can potentially be modified and used for purposes like drug delivery (as in Kotopoulis et al. 2014), this option is rarely available and does not offer much control over ultrasound parameters. The second important aspect for clinical translation is the presence of real-time treatment feedback to control for the desired physical effect on tissue; in the case of microbubbles, it is cavitation. Although simply imaging of the ultrasound contrast agent distribution has been utilized in diagnostic ultrasound for a long time, tools for mapping the degree of cavitation activity of the bubbles have only been reported recently [Jensen et al. 2012; O’Reilly and Hynynen, 2013]. These tools are also ultrasound-based and rely on the emissions of harmonics and broadband ultrasound noise from the collapsing bubbles. In order for the preclinical studies to be immediately translatable to human use, the ability to detect and map cavitation activity should be incorporated in all experiments to obtain the quantitative correlation and dose dependence between the extent of cavitation and the desired effect (e.g. drug uptake). Third, as already mentioned above, large bubbles are confined to vasculature. While this may be sufficient for the applications in well perfused tumors/organs such as liver cancer, it is unlikely to produce considerable benefit in highly fibrotic, poorly vascularized tumors such as pancreatic cancer. Two potential pathways (or a combination thereof) may be used to overcome this problem: the development of more sophisticated, potentially targeted nanobubble and nanodroplet formulations, which would be able to extravasate beyond the vessel wall and perivascular space towards the poorly perfused areas of the tumors. Alternatively, inducing de novo cavitation in targeted tissue could be used. The latter approach does not face the additional regulatory hurdles of FDA approval of the new contrast agent, the high pressures needed for this regime can be easily achieved using existing ultrasound-guided HIFU systems utilized in pulsed mode, and cavitation monitoring can be performed using existing ultrasound-based tools[Li et al. 2014a].
To conclude, major advances have been made in the development and understanding of microbubbles for both diagnostic and therapeutic applications in gastrointestinal oncology. There is clear evidence that microbubbles can be used to enhance drug and gene delivery. However, the question remains as to the composition of the optimal agent and ultrasound parameters that will result in the most effective therapies for specific applications.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Tatiana D. Khokhlova, Division of Gastroenterology, Department of Medicine, University of Washington, Seattle, WA, USA
Yasser Haider, Department of Urology, University of Washington, Seattle, WA, USA.
Joo Ha Hwang, Associate Professor of Medicine, Division of Gastroenterology, Department of Medicine, University of Washington, Box 359773, 325 Ninth Avenue, Seattle, WA 98104, USA.
References
- Adam R., De Gramont A., Figueras J., Guthrie A., Kokudo N., Kunstlinger F., et al. (2012) The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist.17: 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Kreider W., Brayman A., Bailey M., Matula T. (2011) Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett 106: 034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M., Eisenbrey J., Soulen M., Schultz S., Ouma R., White S., et al. (2011) Disposition of ultrasound sensitive polymeric drug carrier in a rat hepatocellular carcinoma model. Acad Radiol 18: 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton P., Morgan K., Klibanov A., Brandenburger G., Ferrara K. (1999) Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans Ultrason Ferroelect Freq Control 46: 220–232. [DOI] [PubMed] [Google Scholar]
- Eisenbrey J., Burstein O., Kambhampati R., Forsberg F., Liu J., Wheatley M. (2010) Development and optimization of a doxorubicin loaded poly(lactic acid) contrast agent for ultrasound directed drug delivery. J Controlled Release 143: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner A., Llovet J., Bruix J. (2012) Hepatocellular carcinoma. Lancet 379: 1245–1255. [DOI] [PubMed] [Google Scholar]
- Gabizon A., Catane R., Uziely B., Kaufman B., Safra T., Cohen R., et al. (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54: 987–992. [PubMed] [Google Scholar]
- Gewirtz D. (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Raichlen J., Forsberg F. (2001) Ultrasound Contrast Agents: Basic Principles and Clinical Applications, 2nd edn. London: Martin Dunitz. [Google Scholar]
- Gramiak R., Shah P. (1968) Echocardiagraphy of the aortic root. Invest Radiol 3: 356–366. [DOI] [PubMed] [Google Scholar]
- Hauff P., Seemann S., Reszka R., Schultze-Mosgau M., Reinhardt M., Buzasi T., et al. (2005) Evaluation of gas-filled microparticles and sonoporation as gene delivery system: feasibility study in rodent tumor models. Radiology 236: 572–578. [DOI] [PubMed] [Google Scholar]
- Havlik R., Jiao L., Nicholls J., Jensen S., Habib N. (2002) Gene therapy for liver metastases. Semin Oncol 29: 202–208. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. (2010) Pancreatic cancer. N Engl J Med 362: 1605–1617. [DOI] [PubMed] [Google Scholar]
- Hijnen N., Langereis S., Grüll H. (2014) Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Delivery Rev 72: 65–81. [DOI] [PubMed] [Google Scholar]
- Hwang J., Brayman A., Reidy M., Matula T., Kimmey M., Crum L. (2005) Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med Biol. 31: 553–564. [DOI] [PubMed] [Google Scholar]
- Ibsen S., Schutt C., Esener S. (2013) Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des, Dev Ther 7: 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C., Ritchie R., Gyöngy M., Collin J., Leslie T., Coussios C. (2012) Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology 262: 252–261. [DOI] [PubMed] [Google Scholar]
- Keller M., Glasheen W., Kaul S. (1989) Albunex: a safe and effective commercially produced agent for myocardial contrast echocardiography. J Am Soc Echocardiogr 2: 48–52. [DOI] [PubMed] [Google Scholar]
- Koda M., Murawaki Y., Mitsuda A., Ohyama K., Horie Y., Suou T., et al. (2000) Predictive factors for intrahepatic recurrence after percutaneous ethanol injection therapy for small hepatocellular carcinoma. Cancer 88: 529–537. [PubMed] [Google Scholar]
- Kotopoulis S., Delalande A., Popa M., Gjertsen B., McCormack E., et al. (2014) Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol Imag Biol 16: 53–62. [DOI] [PubMed] [Google Scholar]
- Kotopoulis S., Dimcevski G., Gilja O., Hoem D., Postema M. (2013) Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med Phys 40: 072902. [DOI] [PubMed] [Google Scholar]
- Kripfgans O., Fowlkes J., Miller D., Eldevik O., Carson P. (2000) Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med Biol 26: 1177–1189. [DOI] [PubMed] [Google Scholar]
- Lanza G., Winter P., Caruthers S., Hughes M., Hu G., Schmieder A., et al. (2010) Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis 13: 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Khokhlova T., Sapozhnikov O., O’Donnell M., Hwang J. (2014a) A new active cavitation mapping technique for pulsed HIFU applications–bubble Doppler. IEEE Trans Ultrason Ferroelectr Freq Control 61: 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Khokhlova T, Wang Y., D’Andrea S., Starr F., Hwang J. (2014b) In vivo enhanced delivery of doxorubicin in mouse pancreatic tumors. In: Proceedings of the 14th International Symposium on Therapeutic Ultrasound Final Program and Abstracts Book, abstract 62. [Google Scholar]
- Liu Q., Zhao H., Wu S., Zhao X., Zhong Y., Li L., et al. (2013) Impact of microbubble-enhanced ultrasound on liver ethanol ablation. Ultrasound Med Biol 39: 1039–1046. [DOI] [PubMed] [Google Scholar]
- Martin K., Dayton P. (2013) Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5: 329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao C., Brayman A., Loeb K., Ye P., Zhou L., Mourad P., et al. (2005) Ultrasound enhances gene delivery of human factor IX plasmid. Human Gene Ther 16: 893–905. [DOI] [PubMed] [Google Scholar]
- Nahire R., Paul S., Scott M., Singh R., Muhonen W., Shabb J., et al. (2012) Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes. Mol Pharmacol 9: 2554–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama N., Kataoka K. (2006) Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther 112: 630–648. [DOI] [PubMed] [Google Scholar]
- Noble M., Kuhr C., Graves S., Loeb K., Sun S., Keilman G., et al. (2013) Ultrasound-targeted microbubble destruction-mediated gene delivery into canine livers. Mol Ther 21: 1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive K., Jacobetz M., Davidson C., Gopinathan A., McIntyre D., Honess D., et al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M., Hynynen K. (2013) A super-resolution ultrasound method for brain vascular mapping. Med Phys 40: 110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Caskey C., Ferrara K. (2009) Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol 54: R27–R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan K., Haworth K., Huang S., Klegerman M., McPherson D., Holland C. (2012) Stability of echogenic liposomes as a blood pool ultrasound contrast agent in a physiologic flow phantom. Ultrasound Med Biol 38: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N. (2012) Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4: 492–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N., Kennedy A., Shea J., Scaife C., Nam K. (2010) Ultrasonic nanotherapy of pancreatic cancer: lessons from ultrasound imaging. Mol Pharmacol 7: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N., Nam K., Gupta R., Gao Z., Mohan P., Payne A., et al. (2011) Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Controlled Release 153: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakima Y., Hayashi S., Yagi Y., Hayakawa A., Tachibana K., Nakao A. (2005) Gene therapy for hepatocellular carcinoma using sonoporation enhanced by contrast agents. Cancer Gene Ther 12: 884–889. [DOI] [PubMed] [Google Scholar]
- Sangro B., Mazzolini G., Ruiz G., Ruiz J., Quiroga J., Herrero I., et al. (2010) A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther 17: 837–843. [DOI] [PubMed] [Google Scholar]
- Sheeran P., Dayton P. (2012) Phase-change contrast agents for imaging and therapy. Current Pharmaceutical Design. 18: 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Brayman A., Chen L., Miao C. (2008) Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther 15: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal P., Iliskovic N. (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905. [DOI] [PubMed] [Google Scholar]
- Tzu-Yin W., Wilson K., Machtaler S., Willmann J. (2013) Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr Pharmaceut Biotechnol 14: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Li S., Liu Z., Tang Y., Wang Z., Gong J., Liu C. (2010) Ultrasound-targeted microbubble destruction mediated herpes simplex virus-thymidine kinase gene treats hepatoma in mice. J Exp Clin Cancer Res 29: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]