Abstract
Fluoropyrimidines form the mainstay in treatment of gastrointestinal malignancies. For decades 5-fluorouracil (5FU), was the major fluoropyrimidine. Currently it is usually given in a combination with leucovorin and oxaliplatin, i.e. FOLFOX, or irinotecan, i.e. FOLFIRI, or all three, i.e. FOLFIRINOX, but gradually it has been replaced by oral fluoropyrimidine prodrug formulations, such as tegafur-uracil and S-1 (both contain ftorafur), and capecitabine (Xeloda®). Novel drugs such as the antivascular endothelial growth factor antibody, bevacizumab, and the anti-epidermal growth factor receptor antibody, cetuximab, are often combined with one of these treatment options. However, when resistance emerged, no alternatives were available. TAS-102, a combination of trifluorothymidine and the thymidine phosphorylase inhibitor TPI in a 1:0.5 ratio, is a novel oral formulation, which is active in 5FU-resistant models, both in vitro and in xenograft models. In addition to inhibition of thymidylate synthase, the major mechanism of action of classical fluoropyrimidines, TAS-102’s major mechanism of action is incorporation into DNA, thereby causing DNA damage. TAS-102 also follows an alternative activation pathway via thymidine kinase, and is not a substrate for dihydropyrimidine dehydrogenase. All together this explains the efficacy in 5FU-resistant models. In early clinical studies, the twice-daily schedule (5 days on, 2 days rest) for 2 weeks every 4 weeks, led to a significant disease control rate in various malignancies. This schedule showed consistent activity in two randomized trials on fluoropyrimidine refractory colorectal cancer patients, reflected by an increase of 2–3 months in overall survival in the TAS-102 group compared with placebo. Considering the impressive preclinical potential of various combinations TAS-102 has the promise to become an alternative for 5FU-resistant cancer.
Keywords: clinical trials, colorectal cancer, combination studies, DNA damage, TAS-102, thymidine phosphorylase inhibitor, trifluorothymidine
Introduction
TAS-102 is an oral formulation that combines several attractive features of the cytotoxic pyrimidine analog 5-trifluoro-2’-deoxythymidine (trifluorothymidine [TFT]; trifluridine [F3TdR]; 5-trifluoromethyl-2’-deoxyuridine [CF3dUrd]; FTD; 2’-deoxy-5-(trifluoromethyl)uridine [F3Thd]) and the potent thymidine phosphorylase (TP) inhibitor TPI (tipiracil hydrochloride), in a molar ratio of 1:0.5 [Temmink et al. 2007b] (Figure 1). It has successfully completed two randomized phase II/III studies [Yoshino et al. 2012; Mayer et al. 2015].
Figure 1.
Composition of TAS-102.
Although TFT was synthesized by Heidelberger and colleagues [Heidelberger et al. 1964], its initial clinical evaluation was discontinued because of a poor pharmacokinetic profile and side effects. However, some of the administration schedules resulted in a reduction of tumor size (8 out of 23 breast cancers and 1 out of 6 colon cancers) [Ansfield and Ramirez, 1971]. It was shown that TFT can be phosphorylated by thymidine kinase to its active monophosphate form mediating cytotoxicity [Heidelberger et al. 1965], and it was as effective against adenocarcinoma cells as 5-fluoro-2’-deoxyuridine (FdUrd) [Heidelberger and Anderson, 1964]. TFT is registered as Viroptic® [De Clercq, 2004], for use against herpes simplex virus infections and was previously approved by the US Food and Drug Administration in 1980 for the treatment of primary keratoconjunctivitis and epithelial keratitis [Carmine et al. 1982]. TAS-102 is currently registered in Japan as Lonsurf® for third-line treatment of colorectal cancer. The current review summarizes the mechanism of action of the individual components of TAS-102, TFT and TPI, and its development as an alternative treatment for colorectal cancer.
TFT-mediated cytotoxicity
TFT shares some similarities to 5-fluorouracil (5FU), but its differences seem responsible for its successful application as an anticancer drug [De Bruin et al. 2006]. The mechanism of action of TFT is depicted in Figure 2. TFT is phosphorylated by thymidine kinase 1 (TK1) to its active monophosphate-derivative TFT-MP, which inhibits thymidylate synthase (TS) [Reyes and Heidelberger, 1965; Sakamoto et al. 2015]. TS is one of the rate-limiting enzymes in pyrimidine de novo deoxynucleotide synthesis and therefore it plays a central role in DNA synthesis as a target for chemotherapeutic approaches [Van Triest and Peters, 1999]. TFT-MP does not form a ternary complex and binds covalently to the active site of TS (tyrosine 146), thereby inhibiting its activity [Santi and Sakai, 1971; Eckstein et al. 1994]. TFT-MP is a potent reversible inhibitor of TS with a Ki of 0.38 nM [Reyes and Heidelberger, 1965], and its activity remains inhibited when a constant influx of TFT is present; removal of the compound leads to a rapid recovery of TS activity [Santi and Sakai, 1971; Temmink et al. 2004]. This is in contrast to the 5FU derivative, 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), which inhibits TS after formation of a ternary complex with 5,10-methylene-tetrahydrofolate (CH2-THF), thereby enhancing and prolonging this inhibition [Peters et al. 2002]. CH2-THF also serves as the co-factor for the reductive methylation of dUMP to thymidine monophosphate (dTMP) (Figure 2). Inhibition of TS leads to intracellular depletion of deoxythymidine triphosphate (dTTP), and subsequent dUMP accumulation, resulting in uracil misincorporation into the DNA leading to DNA damage [Wilson et al. 2014; Peters et al. 2009]. In an animal model continuous treatment with TFT led to an accumulation of dUMP [Tanaka et al. 2014]. Normally dUMP can be broken down to deoxyuridine, which will be excreted into plasma, resulting in increased deoxyuridine plasma levels, which can be considered as a biomarker for effective TS inhibition [Peters et al. 2009]. This effect was also observed in animals and patients treated with a specific TS inhibitor such as raltitrexed, nolatrexed, or pemetrexed [Peters et al. 2009; Ford et al. 2002].
Figure 2.
Metabolism and mechanism of action of TAS-102. Trifluorothymidine (TFT) can be phosphorolyzed by thymidine phosphorylase (TP) to trifluorothymine which can be inhibited by TPI. TAS-102 is the combination of TFT and TPI (a thymidine phosphorylase inhibitor). TFT is phosphorylated by thymidine kinase 1 (TK1) to TFT-MP, which is a reversible inhibitor of thymidylate synthase (TS), which leads to depletion of thymidine monophosphate (dTMP), but an accumulation of 2’-deoxyuridine-5’-monophosphate (dUMP) (and deoxyuridine outside the cell and in plasma). TFT-MP can be phosphorylated to TFT-TP, which can be incorporated into DNA, causing DNA damage, and induce a DNA damage-response signaling, leading to G2-M accumulation and cell death. Depletion of deoxythymidine triphosphate (dTTP) by inhibition of TS, will enhance the incorporation of TFT-TP into DNA. An alternative source for dTMP is thymidine, which can be phosphorylated to dTMP by TK1. However, degradation of thymidine by TP to thymine also leads to the formation of deoxyribose-1-P (dRib-1-P), which can be degraded to deoxyribose (dRibose), and may be responsible for angiogenic effects. TPI will also inhibit this step when patients are treated with TAS-102 (adapted from [Peters et al. 2002]).
In addition to TFT-mediated TS inhibition, TFT itself can be incorporated into DNA [Fujiwara and Heidelberger, 1970], for which evidence is accumulating that this is the main mechanism of action of the drug. TFT is incorporated into DNA as the triphosphate form of TFT (TFT-TP) [Temmink et al. 2005; Emura et al. 2004c], causing cell death due to DNA strand-break formation [Emura et al. 2004d; Suzuki et al. 2011; Matsuoka et al. 2015]. This incorporation into DNA was related to the antitumor activity of TAS-102 in in vivo mouse models [Tanaka et al. 2014]. TAS-102 showed a dose-dependent and similar antitumor effect against 5FU-resistant DLD-1 colon tumors (tumor growth inhibition rate 73.2% at 150 mg/kg/day) and the parent DLD-1 tumors (tumor growth inhibition rate 73.4% at 150 mg/kg/day) [Emura et al. 2004d], in contrast to conventional 5FU (continuous infusion; inhibition rate 28.2% versus 62.3%, respectively), or an analog such as tegafur-uracil (UFT) (inhibition rate 12.9% versus 61.2%, respectively). DLD-1/FdUrd resistant and DLD-1 parent tumors were similarly sensitive to TAS-102, but DLD-1/FdUrd tumors were resistant to UFT. Also another 5FU-resistant model (the gastric cancer xenograft NUGC-3/5FU) was similarly sensitive to oral TFT but cross-resistant to FdUrd and TS-1. Intracellular TFT-TP is rapidly eliminated from tumor cells after the removal of TFT from the culture medium, but TFT incorporation into DNA continued to increase during 8 h TFT exposure of NUGC-3 human gastric cancer cells. TFT was incorporated in a time-dependent manner and not in a concentration-dependent manner [Emura et al. 2004c; Tanaka et al. 2014]. Incorporated TFT into the DNA is retained for at least 80% up to 24 h after the wash-out procedure. These observations indicated that TFT incorporation into DNA is important. This incorporation led to inhibition of chk-1 phosphorylation and an increase in AP sites [Suzuki et al. 2011]. In addition, tumor-bearing mice treated with TAS-102 at a repeated dosing of 75 mg/kg/day or 150 mg/kg/day showed a significantly higher incorporation of TFT into DNA of the tumor compared with single dosing [Emura et al. 2004c; Tanaka et al. 2014], while higher dosing also led to an accumulation of dUMP, indicating inhibition of TS in vivo [Tanaka et al. 2014]. TFT induced pronounced DNA damage due to enhanced DNA fragmentation resulting in most potent antitumor activity. This indicates that multiple daily dosing may result in better clinical benefits for TAS-102-treated cancer patients. However, although it has been reported that TFT-TP is a substrate for DNA polymerase α [Sakamoto et al. 2015], and causes DNA damage, it is not yet known whether TFT-TP is a substrate for other DNA polymerases, and whether it might cause a chain-terminating effect. From the present data it can be concluded that the DNA-targeted effects of TAS-102 are specific for cancer cells, determine the antitumor effects, and are enhanced by the inhibition of TS.
TFT resistance
Most chemotherapeutic regimens in the treatment of gastrointestinal cancer patients are 5FU-based. The presence of a fluoropyrimidine is indispensable to obtain a maximal effect. To improve treatment of colorectal cancer it is important that an alternative chemotherapeutic regimen, such as TAS-102, also exhibits antitumor effects against 5FU-sensitive, and more favorably, against 5FU-resistant tumor cells. Indeed a high dose level of TFT alone (200 mg/kg/day) resulted in tumor growth-inhibition rates of about 70% for both 5FU-sensitive and 5FU-resistant NUGC-3 gastric cancer cells (NUGC-3/5FU) implanted subcutaneously in nude mice [Emura et al. 2004d]. Similar data were found for FdUrd-resistant DLD-1 colorectal cancer cells (DLD-1/FdUrd) in mice treated with 150 mg/kg/day TFT. The resistance mechanisms of both tumors are different [Inaba et al. 1996; Murakami et al. 2000], showing an advantage for TAS-102 over 5FU in 5FU-resistant tumor cells, while TAS-102 was also effective in vivo against 5FU-sensitive tumors from different tissue types, such as human pancreatic and esophageal tumor cells [Emura et al. 2004a, 2004d].
Also in vitro TFT was active against 5FU-resistant DLD-1 cells (DLD-1/5FU) [Emura et al. 2004a]. In DLD-1/TFT cells no increase in TS was found, but TK activity was decreased significantly (37-fold). Also H630 human colorectal carcinoma cells made resistant to TFT using either a long-term continuous exposure schedule (H630-cTFT) or short-term repeated exposure schedule (H630-4TFT), showed different mechanisms of resistance [Temmink et al. 2010]. The H630-4TFT cells exposed to TFT on a short-term basis (250 µM TFT 4 h/week) had normal TS levels, but no TK activity, similar to the DLD-1/TFT-resistant cells. H630-cTFT cells growing in medium ultimately containing 20 µM TFT did not have altered TS and TK levels but showed a disturbed signal transduction with upregulated secretory phospholipase A2 expression. In both H630- TFT-resistant cell lines no change in TP levels was observed, although this was less relevant, because a high TP expression in colorectal cancer cells hardly influences the sensitivity to TFT [Temmink et al. 2005; De Bruin et al. 2003]. In agreement with the DLD-1/TFT cells, both H630-derived TFT-resistant cell lines were not cross-resistant to 5FU or the folate-based direct TS inhibitor GW1843, but the TK-deficient variants were cross-resistant to FdUrd (about 160-fold). In contrast to the DLD-1/5FU, 5FU-resistant H630 cells (H630-R10) with increased TS levels were cross-resistant to FdUrd and TFT, but this was dependent on exposure time [Temmink et al. 2005].
It can be concluded that TAS-102 is active in 5FU-resistant models, while resistance to TFT is multifactorial but different from 5FU. This offers opportunities to use this information to select patients for the best treatment option.
TPI increases bioavailability of TFT in vivo
The main pharmacokinetics of TFT [Dexter et al. 1972] resemble that of most antimetabolites with a short half-life [Peters et al. 1993]. The mean plasma half-life of single TFT injected to cancer patients was less than 15 min [Dexter et al. 1972]; TFT is phosphorolyzed to trifluorothymine (TF-Thy), which can be hydrolyzed to 5-carboxyuracil with the loss of the inorganic fluoride. Initial studies on mice have already demonstrated that the TFT degradation product TF-Thy and its catabolite 5-carboxyuracil were formed mainly in the liver, spleen, and intestines [Heidelberger et al. 1965]. Similar to normal nucleosides [Peters, 2014], TFT can easily cross the blood–brain barrier in low micromolar concentrations and is also predominantly metabolized to TF-Thy in the brain [Pouremad et al. 1999]. Besides TF-Thy, the dihydro-species of TFT and TF-Thy, the reductive catabolites α-trifluoromethyl-β-ureidopropionic acid (F3MUPA) and α-trifluoromethyl-β-alanine (F3MBA) were detected in liver extracts. Low levels of fluoride ions were detected in serum and urine, but also in brain and liver extracts. Early clinical pharmacology studies with TFT showed that the only metabolites detected in urine and serum samples of treated cancer patients were unmodified TFT, TF-Thy, and 5-carboxyuracil [Heidelberger et al. 1965; Dexter et al. 1972].
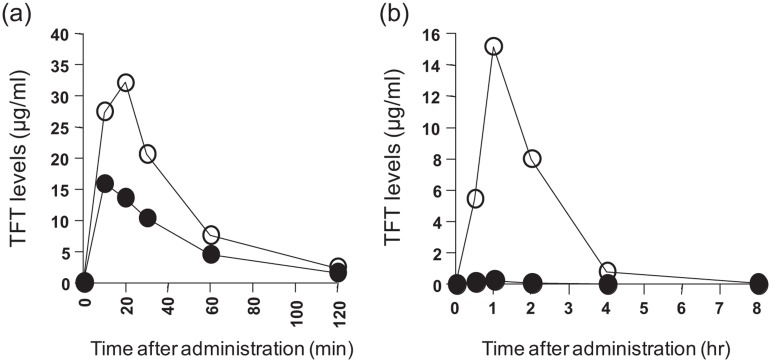
In a series of novel orally active 6-methylene-bridged, uracil-derived inhibitors of human TP [Fukushima et al. 2000; Yano et al. 2004], the most potent human TP inhibitor was the 5-chloro-6-(2-iminopyrrolidin-1-yl)methyl-2,4(1H,3H)-pyrimidine dione hydrochloride (TPI; tipiracil hydrochloride) with an IC50 of 35 nM for TP, which was a poor inhibitor of uridine phosphorylase (UP) (IC50 > 100 µM) [Fukushima et al. 2000], a pyrimidine phosphorylase also known to cleave thymidine (TdR) and other pyrimidine nucleosides. The Ki value for TPI using recombinant human TP was 17 nM. TPI was a potent inhibitor of TFT phosphorolysis in extracts from human liver, small intestine, and tumor tissues. At equimolar doses TPI increased Cmax and area under curve (AUC) of orally administered TFT to rodents or monkeys (10 mg/kg) [Fukushima et al. 2000; Yano et al. 2004], which was most pronounced in monkeys (Figure 3), due to differences in expression of pyrimidine phosphorylases in these animals [Ackland and Peters, 1999; Cao and Pizzorno, 2004; Pugmire and Ealick, 2002]. Pharmacokinetics studies were also designed to optimize the molar drug ratio of TFT:TPI to reach maximal TFT plasma levels. With a TFT:TPI molar ratio of 1:0.5 optimal TFT concentrations in the plasma were obtained [Emura et al. 2005], and this formulation was developed as TAS-102. At a co-administration of 1 M TFT (10 mg/kg) and > 0.5 M TPI, a maximum and constant level of 15 µg TFT/ml was found, associated with a maximally augmented antitumor activity for gastrointestinal cancer cells xenografted into mice [Emura et al. 2005]. Steady levels of TFT in plasma obtained with a divided dosing of TFT within an adequate time period resulted in optimal DNA incorporation and subsequent antitumor activity [Emura et al. 2004c].
Figure 3.
Effect of TPI on trifluorothymidine (TFT) pharmacokinetics. Plasma TFT levels in mice (a) and monkeys (b) were measured following oral administration in the presence and absence of TPI. TFT (black circles) was administered in doses of 50 mg/kg (normal mice) or 10 mg/kg (normal monkeys), or in combination with an equimolar amount of TPI (white circles). Values are means of several experiments (n = six mice; n = three monkeys). (Data adapted from [Fukushima et al. 2000] with permission.)
High TdR levels may reduce the antitumor effect of TFT. Administration of TAS-102 will not only inhibit TFT degradation but can also inhibit TdR degradation, which can lead to an increase of intracellular TdR [Emura et al. 2004b]. TAS-102 or TPI will not affect the concentration of uridine or deoxycytidine, since TPI does not inhibit UP or deoxycytidine degradation. However, it is possible that TAS-102 can increase the deoxyuridine concentration since it causes a dUMP accumulation [Tanaka et al. 2014].
The addition of TPI is only important for in vivo studies, since in vitro studies showed that, despite high TFT phosphorolysis, high TP-expressing colorectal Colo320TP1 and nonsmall cell lung H460TP2 cancer cells are not more resistant to TFT, while addition of TPI did not increase TFT sensitivity [Temmink et al. 2005; De Bruin et al. 2003]. A moderate almost two-fold increase in formation of active TFT metabolites was found, although it was not related to increased incorporation of TFT into DNA [Temmink et al. 2005]. Probably TK1 has favorable enzyme properties for the TFT-activation pathway by being maximally saturated, which is hardly influenced by TFT degradation by TP. TPI only affected TFT-mediated cytotoxicity at very short exposures in cells expressing very high TP [Temmink et al. 2005]. This means that the mode of in vitro cytotoxicity exerted by TFT is also dependent on the exposure times used. The effect of TPI was not dependent on the activity of TP, since all models displayed different activities of TP.
It can be concluded that the addition of TPI improved the pharmacology of TFT drastically, by almost completely inhibiting TFT degradation, prolonging its half-life and drug exposure, and enhanced its incorporation into DNA.
Tumor angiogenesis
TPI is a potent inhibitor of TP, also known as platelet-derived endothelial cell growth factor (PDECGF) [Ackland and Peters, 1999; Moghaddam and Bicknell, 1992], which is an angiogenesis growth factor [Miyazono et al. 1987]. Angiogenesis is an important process in stimulating tumor vascularization and targeting angiogenesis is an important novel therapeutic approach [Ellis, 2004]. Most anti-angiogenic drugs target growth factors (and their receptors) or inhibit endothelial cell proliferation or/and signal transduction. Vascular endothelial growth factor (VEGF), but also fibroblast growth factor (FGF), PDECGF, and their tyrosine kinase receptors are the major regulators of angiogenesis [Manetti and Botta, 2003; George, 2001]. TP/PDECGF overexpression is often associated with VEGF overexpression. Bevacizumab (Avastin®) depletes VEGF levels and is effective in combination with 5FU-based regimens for colorectal cancer. Thymidine exposure of TP-overexpressing cells leads to an increase of various angiogenic factors (bFGF, interleukin-8, and tumor necrosis factor [TNF]-α, but not VEGF), which enhanced migration and invasion of human umbilical vein endothelial cells [Bijnsdorp et al. 2011].
In addition to VEGF, increased TP/PDECGF expression is seen in colorectal tumor tissue compared with normal tissue [Takebayashi et al. 1996b], and high PDECGF/TP levels are a prognostic factor for poor survival in colorectal [Takebayashi et al. 1996a] and gastric cancers [reviewed in De Bruin et al. 2006]. PDECGF/TP is also overexpressed in tumor-infiltrating cells (mainly macrophages) and colon cancer cells themselves [De Bruin et al. 2006; Van Triest et al. 2000; Takahashi et al. 1996]. High blood vessel density is well correlated with increased PDECGF/TP expression and is associated with metastasis formation in several tumors [Takahashi et al. 1996, 2003]. Both VEGF and PDECGF/TP are important in human angiogenesis induced by both tumor and normal cells and therefore these factors and their receptors are good targets for antiangiogenic therapy [De Bruin et al. 2006]. Tumor growth and metastasis are dependent on a sufficient blood supply and therefore inhibition of tumor-induced angiogenesis in combination with classic cytotoxic chemotherapy may be a strategy to improve survival for patients with solid tumors, as was already shown in preclinical models when an anti-angiogenic agent was combined with cytotoxic agents [Zondor and Medina, 2004; Kabbinavar et al. 2005].
The downstream mediator of PDECGF/TP 2-deoxy-D-ribose is rapidly formed [Bijnsdorp et al. 2010a] (Figure 2), and its increased levels promote angiogenesis by enhancing chemotaxis of vascular endothelial cells [Uchimiya et al. 2002], which may confer resistance to apoptosis induced by hypoxia [Ikeda et al. 2002]. Deoxyribose has shown angiogenic properties in various in vitro and in vivo studies [Seeliger et al. 2004; Hotchkiss et al. 2003], and it was shown that it can stimulate invasion and migrations by activation of focal adhesion kinase (FAK) and p70/S6, the downstream kinase of the mechanistic target of rapamycin (mTOR), which regulates cell proliferation, metabolism, and also angiogenesis. mTOR and FAK seem responsible for the invasive potential of TP. TPI can block these processes and, in vivo [Emura et al. 2005; Akiyama et al. 2004; Takao et al. 2000], it was demonstrated that TPI significantly inhibited PDECGF/TP-induced neovascularization in a dose-dependent manner in mice models. In a specific model, with KB cells transfected with TP leading to a clinically not relevant overexpression of TP (KB/TP cells), TPI decreased the growth rate of these KB/TP cells and increased the apoptotic index, indicating a potential antitumor activity of TPI as a single agent [Takao et al. 2000].
TAS-102 was also able to reduce the number of liver metastases in a nude mouse model [Emura et al. 2004a]; TPI alone decreased the chemotactic motility and basement membrane invasion of the above-mentioned KB/TP cells [Takao et al. 2000], suppressed the number of liver metastases macroscopically, and markedly diminished the invasive activity [Sato et al. 2003]. Due to the potent anti-invasive and antimetastatic activity of TPI, TAS-102 may have multiple mechanisms of action in clinical use.
The expression of TP in tumors shows a larger variation between malignancies with the same pathology, but also between different types of tumors [De Bruin et al. 2006]. When TP is low, chemotherapeutic drugs often upregulate PDECGF/TP [Endo et al. 1999; Fukushima et al. 2002]. This is an advantage for the oral 5FU prodrug capecitabine ((+)-pentyl 1-(5-deoxy-β-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate) [Van Cutsem et al. 2001; Hoff et al. 2001], which is activated by PDECGF/TP and for which a low expression negatively influences therapeutic outcome of capecitabine-based chemotherapy [Ackland and Peters, 1999]. However, activation of the capecitabine intermediate 5-fluoro-5’-deoxyuridine (5’DFUR) to 5FU can also be mediated by UP [Cao and Pizzorno, 2004; Temmink et al. 2006a, 2007a], but TPI is a poor inhibitor of UP [Fukushima et al. 2000]. Since TFT is a poor substrate for UP, a high activity of UP will unlikely affect the efficacy of TFT. In vivo data showed that TAS-102 is only effective in inducing cytotoxicity when systemic TPI is present, but acts against both undetectable and high TP-expressing colon cancer cells.
PDECGF/TP clearly promotes angiogenesis, possibly in conjunction with other growth factors. This pro-angiogenic effect seems to be mediated by breakdown products of thymidine, such as deoxyribose-1-phosphate. Inhibition of thymidine phosphorolysis by TPI has an anti-angiogenic effect. Whether inhibition of angiogenesis contributes to the antitumor activity of TAS-102 in patients needs further investigation.
Combination studies
Novel treatment options often consist of combinations of drugs in which current chemotherapeutic regimens are combined with, for example, protein kinase-targeting drugs, immunotherapy, or radiation therapy. TAS-102 is an excellent candidate for combination with other cytotoxic agents. In in vitro studies potential TAS-102 combinations were extensively investigated by combinations with other anticancer agents (Table 1). TFT was combined with other TS inhibitors [Temmink et al. 2006b], oxaliplatin [Temmink et al. 2007d], or irinotecan (CPT-11) [Temmink et al. 2007c], in different schedules in order to determine their interactions. At low folate conditions TFT and folate-based TS inhibitors (e.g. raltitrexed) showed schedule-dependent synergism in growth inhibition, two-sided TS inhibition, and DNA damage induction, whereas at high folate conditions only additive effects were seen [Temmink et al. 2006b].
Table 1.
Effective combinations of trifluorothymidine or TPI (in vitro) or TAS-102 (in vivo) with conventional chemotherapy targeted against DNA and novel therapeutics targeting protein kinases.
| Drug | Combination drug | Model system | In vitro/in vivo | Outcome | Postulated mechanism | Reference |
|---|---|---|---|---|---|---|
| Trifluorothymidine | Oxaliplatin | Colon cancer | In vitro | Synergistic | Increased DNA adducts | Temmink et al. [2007d] |
| TAS-102 | Oxaliplatin | Colon and gastric cancer | In vivo | Synergistic | Nukatsuka et al. [2015a] | |
| Trifluorothymidine | Irinotecan | Colon cancer | In vitro | Synergistic | Increased DNA damage and apoptosis | Temmink et al. [2007c] |
| TAS-102 | Irinotecan | Colon and gastric cancer | In vivo | Nukatsuka et al. [2015b] | ||
| Trifluorothymidine | Docetaxel | Colon cancer | In vitro | Synergistic | Cell-cycle arrest; cell kill | Bijnsdorp et al. [2008] |
| Trifluorothymidine | Antifolates | Colon cancer | In vitro | Synergistic/additive | DNA damage | Temmink et al. [2006b] |
| TPI | Rapamycin | Colon cancer | In vitro | Synergistic | TPI prevents autophagy | Bijnsdorp and Peters [2011] |
| Trifluorothymidine | Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) | Lung cancer | In vitro | Synergistic | Induction caspase pathway | Azijli et al. [2014] |
| Trifluorothymidine | Radiation | Colon cancer | In vitro | Synergistic | Decreased repair DNA damage | El-Naggar et al. [2014] |
| TAS-102 | Radiation | Colon cancer | In vivo | Sensitization | Angiogenesis and decreased DNA repair | Miyatani et al. [2012] |
| Trifluorothymidine | Erlotinib | Colon cancer | In vitro | Synergistic | S-phase arrest; no DNA repair | Bijnsdorp et al. [2010b] |
| TAS-102 | Cetuximab, panitumumab | Colon cancer | In vivo | Tsukihara et al. [2015] | ||
| TAS-102 | Bevacizumab | Colon cancer | In vivo | Increased TFT-phosphates | Tsukihara et al. [2015] |
The sensitivity of colon cancer cells to oxaliplatin could be increased by simultaneous exposure to TFT [Temmink et al. 2007d], resulting in increased Pt-DNA-adduct formation, and subsequent increased DNA damage induction and apoptosis induction. The TFT-oxaliplatin combination was dose-schedule dependent, since TFT pre-incubation decreased oxaliplatin-induced cytotoxicity to colorectal cancer cells. Recently it was also shown that this combination was synergistic in vivo [Nukatsuka et al. 2015a]. For the combination of TFT and SN38, the active metabolite of irinotecan, the most pronounced synergistic interactions were found when colorectal cancer cells were pre-incubated with TFT before SN38 exposure, which resulted in increased DNA strand-break formation and cell death [Temmink et al. 2007c]. Also in several in vivo models of colorectal and gastric cancer the combination of TAS-102 and irinotecan was superior to single agents without increased toxicity [Nukatsuka et al. 2015b]. TFT induces an uncommon effect on the cell cycle (G2-M accumulation and tetraploidy) [Bijnsdorp et al. 2010c], which explains why the combination docetaxel appeared to be schedule dependent. A pre-incubation of docetaxel followed by TFT was synergistic, due to increased cell kill, polynucleation, and mitotic spindle inhibition, accompanied by phosphorylation of chk2 and dephosphorylation of cdc25 [Bijnsdorp et al. 2008]. The reverse sequence was antagonistic. TFT’s mechanism of cell kill was mediated by the intrinsic pathway (via the mitochondrial cytochrome c pathway, leading to caspase 9 cleavage). This is probably related to the TFT-induced p-53-dependent arrest in the G2 phase that was associated with a proteasome-dependent decrease in cyclin B1 [Matsuoka et al. 2015]. It seemed likely that additional induction of the extrinsic pathway via caspase 8 cleavage would be effective. Indeed TNF-related apoptosis-inducing ligand (TRAIL), a specific inducer of the extrinsic pathway, via binding to the death receptors 4 and 5, was synergistic with TFT [Azijli et al. 2014]. Since many colorectal patients are sensitive to the anti-epidermal growth factor receptor (EGFR) antibody cetuximab (in case tumor KRAS is wild type), the combination of TFT with an EGFR inhibitor was also investigated. The small molecule EGFR inhibitor erlotinib showed synergism in cells with a wild-type EGFR and additivity in cells with a mutant EGFR [Bijnsdorp et al. 2010b], erlotinib appeared to inhibit the prosurvival pathway (AKT and MAPK) induced by TFT. Likewise in vivo combinations of TAS-102 with the anti-EGFR antibodies cetuximab and panitumumab resulted in a more than additive activity, while the combination of bevacizumab with TAS-102 was more effective [Tsukihara et al. 2015]. Interestingly bevacizumab increased the formation of TFT phosphate. Since many nucleoside analogs are excellent radiosensitizers, and since 5FU is used for the treatment of patients with rectal cancer, TAS-102 was investigated for its potential radiosensitizing effect [Miyatani et al. 2012]. It was reasoned that TFT would inhibit the repair of radiation-induced DNA damage, and that TPI would have no effect, which was indeed observed in vivo (TAS-102) [Miyatani et al. 2012], and in vitro [El-Naggar et al. 2014].
In summary TAS-102 has the potential to increase the effect of a variety of different drugs, affecting their mechanism of action at critical points such as DNA repair of drug-induced lesions. The synergism of drugs used in common combination in colorectal cancer, such as oxaliplatin and irinotecan, is especially promising.
Clinical trials
Early phase I and II studies
The initial antitumor effects of TFT against colon cancer were reported in 1971, where it was shown that repeated administration of TFT can produce reduction in tumor size of patients with breast and colon cancer [Ansfield and Ramirez, 1971]. However, systemic administration of TFT alone (2.5 mg/kg/day) in divided doses every 3 h for 8–13 days, resulted in severe bone-marrow depression. TFT-mediated side effects were primarily confined to the hematopoietic system with less damage of the gastrointestinal tract compared with 5FU and FdUrd [Ansfield and Ramirez, 1971; Dexter et al. 1972]. The TFT early clinical trials were discontinued because of the short half-life due to its rapid clearance and extensive degradation by PDECGF/TP in vivo. This likely resulted in the moderate antitumor efficacy that was observed (Table 2).
Table 2.
Overview of phase I and II studies with TAS-102 at different schedules.
| Study | Dose/scheme | Maximum tolerated dose | Dose-limiting toxicity | Patients/disease | Efficacy | Reference | |
|---|---|---|---|---|---|---|---|
| Phase I/II | Trifluorothymidine alone 2.5 mg/kg/day | Every 3 h; 8–13 days | 2.5 mg/kg/day | Bone marrow | 6 colon 23 breast | Some short PR | Ansfield et al. [1971] |
| Phase I (9801) | Once daily for 14 days every 3 weeks (50–100 mg/m2/day) | 50 mg/m2/day | Granulocytopenia | 14 various | 4 stable disease | Hong et al. [2006] | |
| Phase I (9802) | Once daily for 5 days for 2 weeks every 4 weeks (50–110 mg/m2/day | 100 mg/m2/day | Granulocytopenia | 24 various | 7 stable disease | Overman et al. [2008b] | |
| Phase I (9803) | Once daily for 5 days for 1 week every 3 weeks (100–180 mg/m2/day) | 160 mg/m2/day | Granulocytopenia | 39 various | 11 stable disease | Overman et al. [2008b] | |
| Phase I (9804) | Twice daily for 5 days for 2 weeks every 4 weeks (50–80 mg/m2/day) | 50 mg/m2/day | Granulocytopenia | 19 breast | 12 stable disease | Green et al. [2006] | |
| Phase I (9805) | Three times daily for 5 days for 2 weeks every 4 weeks (60–80 mg/m2/day) | 70 mg/m2/day | Granulocytopenia | 15 mostly gastrointestinal | 9 stable disease | Overman et al. [2008a] | |
| Phase 1 (J001) | Twice daily for 5 days for 2 weeks every 4 weeks (30–70 mg/m2/day) | 70 mg/m2/day | Neutropenia | 21 gastrointestinal (18 colorectal) | 12 stable disease | Doi et al. [2012] | |
| Phase II (9806) | Twice daily for 5 days for 2 weeks every 4 weeks (50 mg/m2/day) | 18 gastric | 1 stable disease | Mayer et al. [2015] (supplementary data) | |||
The studies 9801–9806 (TAS102-9801 to TAS102-9806) were performed in the US; study J001 was a Japanese study; PR, partial response.
TFT re-entered clinical development as TAS-102, and in several US phase I clinical trials dosing was optimized (summarized in Table 2). In the first phase I trial (TAS102-9801) TAS-102 was orally administered to patients with solid tumors once daily for 14 days every 3 weeks. The maximum-tolerated dose (MTD) was 50 mg/m2/day with granulocytopenia as the dose-limiting toxicity (DLT) at 100 mg/m2/day [Hong et al. 2006]. In this schedule drug accumulation was observed, since the AUC increased 2–2.5-fold after 2 weeks, possibly explaining the toxicity. In 4 out of 14 patients a stable disease (SD) was observed. In the second trial (TAS102-9802) once-daily TAS-102 was given orally (50–110 mg/m2/day) to patients with gastrointestinal malignancies for 5 days/week for 2 weeks repeated every 4 weeks [Overman et al. 2008b]. This schedule with the 2-day treatment rest allowed the administration of higher doses of TAS-102 compared with the continuous daily schedule. Granulocytopenia was dose limiting, while in 7 out of 24 patients SD was observed. In a third phase I study (TAS102-9803) patients with solid tumors received TAS-102 once daily (doses ranging from 100 mg/m2/day to 140 mg/m2/day for 5 days every 3 weeks) in order to determine the MTD [Overman et al. 2008b]. At 120 mg/m2/day patients experienced severe granulocytopenia, which was considered to be the DLT. Other toxicities observed included neutropenia, mild to moderate nausea, vomiting, diarrhea, fatigue, and rash. Out of 39 patients, SD was observed in 11 patients.
Other TAS-102 phase I trials applied two or three times a day administration schedules, since divided daily dosing of TFT resulted in higher antitumor activity in preclinical studies due to increased incorporation of TFT into DNA. Furthermore, the previous once-daily TAS-102 phase I trials showed a short TFT half-life (about 2 h). A phase I trial (TAS102-9805) was carried out at the University of Texas MD Anderson Cancer Center (Houston, TX, USA) using a three-times daily (60–80 mg/m2/day) schedule (5 days/week for 2 weeks repeated every 4 weeks) with 15 patients with solid tumors receiving TAS-102 orally. It was concluded that TAS-102 was well tolerated with manageable hematologic (most common grade 3/4 neutropenia) and nonhematologic (e.g. nausea, vomiting, fatigue, colitis) toxicities [Overman et al. 2008a]. SD was observed in nine patients. The suggested phase II TAS-102 dose was 70 mg/m2/day. Another phase I trial (TAS102-9804) was carried out to determine the phase II dose and the DLT in 19 patients with metastatic breast cancer [Green et al. 2006]. Patients received TAS-102 orally at an initial dose level of 80 mg/m2/day in a twice-daily schedule (5 days/week for 2 weeks repeated every 4 weeks). Clinical activity (12 SD) was seen including prolonged disease control (> 12 weeks). It was concluded that TAS-102 is an active agent against heavily pretreated metastatic breast cancer patients, with primarily hematologic toxicities (mainly grade 3/4 granulocytopenia and grade 4 thrombocytopenia), and the recommended dose was 50 mg/m2/day using this schedule. In a Japanese phase I study (J001) TAS-102 was also given in the same schedule twice daily (30–70 mg/m2/day) to 21 patients with gastrointestinal malignancies (mainly colorectal cancer), resulting in a higher MTD of 70 mg/m2/day, with 11 patients with SD [Doi et al. 2012]. In summary in the US studies grades 1 and 2 toxicity consisted of nausea (66%), fatigue (64%), and neutropenia (55%), which were also seen as grades 3 and 4, but in general treatment-related toxicities were reversible.
In a phase II study in the US (TAS102-9806) 18 patients (most pretreated with a fluoropyrimidine regimen and progressive) with gastric cancer were treated with the twice-daily schedule at 50 mg/m2/day. All patients progressed within four cycles, and the study was closed early (see supplementary data of Mayer and colleagues [Mayer et al. 2015]). The toxicity profile was similar to that of the phase I studies. In general the MTD in US patients was lower than in the Japanese study, which is most likely not due to the ethnic background but to the difference in pretreatment; most US patients received more pretreatments. For example, a median of more than five prior treatments was given to patients with breast cancer in the TAS102-9804 study, including cyclophosphamide, which can compromise the bone-marrow reserve. A phase I study (TAS102-101) in the USA preceding the RECOURSE phase III study was limited to patients with metastatic colon cancer. The dose of 70 mg/m2/day was shown to be tolerable, underlining that toxicity was related to pretreatment.
Pharmacokinetics
The pharmacokinetics of TAS-102 was investigated in the phase I studies but most extensively in the J001 study in which TAS-102 was given at 30–70 mg/m2/day in a twice-daily schedule for 5 days during 2 weeks repeated every 4 weeks [Doi et al. 2012]. The pharmacokinetics in Japanese patients seemed comparable to that in US patients, for example, at 50 mg/m2/day plasma levels were comparable with those of patients in the same schedule [Green et al. 2006]. TFT showed a slightly nonlinear pharmacokinetics, for example, the Cmax increased from 1009 (at 30 mg/m2/day) to 3338 ng/ml at 70 mg/m2/day; for the AUC0–10 these values were 2037–8678 ng*h/ml. The pharmacokinetics was performed on days 1 and 12, enabling the investigation of potential accumulation as was observed in the first phase I studies. Also in this study an accumulation of the drug was observed, which was most pronounced at the highest dose of 70 mg/m2/day, for example, the Cmax (reached between 1.3 and 1.9 h) increased from 3338 ng/ml to 4752 ng/ml. The increase in AUC0–10 was more pronounced (also at the lower levels) and increased from 8678 ng*h/ml to 20,950 ng*h/ml, with a tendency that the T½ also increased, but only up to 1.5-fold, and was about 2 h at 12 days. Interestingly the metabolite trifluorothymine decreased after 12 days to about 60% of that at day 1. The Cmax of TPI was about 70 ng/ml and was reached after about 2.3 h, with no significant difference between day 1 and day 12, although the AUC0–10 tended to increase from 281 ng*h/ml to 317 ng*h/ml at the 70 mg/m2/day dose. For both the Cmax and AUC0–10 a linear significant inverse relationship (r = -0.678 and r = -0.753, respectively; both p < 0.001) was observed with the relative change in neutrophil count. A recent mass-balance study in patients showed that 60% of the dose of TFT was recovered, mostly in urine, and excreted as TFT itself, trifluorothymine, and TFT glucuronide, while TPI was mostly recovered in feces as TPI and partially as 6-hydroxymethyluracil [Lee et al. 2015a, 2015b], which was in line with the early historical data [Heidelberger et al. 1965; Dexter et al. 1972].
The clinical development of TAS-102 was guided by its preclinical pharmacology, showing that a twice-daily administration would yield a better incorporation into DNA, which is responsible for its antitumor activity. This twice-daily schedule of TAS-102 was well tolerated and yielded similar pharmacokinetics in the USA and Japan.
Randomized phase II and III studies
These dose-finding and early efficacy studies were followed by two placebo-controlled studies; a randomized Japanese study (J003) and a worldwide phase III study (RECOURSE protocol) (Figure 4). In both studies the efficacy of TAS-102 was evaluated in patients with metastatic colorectal cancer, progressive on at least two chemotherapeutic regimens. In the J003 study [Yoshino et al. 2012], all patients (112 in the TAS-102 group and 57 in the placebo group) were refractory or intolerant on treatment regimens including a fluoropyrimidine, oxaliplatin, and irinotecan. The majority of the patients (87 in the TAS-102 and 47 in the placebo group) also received the anti-VEGF antibody bevacizumab and a subgroup the anti-EGFR antibody, cetuximab (71 in the TAS-102 and 34 in the placebo group, although 54 and 24 patients were KRAS wild-type, respectively). TAS-102 was given orally twice daily (70 mg/m2/day) for 5 days a week with 2 days rest for 2 weeks and repeated after 4 weeks. In the Japanese study the disease control rate (DCR) was 43.8% versus 10.5% (p < 0.0001), respectively. The overall survival (OS) was 9.0 months and 6.6 months (hazard ratio [HR] = 0.56; 95% confidence interval: 0.39–0.81; p = 0.0011), respectively. For progression-free survival (PFS) these values were 2.0 months and 1.0 months (HR = 0.41), respectively. In the TAS-102 group an improved OS was observed in patients with wild-type and mut-KRAS, but it was more pronounced and consistent in the mut-KRAS group (HR wt/mt = 1.48; p = 0.045). The safety profile was comparable with that observed in the earlier US and Japanese phase I and II studies, with bone-marrow suppression and gastrointestinal events.
Figure 4.
Efficacy of TAS-102 in colorectal cancer patients, progressive on multiple treatment regimens including a fluoropyrimidine, irinotecan, or oxaliplatin. The upper curve shows the overall survival curve of the Japanese J003 study [Yoshino et al. 2012], while the lower curve shows the overall survival curves of the worldwide RECOURSE study [Mayer et al. 2015]. (Figures reproduced with permission.)
In the RECOURSE study a similar protocol was used as in the J003 study, using the same dose (70 mg/m2/day), randomized in a 2:1 ratio (534 versus 266 patients) [Mayer et al. 2015]. All patients were progressive on systemic anticancer treatment (e.g. fluoropyrimidines, oxaliplatin, irinotecan, bevacizumab), having received at least two regimens but the majority received four regimens. The KRAS wild-type patients (278 versus 144 in each arm, respectively) received an anti-EGFR antibody and a subgroup also received regorafenib (91 and 53, respectively). Also in this worldwide study (Japan, USA, Europe, Australia) TAS-102 showed favorable activity in view of OS (7.1 versus 5.3 months; HR = 0.68; p < 0.001), PFS (2.0 versus 1.7 months; HR = 0.48; p < 0.001), and a DCR of 44% versus 16% (p < 0.001). Even when corrected for three prognostic factors (i.e. time since diagnosis of first metastasis, Eastern Cooperative Oncology Group (ECOG) performance status, and number of metastatic sites), the effect of TAS-102 treatment was maintained (HR = 0.69). The effect was independent of pretreatment (e.g. KRAS status, anti-EGFR antibody treatment, regorafenib). Also in this worldwide study, the safety was comparable with the earlier phase I and II studies, with hematologic toxicity in the TAS-102 group (e.g. grade 3 neutropenia in 38% of the patients), but no toxicities typical for fluoropyrimidines, such as hand–foot syndrome (typical for capecitabine), stomatitis, or coronary spasm. A population pharmacokinetic analysis showed that pharmacokinetic parameters did not vary with race, age, gender, or hepatic functions, that dosing according to body surface area is adequate; renal function was the primary determinant of the pharmacokinetics of TAS-102 indicating that renal function should be monitored in patients on TAS-102 treatment [Cleary et al. 2015].
The two randomized studies yielded almost completely identical results, with a comparable increase in OS and a similar safety profile.
Conclusion and future directions
The novel formulation TAS-102 is promising because of its activity in colorectal cancer patients progressive on classical fluoropyrimidine therapies. Its mechanism of action is distinct from 5FU as has been shown in various studies [Peters and Bijnsdorp, 2012]. In multiple studies it has been shown that 5FU’s activity is related to the inhibition of TS, but not on its incorporation into RNA or DNA. Although TAS-102 inhibits TS (although transiently), its antitumor activity was most clearly related to its incorporation into DNA. It is not clear whether its angiogenic effects contributed to the efficacy of the treatment. Other differences include the different activation pathways. 5FU is dependent on either a phosphoribosyl transferase or UP/uridine kinase, but TFT on TK1. Interestingly, 5FU is protected by autophagy, but TFT is not and is therefore several fold more active in clonogenic assays, which reveal such a difference [Bijnsdorp et al. 2010c].
Future studies should not only explore combinations, but also identify predictive parameters to select patients likely to benefit from TAS-102. These include the equilibrative nucleoside transporter and concentrative nucleoside transporter [Takahashi et al. 2015], and its activation (catalyzed by TK1) [Sakamoto et al. 2015], while positron emission tomography, using 3’-fluoro-3’[18F]-thymidine (FLT), may help to characterize these processes in patients [Lee et al. 2015a, 2015b] TAS-102 appeared to upregulate FLT accumulation in tumors. Evaluation of DNA damage in surrogate tissues and plasma thymidine can reveal limiting metabolic and mechanistic factors, while accumulation of sugars (e.g. deoxyribose) in plasma can give some insight into potential other effects, such as on angiogenesis. Due to the inclusion of TPI, which has clearly shown anti-angiogenic effects in preclinical studies, evaluation of several other angiogenic factors could be included.
Although TAS-102 showed benefit against gastrointestinal malignancies, preclinical studies clearly showed efficacy against other cancers, while its efficacy in preclinical models shows great potential in future clinical drug combination studies, both with conventional cytotoxic agents and signaling targeted therapy, as well as radiotherapy. A combination of these treatments may help to prevent recurrence of tumors due to drug resistance. Specifically TAS-102 has great potential to improve the treatment of gastrointestinal malignancies when combined with other agents, such as with the VEGF inhibitor bevacizumab or the DNA synthesis inhibitors oxaliplatin or irinotecan. Recently the first promising results of the combination of TAS-102 (70 mg/m2/day) and bevacizumab were reported [Kuboki et al. 2015], showing a DCR of 64% by central assessment and 72% by investigator assessment, a median OS of 11 months, no drug–drug interaction, and acceptable toxicity. Other possibilities are combining TAS-102 with the anti-EGFR antibodies cetuximab or panitumumab or with the small-molecule inhibitors of the EGFR tyrosine kinase domain gefitinib or erlotinib, or one of the new second- or third-generation EGFR inhibitors.
Footnotes
Conflict of interest statement: The author received consulting fees from Taiho Pharmaceutical and in the past (more than 4 years ago) financial support for research.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Ackland S., Peters G. (1999) Thymidine phosphorylase: its role in sensitivity and resistance to anticancer drugs. Drug Resist Update 2: 205–214. [DOI] [PubMed] [Google Scholar]
- Akiyama S., Furukawa T., Sumizawa T., Takebayashi Y., Nakajima Y., Shimaoka S., et al. (2004) The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci 95: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansfield F., Ramirez G. (1971) Phase I and II studies of 2’-deoxy-5-(trifluoromethyl)-uridine (NSC-75520). Cancer Chemother Rep 55: 205–208. [PubMed] [Google Scholar]
- Azijli K., Van Roosmalen I., Smit J., Pillay S., Fukushima M., de Jong S., et al. (2014) The novel thymidylate synthase inhibitor trifluorothymidine (TFT) and TRAIL synergistically eradicate non-small cell lung cancer cells. Cancer Chemother Pharmacol 73: 1273–1278. [DOI] [PubMed] [Google Scholar]
- Bijnsdorp I., Azijli K., Jansen E., Wamelink M., Jakobs C., Struys E., et al. (2010a) Accumulation of thymidine-derived sugars in thymidine phosphorylase overexpressing cells. Biochem Pharmacol 80: 786–796. [DOI] [PubMed] [Google Scholar]
- Bijnsdorp I., Capriotti F., Kruyt F., Losekoot N., Fukushima M., Griffioen A., et al. (2011) Thymidine phosphorylase in cancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br J Cancer 104: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnsdorp I., Kruyt F., Fukushima M., Smid K., Gokoel S., Peters G. (2010b) Molecular mechanism underlying the synergistic interaction between trifluorothymidine and the epidermal growth factor receptor inhibitor erlotinib in human colorectal cancer cell lines. Cancer Sci 101: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnsdorp I., Kruyt F., Gokoel S., Fukushima M., Peters G. (2008) Synergistic interaction of trifluorothymidine with docetaxel (Taxotere®) after sequential scheduling. Cancer Sci 99: 2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnsdorp I., Peters G. (2011) Deoxyribose protects against rapamycin induced cytotoxicity in colorectal cancer cells in vitro. Nucleosides Nucleotides Nucleic Acids 30: 1197–1202. [DOI] [PubMed] [Google Scholar]
- Bijnsdorp I., Peters G., Temmink O., Fukushima M., Kruyt F. (2010c) Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells. Int J Cancer 126: 2457–2468. [DOI] [PubMed] [Google Scholar]
- Cao D., Pizzorno G. (2004) Uridine phosphorylase: an important enzyme in pyrimidine metabolism and fluoropyrimidine activation. Drugs Today (Barc) 40: 431–443. [DOI] [PubMed] [Google Scholar]
- Carmine A., Brogden R., Heel R., Speight T., Avery G. (1982) Trifluridine: a review of its antiviral activity and therapeutic use in the topical treatment of viral eye infections. Drugs 23: 329–353. [DOI] [PubMed] [Google Scholar]
- Cleary J., Mayer R., Van Cutsem E., Yamashita F., Yoshisue K. Ieiri, I.et al. (2015) Population pharmacokinetic (PK) analysis of TAS-102 in patients (pts) with metastatic colorectal cancer (mCRC): results from 3 phase 1 trials and the phase 3 RECOURSE trial. J Clin Oncol 33(Suppl.): abstract 2579. [Google Scholar]
- De Bruin M., Temmink O., Hoekman K., Pinedo H., Peters G. (2006) Role of platelet derived endothelial cell growth factor/thymidine phosphorylase in health and disease. Cancer Ther 4: 99–124. [Google Scholar]
- De Bruin M., van Capel T., van der Born K., Kruyt F., Fukushima M., Hoekman K., et al. (2003) Role of platelet-derived endothelial cell growth factor/thymidine phosphorylase in fluoropyrimidine sensitivity. Br J Cancer 88: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. (2004) Antiviral drugs in current clinical use. J Clin Virol 30: 115–133. [DOI] [PubMed] [Google Scholar]
- Dexter D., Wolberg W., Ansfield F., Helson L., Heidelberger C. (1972) The clinical pharmacology of 5-trifluoromethyl-2’-deoxyuridine. Cancer Res 32: 247–253. [PubMed] [Google Scholar]
- Doi T., Ohtsu A., Yoshino T., Boku N., Onozawa Y., Fukutomi A., et al. (2012) Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 107: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein J., Foster P., Finer-Moore J., Wataya Y., Santi D. (1994) Mechanism-based inhibition of thymidylate synthase by 5-(trifluoromethyl)-2’-deoxyuridine 5’-monophosphate. Biochemistry 33: 15086–15094. [DOI] [PubMed] [Google Scholar]
- Ellis L. (2004) Angiogenesis and its role in colorectal tumor and metastasis formation. Semin Oncol 31: 3–9. [DOI] [PubMed] [Google Scholar]
- El-Naggar M., Ebbing E., Bijnsdorp I., Van den Berg J., Peters G. (2014) Radiosensitization by thymidine phosphorylase inhibitor in thymidine phosphorylase negative and overexpressing bladder cancer cell lines. Nucleosides Nucleotides Nucleic Acids 33: 413–421. [DOI] [PubMed] [Google Scholar]
- Emura T., Murakami Y., Nakagawa F., Fukushima M., Kitazato K. (2004a) A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med 13: 545–549. [PubMed] [Google Scholar]
- Emura T., Nakagawa F., Fujioka A., Ohshimo H., Kitazato K. (2004b) Thymidine kinase and thymidine phosphorylase level as the main predictive parameter for sensitivity to TAS-102 in a mouse model. Oncol Rep 11: 381–387. [PubMed] [Google Scholar]
- Emura T., Nakagawa F., Fujioka A., Ohshimo H., Yokogawa T., Okabe H., et al. (2004c) An optimal dosing schedule for a novel combination antimetabolite, TAS-102, based on its intracellular metabolism and its incorporation into DNA. Int J Mol Med 13: 249–255. [PubMed] [Google Scholar]
- Emura T., Suzuki N., Fujioka A., Ohshimo H., Fukushima M. (2005) Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol 27: 449–455. [PubMed] [Google Scholar]
- Emura T., Suzuki N., Yamaguchi M., Ohshimo H., Fukushima M. (2004d) A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 25: 571–578. [PubMed] [Google Scholar]
- Endo M., Shinbori N., Fukase Y., Sawada N., Ishikawa T., Ishitsuka H., et al. (1999) Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5’-deoxy-5-fluorouridine by cyclophosphamide in mammary tumor models. Int J Cancer 83: 127–134. [DOI] [PubMed] [Google Scholar]
- Ford H., Mitchell F., Cunningham D., Farrugia D., Hill M., Rees C., et al. (2002) Patterns of elevation of plasma 2’-deoxyuridine, a surrogate marker of thymidylate synthase (TS) inhibition, after administration of two different schedules of 5-fluorouracil and the specific TS inhibitors raltitrexed (Tomudex) and ZD9331. Clin Cancer Res 8: 103–109. [PubMed] [Google Scholar]
- Fujiwara Y., Heidelberger C. (1970) Fluorinated pyrimidines. 38. The incorporation of 5-trifluoromethyl-2’-deoxyuridine into the deoxyribonucleic acid of vaccinia virus. Mol Pharmacol 6: 281–291. [PubMed] [Google Scholar]
- Fukushima M., Okabe H., Takechi T., Ichikawa W., Hirayama R. (2002) Induction of thymidine phosphorylase by interferon and taxanes occurs only in human cancer cells with low thymidine phosphorylase activity. Cancer Lett 187: 103–110. [DOI] [PubMed] [Google Scholar]
- Fukushima M., Suzuki N., Emura T., Yano S., Kazuno H., Tada Y., et al. (2000) Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2’-deoxyribonucleosides. Biochem Pharmacol 59: 1227–1236. [DOI] [PubMed] [Google Scholar]
- George D. (2001) Platelet-derived growth factor receptors: a therapeutic target in solid tumors. Semin Oncol 28: 27–33. [PubMed] [Google Scholar]
- Green M., Pusztai L., Theriault R., Adinin R., Hofweber M., Fukushima M., et al. (2006) Phase I study to determine the safety of oral administration of TAS-102 on a twice daily (BID) schedule for five days a week (wk) followed by two days rest for two wks, every (Q) four wks in patients (pts) with metastatic breast cancer (MBC). Proc Am Soc Clin Oncol (abstract 10576). [Google Scholar]
- Heidelberger C., Anderson S. (1964) Fluorinated pyrimidines. XXI. The tumor inhibitory activity of 5-trifluoromethyl-2’-deoxyuridine. Cancer Res 24: 1979–1985. [PubMed] [Google Scholar]
- Heidelberger C., Boohar J., Kampschroer B. (1965) Fluorinated pyrimidines. XXIV. In vivo metabolism of 5-trifluoromethyluracil-2-C-14 and 5-trifluoromethyl-2’-deoxyuridine-2-C-14. Cancer Res 25: 377–381. [PubMed] [Google Scholar]
- Heidelberger C., Parsons D., Remy D. (1964) Syntheses of 5-trifluoromethyluracil and 5-trifluoromethyl-2’-deoxyuridine. J Med Chem 55: 1–5. [DOI] [PubMed] [Google Scholar]
- Hoff P., Ansari R., Batist G., Cox J., Kocha W., Kuperminc M., et al. (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19: 2282–2292. [DOI] [PubMed] [Google Scholar]
- Hong D., Abbruzzese J., Bogaard K., Lassere Y., Fukushima M., Mita A., et al. (2006) Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer 107: 1383–1390. [DOI] [PubMed] [Google Scholar]
- Hotchkiss K., Ashton A., Schwartz E. (2003) Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. J Biol Chem 278: 19272–19279. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Furukawa T., Kitazono M., Ishitsuka K., Okumura H., Tani A., et al. (2002) Molecular basis for the inhibition of hypoxia-induced apoptosis by 2-deoxy-D-ribose. Biochem Biophys Res Commun 291: 806–812. [DOI] [PubMed] [Google Scholar]
- Inaba M., Mitsuhashi J., Sawada H., Miike N., Naoe Y., Daimon A., et al. (1996) Reduced activity of anabolizing enzymes in 5-fluorouracil-resistant human stomach cancer cells. Jpn J Cancer Res 87: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbinavar F., Hambleton J., Mass R., Hurwitz H., Bergsland E., Sarkar S. (2005) Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 23: 3706–3712. [DOI] [PubMed] [Google Scholar]
- Kuboki Y., Nishina T., Shinozaki E., Yamazaki K., Shitara K., Okamoto W., et al. (2015) An investigator initiated multicenter phase I/II study of TAS-102 with bevacizumab for metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE). J Clin Oncol 33(Suppl.): abstract 3544. [DOI] [PubMed] [Google Scholar]
- Lee H., Oh S., Lee E., Chung J., Kim Y., Ryu J., et al. (2015a) Positron emission tomography imaging of human colon cancer xenografts in mice with [(18)F]fluorothymidine after TAS-102 treatment. Cancer Chemother Pharmacol 75: 1005–1013. [DOI] [PubMed] [Google Scholar]
- Lee J., Seraj J., Yoshida K., Narurkar M., Mizuguchi H. Strychor, S.et al. (2015b) Mass balance study of TAS-102 using 14C labeling analyzed by accelerator mass spectrometry. J Clin Oncol 33(Suppl.): abstract 2563. [Google Scholar]
- Manetti F., Botta M. (2003) Small-molecule inhibitors of fibroblast growth factor receptor (FGFR) tyrosine kinases (TK). Curr Pharm Des 9: 567–581. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Iimori M., Niimi S., Tsukihara H., Watanabe S., Kiyonari S., et al. (2015) Trifluridine induces p53-dependent sustained G2 phase arrest with its massive misincorporation into DNA and few DNA strand breaks. Mol Cancer Ther 14: 1004–1013. [DOI] [PubMed] [Google Scholar]
- Mayer R., Van Cutsem E., Falcone A., Yoshino T., Garcia-Carbonero R., Mizunuma N., et al. (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372: 1909–1919. [DOI] [PubMed] [Google Scholar]
- Miyatani T., Kurita N., Utsunomiya T., Iwata T., Nishioka M., Yoshikawa K., et al. (2012) Platelet-derived endothelial cell growth factor/thymidine phosphorylase inhibitor augments radiotherapeutic efficacy in experimental colorectal cancer. Cancer Lett 318: 199–205. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Okabe T., Urabe A., Takaku F., Heldin C. (1987) Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem 262: 4098–4103. [PubMed] [Google Scholar]
- Moghaddam A., Bicknell R. (1992) Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry 31: 12141–12146. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Kazuno H., Emura T., Tsujimoto H., Suzuki N., Fukushima M. (2000) Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int J Oncol 17: 277–283. [DOI] [PubMed] [Google Scholar]
- Nukatsuka M., Nakagawa F., Matsuoka K., Tsukihara K., Takechi T. (2015a) Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with oxaliplatin on human colorectal or gastric cancer and 5FU-resistant gastric cancer xenografts. Proc Annu Meet Am Assoc Cancer Res 56: abstract 2557. [Google Scholar]
- Nukatsuka M., Nakagawa F., Saito H., Sakata M., Uchida J., Takechi T. (2015b) Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102 with irinotecan hydrochloride on human colorectal and gastric cancer xenografts. Anticancer Res 35: 1437–1445. [PubMed] [Google Scholar]
- Overman M., Kopetz S., Varadhachary G., Fukushima M., Kuwata K., Mita A., et al. (2008a) Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest 26: 794–799. [DOI] [PubMed] [Google Scholar]
- Overman M., Varadhachary G., Kopetz S., Thomas M., Fukushima M., Kuwata K., et al. (2008b) Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Invest New Drugs 26: 445–454. [DOI] [PubMed] [Google Scholar]
- Peters G. (2014) Novel developments in the use of antimetabolites. Nucleosides Nucleotides Nucleic Acids 33: 358–374. [DOI] [PubMed] [Google Scholar]
- Peters G., Backus H., Freemantle S., van Triest B., Codacci-Pisanelli G., van der Wilt C., et al. (2002) Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 1587: 194–205. [DOI] [PubMed] [Google Scholar]
- Peters G., Bijnsdorp I. (2012) TAS-102: more than an antimetabolite. Lancet Oncol 13: e518–e519. [DOI] [PubMed] [Google Scholar]
- Peters G., Honeywell R., Leon L., Van Groeningen C., Jansen G., Ylstra B., et al. (2009) Role of pharmacodynamic, pharmacogenetic and pharmacogenomic biomarkers of cancer chemotherapy with antifolates. Pteridines 20: s115–s127. [Google Scholar]
- Peters G., Schornagel J., Milano G. (1993) Clinical pharmacokinetics of anti-metabolites. Cancer Surv 17: 123–156. [PubMed] [Google Scholar]
- Pouremad R., Bahk K., Shen Y., Knop R., Wyrwicz A. (1999) Quantitative 19F NMR study of trifluorothymidine metabolism in rat brain. NMR Biomed 12: 373–380. [DOI] [PubMed] [Google Scholar]
- Pugmire M., Ealick S. (2002) Structural analyses reveal two distinct families of nucleoside phosphorylases. Biochem J 361: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes P., Heidelberger C. (1965) Fluor-inated pyrimidines. XXVI. Mammalian thymidylate synthetase: its mechanism of action and inhibition by fluorinated nucleotides. Mol Pharmacol 1: 14–30. [PubMed] [Google Scholar]
- Sakamoto K., Yokogawa T., Ueno H., Oguchi K., Kazuno H., Ishida K., et al. (2015) Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int J Oncol 46: 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D., Sakai T. (1971) Thymidylate synthetase. Model studies of inhibition by 5-trifluoromethyl-2’-deoxyuridylic acid. Biochemistry 10: 3598–3607. [DOI] [PubMed] [Google Scholar]
- Sato J., Sata M., Nakamura H., Inoue S., Wada T., Takabatake N., et al. (2003) Role of thymidine phosphorylase on invasiveness and metastasis in lung adenocarcinoma. Int J Cancer 106: 863–870. [DOI] [PubMed] [Google Scholar]
- Seeliger H., Guba M., Koehl G., Doenecke A., Steinbauer M., Bruns C., et al. (2004) Blockage of 2-deoxy-D-ribose-induced angiogenesis with rapamycin counteracts a thymidine phosphorylase-based escape mechanism available for colon cancer under 5-fluorouracil therapy. Clin Cancer Res 10: 1843–1852. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nakagawa F., Nukatsuka M., Fukushima M. (2011) Trifluorothymidine exhibits potent antitumor activity via the induction of DNA double-strand breaks. Exp Ther Med 2: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Bucana C., Liu W., Yoneda J., Kitadai Y., Cleary K., et al. (1996) Platelet-derived endothelial cell growth factor in human colon cancer angiogenesis: role of infiltrating cells. J Natl Cancer Inst 88: 1146–1151. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ellis L., Mai M. (2003) The angiogenic switch of human colon cancer occurs simultaneous to initiation of invasion. Oncol Rep 10: 9–13. [PubMed] [Google Scholar]
- Takahashi K., Yoshisue K., Chiba M., Nakanishi T., Tamai I. (2015) Involvement of concentrative nucleoside transporter 1 in intestinal absorption of trifluridine using human small intestinal epithelial cells. J Pharm Sci 104: 3146–3153. [DOI] [PubMed] [Google Scholar]
- Takao S., Akiyama S., Nakajo A., Yoh H., Kitazono M., Natsugoe S., et al. (2000) Suppression of metastasis by thymidine phosphorylase inhibitor. Cancer Res 60: 5345–5348. [PubMed] [Google Scholar]
- Takebayashi Y., Akiyama S., Akiba S., Yamada K., Miyadera K., Sumizawa T., et al. (1996a) Clinicopathologic and prognostic significance of an angiogenic factor, thymidine phosphorylase, in human colorectal carcinoma. J Natl Cancer Inst 88: 1110–1117. [DOI] [PubMed] [Google Scholar]
- Takebayashi Y., Yamada K., Miyadera K., Sumizawa T., Furukawa T., Kinoshita F., et al. (1996b) The activity and expression of thymidine phosphorylase in human solid tumours. Eur J Cancer 32A: 1227–1232. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Sakamoto K., Okabe H., Fujioka A., Yamamura K., Nakagawa F., et al. (2014) Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 32: 2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmink O., Bijnsdorp I., Prins H., Losekoot N., Adema A., Smid K., et al. (2010) Trifluorothymidine resistance is associated with decreased thymidine kinase equilibrative nucleoside transporter expression or increased secretory phospholipase A2. Mol Cancer Ther 9: 1047–1057. [DOI] [PubMed] [Google Scholar]
- Temmink O., Comijn E., Fukushima M., Peters G. (2004) Intracellular thymidylate synthase inhibition by trifluorothymidine in FM3A cells. Nucleosides Nucleotides Nucleic Acids 23: 1491–1494. [DOI] [PubMed] [Google Scholar]
- Temmink O., De Bruin M., Comijn E., Fukushima M., Peters G. (2005) Determinants of trifluorothymidine sensitivity and metabolism in colon and lung cancer cells. Anticancer Drugs 16: 285–292. [DOI] [PubMed] [Google Scholar]
- Temmink O., De Bruin M., Laan A., Turksma A., Cricca S., Masterson A., et al. (2006a) The role of thymidine phosphorylase and uridine phosphorylase in (fluoro)pyrimidine metabolism in peripheral blood mononuclear cells. Int J Biochem Cell Biol 38: 1759–1765. [DOI] [PubMed] [Google Scholar]
- Temmink O., de Bruin M., Turksma A., Cricca S., Laan A., Peters G. (2007a) Activity and substrate specificity of pyrimidine phosphorylases and their role in fluoropyrimidine sensitivity in colon cancer cell lines. Int J Biochem Cell Biol 39: 565–575. [DOI] [PubMed] [Google Scholar]
- Temmink O., Emura T., De Bruin M., Fukushima M., Peters G. (2007b) Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci 98: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmink O., Hoebe E., Fukushima M., Peters G. (2007c) Irinotecan-induced cytotoxicity to colon cancer cells in vitro is stimulated by pre-incubation with trifluorothymidine. Eur J Cancer 43: 175–183. [DOI] [PubMed] [Google Scholar]
- Temmink O., Hoebe E., van der Born K., Ackland S., Fukushima M., Peters G. (2007d) Mechanism of trifluorothymidine potentiation of oxaliplatin-induced cytotoxicity to colorectal cancer cells. Br J Cancer 96: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmink O., Hoogeland M., Fukushima M., Peters G. (2006b) Low folate conditions may enhance the interaction of trifluorothymidine with antifolates in colon cancer cells. Cancer Chemother Pharmacol 57: 171–179. [DOI] [PubMed] [Google Scholar]
- Tsukihara H., Nakagawa F., Sakamoto K., Ishida K., Tanaka N., Okabe H., et al. (2015) Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep 33: 2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Furukawa T., Okamoto M., Nakajima Y., Matsushita S., Ikeda R., et al. (2002) Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose. Cancer Res 62: 2834–2839. [PubMed] [Google Scholar]
- Van Cutsem E., Twelves C., Cassidy J., Allman D., Bajetta E., Boyer M., et al. (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19: 4097–4106. [DOI] [PubMed] [Google Scholar]
- Van Triest B., Peters G. (1999) Thymidylate synthase: a target for combination therapy and determinant of chemotherapeutic response in colorectal cancer. Oncology 57: 179–194. [DOI] [PubMed] [Google Scholar]
- Van Triest B., Pinedo H., Blaauwgeers J., van Diest P., Schoenmakers P., Voorn D., et al. (2000) Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res 6: 1063–1072. [PubMed] [Google Scholar]
- Wilson P., Danenberg P., Johnston P., Lenz H., Ladner R. (2014) Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol 11: 282–298. [DOI] [PubMed] [Google Scholar]
- Yano S., Kazuno H., Sato T., Suzuki N., Emura T., Wierzba K., et al. (2004) Synthesis and evaluation of 6-methylene-bridged uracil derivatives. Part 2: optimization of inhibitors of human thymidine phosphorylase and their selectivity with uridine phosphorylase. Bioorg Med Chem 12: 3443–3450. [DOI] [PubMed] [Google Scholar]
- Yoshino T., Mizunuma N., Yamazaki K., Nishina T., Komatsu Y., Baba H., et al. (2012) TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 13: 993–1001. [DOI] [PubMed] [Google Scholar]
- Zondor S., Medina P. (2004) Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother 38: 1258–1264. [DOI] [PubMed] [Google Scholar]