Abstract
Hormone receptor positive tumors represent the most common form of breast cancer and account for most of the deaths from the disease. Endocrine therapy represents the main initial therapeutic strategy for these patients and has been associated with significant clinical benefits in a majority of patients. While in early stages endocrine therapy is administered as part of a curative approach once clinical metastases develop, the disease is considered incurable and the main management objectives are tumor control and quality of life. The two major clinical paradigms of always indicating endocrine therapy in the absence of visceral crises and sequencing endocrine treatments have been guiding our therapeutic approach to these patients. However, for many decades, we have delivered endocrine therapy with a ‘one size fits all’ approach by applying agents that interfere with hormone receptor signaling equally in every clinical patient scenario. We have been unable to incorporate the well-known biologic principle of different degrees of hormone receptor dependency in our therapeutic recommendations. Recent developments in the understanding of molecular interactions of hormone signaling with other important growth factor, metabolic and cell division pathways have opened the possibility of improving results by modulating hormone signaling and interfering with resistance mechanisms yet to be fully understood. Unfortunately, limitations in the design of trials conducted in this area have made it difficult to develop predictive biomarkers and most of the new combinations with targeted agents, even though showing improvements in clinical endpoints, have been directed to an unselected population of patients. In this review we explore some of the current and most relevant literature in the management of hormone receptor positive advance breast cancer.
Keywords: advanced breast cancer, breast neoplasms, drug resistance, hormone receptor
Introduction
Approximately 60–80% of breast cancer cases in Western countries are hormone receptor positive (HR+) and endocrine therapy (ET) is the mainstay for treatment of these patients [Huang et al. 2005]. Recent evidence suggests that the proportion of patients with HR+ disease may be increasing, particularly in premenopausal patients [Anderson et al. 2011]. Considering the worldwide prevalence of the disease, it is plausible that among all of available oncology treatments, ET for breast cancer has a greater global impact compared with all other therapeutic interventions in cancer medicine [Sledge et al. 2015].
Even though most early stage HR+ patients receive adjuvant ET with curative intent, approximately 30% of them will eventually experience relapse with metastatic disease [EBCTCG, 2005]. Furthermore, about 5–10% of breast cancer patients do present with stage IV disease at diagnosis [SEER Database, 2014; Lee, 2014]. Advanced breast cancer (ABC) is currently incurable and the main objective of therapy is to palliate symptoms and prolong survival while maintaining a good quality of life.
In the past 10 years, the aromatase inhibitors (AIs) anastrozole, letrozole and exemestane have largely replaced tamoxifen as adjuvant treatment of early breast cancer in postmenopausal women [Coombs et al. 2004; Dowsett, 2010]. Therefore, strategies to overcome resistance in the setting of previous long-term AI induced estrogen deprivation are needed. Although currently available ET agents are generally effective and well tolerated, not all patients benefit equally. Predictive biomarkers should facilitate a more rational approach for HR+ breast cancer. A better understanding of the features underlying heterogeneity, as well as the mechanisms of resistance to ET, is essential for the development of novel therapies. This review details the current and most relevant evidence that supports optimal management of HR+ metastatic breast cancer as well as future directions of this field.
First-line treatment
ET is the preferred option for treatment of HR+ ABC, even in the presence of visceral disease, unless there is proven endocrine resistance or visceral crisis, defined as severe organ dysfunction as assessed by signs and symptoms, laboratory studies and rapid progression of disease [Cardoso et al. 2014].
Current armamentarium of ET includes selective estrogen receptor (ER) modulators (SERMs), AIs and ER downregulators (SERDs). Recently added targeted agents that modulate endocrine responses are discussed later. Although sequencing of ET is the recommended approach, few randomized trials have directly compared the effects of changing the order in which different agents are given. Unfortunately, even after many decades of trials we still lack definitive recommendations regarding the optimal ET sequencing in patients with ABC [Barrios et al. 2012].
Tamoxifen, the earliest selective ER modulator in clinical use, was first described in the treatment of ABC in 1971 [Cole et al. 1971]. For decades, tamoxifen has been the standard of care for ER+ ABC with consistent efficacy and a favorable toxicity profile. A large review of 86 clinical trials involving more than 5000 tamoxifen treated patients described an overall response rate (ORR) of 34% with an additional 19% of patients achieving stable disease for at least 6 months [Litherland and Jackson, 1988].
In postmenopausal women in whom estrogen synthesis occurs mainly in peripheral tissues, third-generation AIs (anastrozole, letrozole and exemestane) have demonstrated efficacy while decreasing circulating estrogen levels [Lonning and Eeikesal, 2013; Smith and Dowsett, 2003]. Exemestane is a steroidal AI that binds irreversibly to aromatase, whereas the nonsteroidal AIs, anastrozole and letrozole, have shown to bind reversibly to the enzyme [Buzdar et al. 2002]. Although the mechanisms remain unclear, steroidal and nonsteroidal AIs are not fully cross-resistant [Miller et al. 2008]. While there is no clinical evidence suggesting that there is a better AI, in a large meta-analysis including 8504 patients AIs documented superior survival compared with tamoxifen [hazard ratio (HR) 0.89; 95% confidence interval (CI): 0.80–0.99] [Mauri et al. 2006].
The third available therapeutic strategy is directed against the ER itself and is exemplified by fulvestrant, a SERD that blocks ER dimerization and DNA binding, inhibits nuclear uptake and increases the turnover and degradation of ER leading to inhibition of estrogen signaling. Fulvestrant (250 mg) demonstrated it was as effective as anastrozole in tamoxifen failures [Robertson et al. 2003]. More recent data suggest that treatment with higher doses of fulvestrant improves disease control and has a survival advantage compared with anastrozole [Di Leo et al. 2010, 2014; Kuter et al. 2012; Robertson et al. 2014a]. Fulvestrant 500 mg was compared with anastrozole in the phase II FIRST trial (n = 205), which demonstrated both improvements in time to progression (TTP) [Robertson et al. 2009] and overall survival (OS) [Robertson et al. 2014b]. Median TTP was 23.4 months for fulvestrant versus 13.1 months for anastrozole with a 34% reduction in risk of progression (HR 0.66; 95% CI: 0.47–0.92; p = 0.01). Median OS was 54 months for fulvestrant versus 48 months for anastrozole (HR 0.70; 95% CI: 0.50–0.98; p = 0.041). This was the first trial to suggest that an alternative ET may be more effective than an AI in the first-line setting for ABC [Robertson et al. 2014b]. The ongoing confirmatory phase III FALCON trial [ClinicalTrials.gov identifier: NCT01602380] has completed accrual with results expected in 2016. Patients included in this trial are mostly treatment naïve and have not received previous ET.
Combination of endocrine therapies
As the available anti-endocrine drugs have different mechanisms of action, combination of agents is a logical approach to improve the effectiveness of ET. Conflicting results have been reported from the comparison of the combination of fulvestrant (250 mg) with anastrozole versus anastrozole as single agent. The FACT trial reported no clinical advantage with the combination [Bergh et al. 2012], while the SWOG S0226 trial reported advantages in PFS and OS favoring the combination in 694 ABC patients [Mehta et al. 2012]. Subgroup analysis of this trial suggested that the benefits were largely restricted to women who had not received adjuvant tamoxifen and differences in the population included probably explain the conflicting results in the two studies. Furthermore, results of the second-line SoFEA trial showed that combining fulvestrant to anastrozole is not more effective that fulvestrant alone or exemestane alone in HR+ ABC that has progressed during therapy with a nonsteroidal AI. A subgroup analysis suggested those patients with tumors with both ER and PR positivity, favoring a more endocrine-sensitive phenotype, may obtain greater benefit [Johnston et al. 2013]. Based on these data, it could be hypothesized that patients with ET-naïve ABC and those with highly endocrine-sensitive tumors could derive the largest benefit from combination ET. However, in our opinion, we should wait for further evidence before considering the combination of AIs and fulvestrant in routine clinical practice [Migliaccio et al. 2015]. First-line ET trials are summarized in Table 1.
Table 1.
Main randomized clinical trials of endocrine therapies as first-line treatment in advanced breast cancer.
| Study/arms | n | ORR (%) | CBR (%) | Median TTP or PFS (months) | Median OS (months) |
|---|---|---|---|---|---|
| Anz versus Tam [Nabholtz et al. 2000] | 353 | 2117 | 5946 | 115.6 * | 3332 |
| Let versus Tam [Mouridsen et al. 2001] | 907 | 3221 * | 5038 * | 9.46.0 * | 3432 |
| Exem versus Tam [Paridaens et al. 2008] | 391 | 46 | – | 9.95.8 * | 3743 |
| Fulv 250 mg versus Tam [Howell et al. 2004] | 578 | 31.633.9 | 54.362 | 6.88.3 | 36.938.7 |
| Fulv 250 mg versus Anz [Osborne et al. 2003] | 400 | 17.5 | 42.2 | 5.4 | – |
| 17.5 | 36.1 | 3.4 | |||
| FACT trial Fulv LD + Anz versus Anz [Bergh et al. 2012] | 514 | 31.833.6 | 55.055.1 | 10.810.2 | 37.838.2 |
| SWOG trial Fulv LD + Anz versus Anz [Mehta et al. 2012] | 707 | – | – | 1513.5 * | 47.741.3* |
| FIRST trial** Fulv HD versus Anz [Robertson et al. 2009, 2014b] | 205 | 3635.5 | 72.567 | 23.413.1* | 54.148.4* |
| PALOMA-01** Let + palbociclib versus Let [Finn et al. 2015] | 165 | 4333 | 8147* | 20.210.2* | -– |
Statistically significant difference.
Randomized phase II.
Anz, anastrozole; CBR, clinical benefit rate; Exem, exemestane; Fulv, fulvestrant; Let, letrozole; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Tam, tamoxifen; TTP, time-to-progression.
Combination of endocrine and targeted therapies in the first-line setting
The combination of targeted therapy and ET is an evolving field. Preclinical evidence suggests that targeting mechanisms of ET resistance, such as the PIK3CA/AKT/mTOR pathway, the cell cycle machinery and the cross talk between HR and growth factor receptor signaling, among others, might increase or restore endocrine sensitivity. Despite stimulating data from preclinical studies with a variety of agents and targets, only recently have randomized clinical trials demonstrated significant benefit with these approaches. Anti-human epidermal growth factor receptor type 2 (anti-HER2) agents, mammalian target of rapamycin (mTOR) and cyclin-dependent kinase (CDK) 4/6 inhibitors are already incorporated in the clinical care of patients with HR+ ABC both in first- and second-line settings. Trials with PIK3CA inhibitors and anti-vascular endothelial growth factor (anti-VEGF) agents have also been presented. Table 3 reviews selected ‘positive’ trials and Table 4 reviews selected ongoing trials evaluating the strategy of combining targeted agents to avoid or reverse resistance to ET.
Table 3.
Main phase III trials of endocrine therapies as second-line treatment in advanced breast cancer.
| Study/Arms | n | ORR (%) | Median TTP or PFS (months) | Median OS (months) |
|---|---|---|---|---|
| CONFIRM [Di Leo et al. 2010, 2014] | 736 | 9.1 | 6.5 | 25.2 |
| Fulv HD versus Fulv 250 mg | 10.2 | 5.5 * | 22.8 | |
| EFECT [Chia et al. 2008] | 693 | 7.4 | 3.7 | – |
| Fulv LD versus Exem | 6.7 | 3.7 | ||
| SOFEA [Johnston et al. 2013] | 703 | 7.4 | 4.4 | 20.2 |
| Fulv LD + Anz versus Fulv LD versus Exem | 6.9 3.6 | 4.83.4 | 19.421.6 | |
| BOLERO-2 [Baselga et al. 2012] | 724 | 9.5 | 7.8 | 31.0 |
| Everolimus + Exem versus Exem | 0.4* | 3.2* | 26.6 | |
| PALOMA-3 [Turner et al. 2015] | 521 | 10.4 | 9.2 | – |
| Fulv HD+ palboc versus Fulv HD | 6.3 | 3.8* |
Anz, anastrozole; Exem; exemestane; Fulv, HD, high dose; LD, low dose; ORR, overall response rate; OS, overall survival; palboc, palbociclib; PFS, progression-free survival; TTP, time-to-progression.
Table 4.
Selected positive trials in endocrine therapy resistance.
| Resistance target or pathway | Agent (stage of development) | ER agent ET setting | Median PFS (months) | Reference |
|---|---|---|---|---|
| CDK4/6 | Palbociclib (phase II) | Letrozole First-line | 20.2 versus 10.2 | Finn et al. [2015] |
| CDK4/6 | Palbociclib (phase III) | Fulvestrant Second-line | 9.2 versus 3.8 | Turner et al. [2015] |
| HER2 | Trastuzumab (phase III) | Anastrozole First-line | 4.8 versus 2.4 | Kaufmann et al. 2009] |
| HER2 | Lapatinib (phase III) | Letrozole First-line | 8.3 versus 3.0 | Johnston et al. [2009] |
| mTOR | Everolimus (phase III) | Exemestane Second-line | 7.8 versus 3.2 | Baselga et al. [2012] |
| mTOR | Everolimus (phase II) | Tamoxifen Second-line | 8.6 versus 4.5 | Bachelot et al. [2012] |
| PI3K | Pictisilib (phase II) | Fulvestrant Second-line | 6.6 versus 5.1 | Krop et al. [2015] |
| Vascular endothelial growth factor | Bevacizumab (phase III) | Letrozole First-line | 20 versus 16 | Dickler et al. [2015] |
| Proteasome | Bortezomib (phase II) | Fulvestrant Second-line | 12mo PFS 28% versus 14% | Adelson et al. [2014] |
| Histone deacetylase | Entinostat (phase II) | Exemestane Second-line | 4.3 versus 2.3 | Yardley et al. [2013] |
| Src | Dasatinib (phase II) | Letrozole First-line | 20.1 versus 9.9 | Paul et al. [2013] |
CDK, cyclin-dependent kinase; ER, endocrine receptor; ET, endocrine therapy; HDAC, histone deacetylase; HER2, human epidermal growth factor receptor type 2; mTOR, mammalian target of rapamycin; PFS, progression-free survival.
CDK4/6 inhibitors
Analysis by the Cancer Genome Atlas (TCGA) demonstrated an association between deregulated cyclin D, CDK4/6 and retinoblastoma (Rb) interaction and luminal B cancer [Ma and Ellis, 2013]. The activation of CDK4/6 by cyclin D leads to Rb phosphorylation and progression of the cell cycle into S phase and is associated with resistance to ET [Thangavel et al. 2011]. Therefore, CDK4/6 inhibitors have the potential to improve the efficacy of ET. Palbociclib (PD-0332991) is an oral, small-molecule inhibitor of CDK4/6 with preclinical evidence of growth-inhibitory activity in HR+ breast cancer cells and synergy with anti-estrogens [Turner et al. 2015].
The PALOMA-1 trial [Finn et al. 2015], was a randomized phase II study that included postmenopausal women with advanced HR+ and HER2 negative tumors with no previous systemic treatment for ABC (see Table 3). Patients could have completed adjuvant therapy with an AI more than 12 months before enrolment (only 32% of patients had received prior adjuvant ET). Patients were randomized to a combination of palbociclib (125 mg daily on days 1–21 every 28 days) and letrozole versus letrozole alone. The addition of palbociclib to letrozole in this very endocrine sensitive population significantly improved PFS that was 20.2 months for the combination versus 10.2 months for letrozole alone (HR 0.48; 95% CI: 0.31–0.74; p = 0.0004). Additionally, the proportion of patients with objective responses and clinical benefit was greater in the combination group. Palbociclib demonstrated excellent tolerability and safety profile, the only frequent adverse event being neutropenia, which occurred in 54% of the patients receiving palbociclib. No cases of febrile neutropenia were reported and the discontinuation rate of palbociclib was very low. A phase III study (PALOMA-2) has completed recruitment and results are awaited. Based on of these results and before any confirmatory information, the combination of palbociclib plus letrozole received US Food and Drug Administration (FDA) accelerated approval as a first-line alternative for HR+ ABC in February 2015. Abemaciclib (LY2853219) and ribociclib (LEE011) are additional CDK4/6 inhibitors being explored in clinical trials (see Table 5).
Table 5.
Selected ongoing phase III trials investigating targeted therapies in combination with ET.
| Target | Agent | ET agent | ClinicalTrials.gov identifier | Estimated enrollment (n) |
|---|---|---|---|---|
| CDK4/6 | Palbociclib | Letrozole | NCT01740427 | 450 |
| PALOMA2 | ||||
| CDK4/6 | Ribociclib | Letrozole | NCT01958021 | 650 |
| MONALEESA 2 | ||||
| CDK4/6 | Abemaciclib | Letrozole or anastrozole | NCT02246621 | 450 |
| MONARCH 3 | ||||
| PI3K | BKM120 | Fulvestrant | NCT01633060 | 615 |
| PI3K | Taselisib | Fulvestrant | NCT02340221 | 600 |
| Sandpiper | ||||
| mTOR | Everolimus | Fulvestrant and anastrozole | NCT02137837 | 825 |
| S1222 | ||||
| HER2 | Lapatinib and trastuzumab | Aromatase inhibitor | NCT01160211 | 525 |
| HDAC | Entinostat | Exemestane | NCT02115282 | 600 |
CDK, cyclin-dependent kinase; ET, endocrine therapy; HDAC, histone deacetylase; HER2, human epidermal growth factor receptor type 2; mTOR, mammalian target of rapamycin.
Anti-angiogenesis in combination with ET
Preclinical data suggest estradiol modulates angiogenesis under both physiologic and pathologic conditions. High VEGF levels in breast tumors have been associated with decreased response to ET. The anti-VEGF monoclonal antibody bevacizumab has been extensively studied in the treatment of both HR+ and negative ABC. A recent systematic review of 14 phase III trials evaluating bevacizumab including more than 4400 patients with ABC showed consistent benefits in relapse rate (RR) and PFS; however, no trial demonstrated OS improvement [Kümler et al. 2014]. Recently, two phase III randomized trials evaluated whether bevacizumab could potentially delay the emergence of resistance to ET. The LEA trial tested the addition of bevacizumab to letrozole or fulvestrant in first-line therapy of postmenopausal patients with HR+ HER2 negative ABC. Despite an improved ORR, both TTF and OS were comparable in the two arms [Martín et al. 2015]. The similarly designed CALGB 40503 trial found a statistically significant improvement in terms of PFS with the combination of bevacizumab and letrozole versus letrozole alone, 20 months versus 16 months respectively (HR = 0.74; 95% CI: 0.58–0.95; p = 0.016) [Dickler et al. 2015]. Again, no difference in OS was reported. In both trials, there were increased grade 3 and 4 toxicities with the addition of the anti-angiogenic agent, the most frequently reported being hypertension and proteinuria. In spite of these results, the role of bevacizumab remains undefined and should not be recommended in combination with ET outside of a clinical trial.
ER+ HER2+ ABC
There is compelling preclinical evidence for a clinically significant cross talk between endocrine signaling and some of the growth factor pathways. Studies have shown that combinations of ET and anti-HER2 treatment (trastuzumab and lapatinib) are feasible and effective, doubling clinical benefit rate (CBR) and TTP compared with ET monotherapy (see Table 3) [Johnston et al. 2009; Kaufmann et al. 2009]. Yet, there are no randomized trials comparing the combinations with anti-HER agents alone in ABC. Importantly, there are no randomized data on whether to continue anti-HER2 therapy with endocrine agents as is currently recommended for anti-HER2 therapies following an initial response to chemotherapy [Barrios et al. 2012]. Still, notwithstanding the lack of controlled trials, a recent American Society of Clinical Oncology (ASCO) guideline states that, for patients with HER2-positive and ER-positive ABC, clinicians may recommend either standard first-line therapy or, for selected patients, endocrine therapy plus HER2-targeted therapy or endocrine therapy alone [Giordano et al. 2014]. Accordingly, we feel that combining anti-HER2 agents and ET is a valid alternative for the treatment of ER+ HER2+ ABC when chemotherapy is not indicated and this strategy could also be considered as maintenance therapy after initial response to chemotherapy and anti-HER2 therapy.
Second-line treatment
The current unavailability of predictive biomarkers defines the need to consider clinical factors when selecting the best hormonal treatment for progressive disease after previous ET (see Table 2). Individual tumors respond differently to different endocrine agents and being able to select which agent an individual patient’s cancer is most sensitive to is a realistic, as well as a clinically worthwhile, goal [Barrios et al. 2012].
Table 2.
Factors to consider when selecting endocrine therapy for patients with advanced breast cancer.
| Patient | Age |
| Menopausal statusComorbiditiesPerformance statusExpectations and preferencesToxicities to previous treatmentsAdherence, compliance | |
| Tumor | Histological subtypeExpression of hormone receptorsHER2 amplificationIntrinsic subtypePredictive biomarkers * |
| Disease | Site of metastasisTumor burdenSymptomatology and/or need for rapid responsePrevious endocrine treatmentDisease-free interval on adjuvant settingResponse to previous ETDuration of response to previous ET |
| Agent | Mechanism of actionExpected toxicitiesPharmacological interactionsAvailabilityCostRoute of administration |
| Other issues | Availability of clinical researchExisting guidelinesFinancial hardshipSocial support |
Predictive biomarkers for endocrine therapy in HR+ ABC are not available and developments in this field should be a priority of future research.
ABC, advanced breast cancer; ET, endocrine therapy; HER2, human epidermal growth factor receptor type 2; HR+, hormone receptor positive.
One of the major hurdles that have hampered the development of ET is the lack of a clear understanding of the mechanisms of resistance that follow our interference with HR signaling. From the clinical point of view, intrinsic or primary resistance has been defined as recurrence within the first 2 years of adjuvant ET or progressive disease within 6 months of starting ET in the advanced setting. However, acquired or secondary resistance is considered with a recurrence after the first 2 years of adjuvant ET or disease progression more than 6 months after initiation of ET in the advanced setting [Cardoso et al. 2014]. These definitions, although imperfect and arbitrary, have been useful in some clinical trials to stratify patient populations [Nagaraj and Ma, 2015; Mauri et al. 2006; Ma et al. 2015].
The existence of agent-selective resistance mechanisms can be inferred from the clinical observation that acquired resistance to one endocrine agent does not preclude a response to another from a different therapeutic class [Ellis, 2004]. In addition, the superior efficacy of an AI versus tamoxifen implies that some tumors with intrinsic tamoxifen resistance remain sensitive to estrogen deprivation. In a first series of randomized trials, each of the third-generation AIs were shown to be superior in efficacy and/or safety profile to megestrol acetate or to the first-generation AI aminoglutethimide as second-line therapy for postmenopausal women progressing on tamoxifen [Buzdar et al. 1998; Dombernowsky et al. 1998; Gershanovich et al. 1998; Kaufmann et al. 2000; Schiavon and Smith, 2013] (Table 3). After AI failure, limited data suggest that tamoxifen is of clinical benefit in approximately 50% of patients, with 10% or less achieving an objective response [Thürlimann et al. 2003; Schiavon and Smith, 2013]. In the TAMRAD study, patients treated with tamoxifen alone after AI failure had a 6 month CBR of 42% and a median TTP of 5.4 months [Bachelot et al. 2012].
A number of trials have explored the recommended sequential administration of ET without clearly defining an optimal or rational sequence. Of note, we have abundant information regarding the molecular profile of primary breast cancer [Ma and Ellis, 2013], but very little information on the molecular changes that characterize or lead to recurrent disease. Most of the clinical trials have not been able to effectively collect or analyze data or tissue that would inform on specific resistance mechanisms on an individual patient which could potentially help us while selecting the most appropriate subsequent therapy. As an example, emerging information suggests that, while mutations in the estrogen receptor 1 (ESR1) gene are rare in primary tumors, their incidence increases significantly (11–55%) in patients after exposure to AIs [Merenbakh-Lamin et al. 2013; Robinson et al. 2013; Toy et al. 2013; Jeselsohn et al. 2014].
Different strategies have been explored in previously treated patients with no definitive improvements in outcome. For example, fulvestrant 250 mg has been compared with AIs [Osborne et al. 2003; Robertson et al. 2003] and has been combined with AIs with no clear demonstration of benefit [Bergh et al. 2012; Mehta et al. 2012]. However, three strategies are worth mentioning as they may suggest new alternatives to improve treatment results for these patients.
Increasing the dose of fulvestrant
The strategy of increasing the dose of fulvestrant has been explored in patients with prior exposure to ET. The CONFIRM study was a multicenter, double-blind, phase III trial that included 736 postmenopausal women with ER+ ABC with progression after tamoxifen or AIs [Di Leo et al. 2010]. Patients were randomized to receive 500 mg or 250 mg of fulvestrant (days 0, 14, 28 and every 28 days thereafter). Results showed that albeit with similar ORR and CBR, the 500 mg dose was associated with a small (6.5 months versus 5.5 months) but statistically significant longer PFS (HR = 0.80; 95% CI: 0.68, 0.94; p = 0.006). OS was not different at the time of the initial report, but with longer follow up, an unplanned analysis [Di Leo et al. 2014] showed a significant 4.1 months difference in favor of the higher dose of fulvestrant. There were no differences in the toxicity profile. The suggested benefit in terms of OS is unique, once very few therapies have demonstrated improvements in survival in patients with HR+ ABC in randomized comparisons. After the publication of these results, fulvestrant at 500 mg has become the preferred schedule for this drug in clinical trials [Ciruelos et al. 2014]. Table 2 shows selected second-line ET trials.
PIK3CA/AKT/mTOR inhibitors
Abnormalities in PIK3CA are the most frequent molecular alteration in HR+ breast cancer identified in 45% and 29% of luminal A and B tumors, respectively. Other components of the PI3K/AKT/mTOR pathway are frequently altered as well [Ellis et al. 2012; The Cancer Genome Atlas Network, 2012; Ma and Ellis, 2013]. Activation of the PIK3CA pathway has been shown to regulate ER expression [Creighton et al. 2010] and has been associated with sensitivity to ET [Ellis et al. 2010]. This may explain the synthetic lethality of combined PIK3CA and ER inhibition [Crowder et al. 2009]. Inhibiting this pathway seems a very reasonable approach to explore. The mTOR serine-threonine kinase regulates a number of metabolic processes in the cell integrating cell growth, cellular proliferation, survival and motility signals mediated by ER, HER2 and other tyrosine kinase receptors [Ma et al. 2015]. Deregulation of mTOR function occurs in various tumor types, including breast cancer, and has been associated with cancer pathogenesis, disease progression and treatment resistance [Martin et al. 2013]. Extensive preclinical data indicate cross talk between the HR and the PI3K/Akt/mTOR signaling pathways [Campbell et al. 2001; Degraffenried et al. 2004].
Interestingly, the inhibition of mTOR in the first-line setting was not associated with benefits over Et alone in the phase III HORIZON trial [Wolff et al. 2013]. Conversely, the BOLERO2 trial, a randomized phase III study, compared everolimus (mTOR1 inhibitor) and exemestane versus exemestane and placebo in 724 patients with HR+ ABC with recurrence or progression while receiving or within 12 months of completing a nonsteroidal AI in the adjuvant setting or progressing during therapy for metastatic disease [Baselga et al. 2012]. A significant improvement in PFS of 7.8 months in the everolimus arm versus 3.2 months with placebo was demonstrated (HR 0.45; 95% CI: 0.38–0.54; p < 0.0001). There was no statistically improvement in OS [Piccart et al. 2014]. Given the remarkable PFS benefit, everolimus was approved by the FDA for the treatment of HR+ ABC in combination with exemestane after failure to letrozole or anastrozole. The addition of everolimus resulted in more toxicity with a significantly higher proportion of discontinuations and grade 3/4 adverse effects in the everolimus arm such as stomatitis, noninfectious pneumonitis and hyperglycemia that are usually not seen with the AI as a single agent [Piccart et al. 2014].
The identification of predictive biomarkers for PIK3CA/mTOR inhibition remains an unmet need, since next-generation sequencing efforts in the BOLERO2 trial could not show a relationship between somatic mutation patterns especially in the catalytic subunit of PIK3CA and clinical outcomes [Hortobagyi et al. 2013; Loi et al. 2013]. An important caveat is that mutational status was assessed mainly on primary tumor tissues and many studies have shown discordant rates in PIK3CA mutational status between primary tumors and metastases [Gonzales-Angulo et al. 2011], suggesting that molecular pathways alterations should be re-analyzed in the metastatic setting. The feasibility of assessing somatic genetic abnormalities, such as PIK3CA mutations, through ‘liquid biopsies’ in circulating tumor cells [Deng et al. 2014] and circulating free DNA [Board et al. 2010] has already been demonstrated. A recently reported exploratory analysis from the phase II TAMRAD trial demonstrated correlations between everolimus efficacy and mTORC1 activation that need validation [Treilleux et al. 2015]. The combination of everolimus and exemestane should be considered an appropriate second line treatment option for patients who have demonstrated benefit from previous lines of ET [Migliaccio et al. 2015; Baselga et al. 2012].
Following the development of the mTOR inhibitors, drugs targeting other components of the pathway are in development and include AKT and PIK3CA inhibitors, as well dual kinase inhibitors targeting both mTOR and PIK3CA [Hasson et al. 2013; Cavazzoni et al. 2012; Sanchez et al. 2011]. Despite encouraging preclinical studies, however, clinical evidence of benefit is still limited. The recently presented FERGI trial was the first randomized phase II study to evaluate the combination of ET with a PIK3CA inhibitor in postmenopausal women with AI resistant HR+ ABC [Krop et al. 2015]. This trial showed that adding pictisilib (GDC0941) to fulvestrant was associated with a nonsignificant PFS improvement from 3.8 to 6.2 months (HR 0.77; 95% CI: 0.50–1.19). PIK3CA mutation status was not associated with outcome. The relationship between PIK3CA inhibition and other mutations and the efficacy of the next generation of inhibitors of this pathway needs to be addressed prospectively and studies are ongoing [Juric et al. 2012].
Combination of ET with CDK4/6 inhibitors
As previously presented, CDK4/6 inhibitors seem to modulate ET. The recently reported PALOMA3 trial demonstrates that palbociclib is associated with significant benefit in previously treated HR+ patients. This double-blind phase III study randomized 521 patients with advanced HR+ HER2 negative ABC with progression after prior ET to receive palbociclib and fulvestrant or placebo and fulvestrant. This study allowed the inclusion of premenopausal or perimenopausal women receiving goserelin. The study was stopped earlier after a planned interim analysis demonstrated efficacy with a median PFS of 9.2 months with palbociclib plus fulvestrant and 3.8 months with fulvestrant (HR 0.42; 95% CI: 0.32 to 0.56; p < 0.001). As expected, more neutropenia, usually not associated with standard ET (78.8% versus 3.5%), was seen with the combination. Palbociclib-treated patients maintained quality of life and the rate of discontinuation due to adverse events was similar to the placebo arm [Turner et al. 2015]. At the time of this analysis, the number of deaths was insufficient for a definitive assessment of differences in OS. Unfortunately, in spite of the early effort in the PALOMA1 study in trying to identify a molecularly selected population of patients (cyclin D1 amplification or p16 loss), so far we have not been able to pinpoint the patient population that benefits the most from CDK4/6 inhibition [Finn et al. 2015].
Other strategies
The evolving nature of our understanding of the complexities of hormonal signaling has fueled the development of many other potential strategies to overcome resistance and improve the clinical results of ET in ABC. Epigenetic modulation of gene expression has been addressed in HR+ ABC patients [Ma et al. 2015]. AI resistance is characterized by estrogen-independent growth and mechanisms of resistance may include decreased ER expression. Entinostat, a selective histone deacetylase (HDAC) inhibitor, leads to increased expression of both ERα and aromatase in preclinical studies. Initial positive results of a phase II trial [Yardley et al. 2013] in combination with exemestane are being confirmed in a phase III trial. Other than the classical AIs, SERDs and SERMs, anti-angiogenesis, mTOR inhibition, CDK4/6 inhibition and epigenetic modulation addressed in our discussion, the role of anti-androgens [Fioretti et al. 2014], apoptosis evasion [Signore et al. 2013], interactions with the tumor microenvironment [Dittmerj, 2014], ESR1 mutations [Robinson et al. 2013; Toy et al. 2013], as well as other tyrosine kinase inhibitors (TKIs) and growth factor signaling pathways are also being studied [Paul et al. 2013; Adelson et al. 2014]. Table 4 summarizes selected ‘positive’ trials and Table 5 reviews ongoing phase III trials of targeted therapies combined with ET.
Not to be forgotten, a number of ET alternatives have been used with varying success over the years and remain options to consider (while delaying chemotherapy) in the progressing patient that persists with clinical evidence of endocrine sensitivity. Progestins (megestrol acetate, medroxyprogesterone) have a reported RR of 25% but are associated with weight gain, fluid retention and an incidence of thromboembolic events [Abrams et al. 1999; Bines et al. 2014]. Androgens (testosterone, fluoxymesterone, danazol) are rarely used but have reported responses albeit associated with significant adverse events [Coombs et al. 1980; Manni et al. 1981]. Estrogens (diethylstilbestrol) have been paradoxically used to treat HR+ ABC showing similar efficacy to tamoxifen but higher toxicity [Ingle et al. 1981; Ellis et al. 2009; Iwase et al. 2013]. Recent preclinical evidence that estrogen therapy may be effective in the setting of ESR1 amplification needs validation [Li et al. 2013]. Despite the low number of patients studied and lack of randomized prospective data, withdrawal of ET and observation as is selectively practiced in prostate cancer is another potential strategy for breast cancer patients [Chavarri-Guerra et al. 2014].
Premenopausal patients
In premenopausal patients with HR+ ABC, tamoxifen or suppression of estrogen synthesis have been standard treatments for decades [Beatson, 1896]. Tamoxifen has shown comparable efficacy (RR, OS) to oophorectomy [Buchanan et al. 1986; Boccardo et al. 1994]. In a meta-analysis of 4 studies (n = 506), the combination of luteinizing hormone-releasing hormone A (LHRH-A) plus tamoxifen resulted in significantly prolonged PFS and OS [Klijn et al. 2001] relative to either agent alone. AIs are not suitable for use alone in premenopausal women and can only be administered in combination with ovarian function suppression (OFS). Adding indirect evidence to this discussion, the practice-changing SOFT and TEXT trials demonstrate that some perimenopausal patients with early stage breast cancer have a PFS benefit with OFS in combination with an AI in comparison with tamoxifen in the adjuvant setting [Pagani et al. 2014]. Whether OFS alone is as effective remains to be explored. After progression on tamoxifen and with indication of further ET, the National Comprehensive Cancer Network (NCCN) guideline and a limited amount of clinical data [Klijn et al. 2000; Carlson et al. 2010; Park et al. 2010; NCCN, 2015] suggest that premenopausal and perimenopausal patients with ABC should be treated with OFS, either with LHRH-A or through surgical oophorectomy, and cared for as if they were postmenopausal patients.
Discussion
HR+ ABC represents a significant clinical problem that is responsible for most of breast cancer deaths. Historically, ET has been an effective therapeutic approach associated with both efficacy and limited toxicity. Sequential administration of available endocrine treatments has been recommended until demonstration either of the need for a rapid response or evidence of clinical resistance leading to indication of chemotherapy.
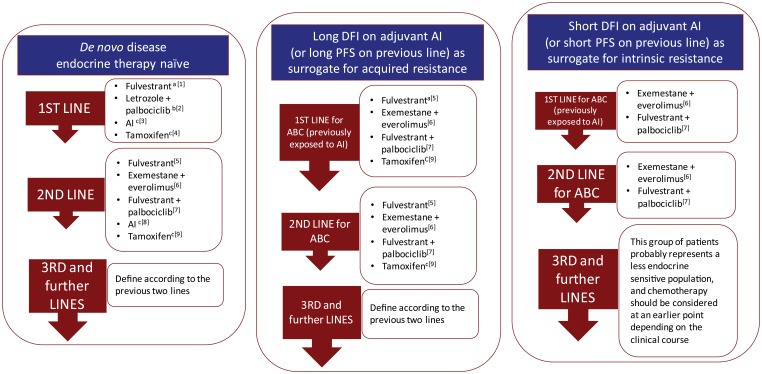
However, our clinical approach to these patients has significant limitations. We tend to treat HR+ patients as having the same endocrine sensitivity not considering tumor heterogeneity or disease progression associated changes. A recurrence, many years after the initial diagnosis, is routinely managed on the basis of the primary tumor information disregarding the fact that cancer evolves. At the same time, we have not been able to define the optimal sequencing of the available endocrine treatment alternatives. The denomination of ‘first’ or ‘second line’ trials is often confusing as most frequently relates to the disease and not the line of therapy. Many so-called first-line trials include patients with previous exposure to endocrine treatments and result in outcomes of difficult biological interpretation. For the most part there is very little ‘rational’ on our current process of selecting endocrine therapy. Please refer to Figure 1 as an arbitrary and very personal approach at this challenging issue.
Figure 1.
Suggested endocrine therapy sequencing alternatives in patients with HR+ advanced breast cancer.
General comments to interpret Figure 1
- The sequence of the treatment alternatives in each line text box does not represent a particular preference order.
- Subsequent use of ET should always take into account previous lines of treatment as well as the type and duration of response to previous ET.
- Intrinsic or primary resistance has been defined as recurrence within the first 2 years of adjuvant ET or progressive disease within 6 months of starting ET in the advanced setting.
- Acquired or secondary resistance has been defined as recurrence after the first 2 years of adjuvant ET or disease progression more than 6 months after initiation of ET in the advanced setting. These definitions, although imperfect and somewhat arbitrary, have been useful in some clinical trials to analyze and stratify patient populations.
- These suggestions refer to postmenopausal patients. Specific management of the premenopausal population is addressed in the text.
- These suggestions do not take into consideration acess and regulatory issues in the dfferent regions of the world.
Specific comments
aFulvestrant (500 mg) use in the first-line treatment of HR+ ABC is based on the randomized phase II FIRST trial. The ongoing confirmatory phase III FALCON trial has completed accrual.
bLetrozole in combination with palbociclib in the first-line treatment of HR+ ABC is based on the randomized phase II PALOMA-1 trial. The ongoing confirmatory phase III PALOMA 2 trial has completed accrual.
cDespite the evidence of superior outcomes with other alternatives, our personal opinion is that an AI or tamoxifen remain reasonable options as first-line or second-line ET for selected patients with HR+ ABC, particularly in the very endocrine sensitive population.
dThis combination demonstrated significant PFS advantage in a second-line phase III trial.
eIn this setting patients may have more endocrine resistant disease and would probably benefit more from treatment strategies that could modulate endocrine resistance rather than sequential single agent ET.
fThe sequential use of the combinations with CDK4/6 inhibitors and mTOR inhibitors has not been addressed in clinical trials and there is no definitive information on response rates or benefit in this setting. Chemotherapy remains an alternative for these most endocrine resistant situations. However, the occasional patient that remains clinically stable with slowly progressive disease in spite previous ET failure could still be considered for treatment with a combination with CDK4/6 or mTOR inhibitor depending on previous exposure.
ABC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin-dependent kinase; DFI, disease-free interval; ET, endocrine therapy; HR, hormone receptor; mTOR, mammalian target of rapamycin; PFS, progression-free survival;
Emerging preclinical and clinical evidence expanding on the complexities of endocrine receptor signaling and the interactions with other pathways have generated different alternatives that used in combination with standard ETs have resulted in improved results. However, as previously discussed, these new combinations (i.e. AI plus mTOR inhibitor or CDK4/6 inhibitor) are at the same time associated with a particular toxicity profile not commonly seen with standard ET. Furthermore, these new medications add significantly to the price tag of managing these patients. The issue of cost and more importantly the value of oncology treatments have been the focus of recent discussions that are extremely important if we are going to incorporate these advances in our clinical practice [Cherny et al. 2015; Schnipper et al. 2015].
Finally, and most importantly, we lack definitive information on the molecular characterization of progressing tumors and mechanisms of resistance to ET. We need to generate predictive biomarkers to guide more personalized care for these patients. Technical developments in sequencing circulating tumor DNA among other ongoing efforts attempting to define changes induced by previous treatments, will allow us to better understand what happens after we interfere with HR signaling. To further advance in this area we feel is essential for ongoing clinical trials to have properly planned and well-designed translational components to answer these pressing questions.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.H.B. has received clinical research funding, consulting and honoraria from Astra Zeneca, Novartis, Pfizer, Roche/Genentech and Lilly. T.R. declares no conflicts of interest in preparing this article.
Contributor Information
Tomas Reinert, Instituto do Câncer, Sistema de Saúde Mãe de Deus, Porto Alegre, RS, Brazil.
Carlos H. Barrios, PUCRS School of Medicine, Department of Medicine, Padre Chagas 66/203, CEP 90 570 080, Porto Alegre, RS, Brazil.
References
- Abrams J., Aisner J., Cirrincione C., Berry D., Muss H., Cooper M., et al. (1999) Dose-response trial of megestrol acetate in advanced breast cancer: cancer and leukemia group B phase III study 8741. J Clin Oncol 17: 64–73. [DOI] [PubMed] [Google Scholar]
- Adelson K., Raptis G., Sparano J., Germain D. (2014) Randomized phase II study of fulvestrant versus fulvestrant plus bortezomib in postmenopausal women with estrogen receptor (ER) positive, aromatase-inhibitor (AI) resistant metastatic breast cancer (MBC): New York Cancer Consortium trial P8457. Cancer Res 71: OT3-01-01. [Google Scholar]
- Anderson W., Katki H., Rosenberg P. (2011) Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot T., Bourgier C., Cropet C., Ray-Coquard I., Ferrero J., Freyer G., et al. (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30: 2718–2724. [DOI] [PubMed] [Google Scholar]
- Barrios C., Forbes J., Jonat W., Conte P., Gradishar W., Buzdar A., et al. (2012) The sequential use of endocrine treatment for advanced breast cancer: where are we? Ann Oncol 23: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J., Campone M., Piccart M., Burris H., 3rd, Rugo H., Sahmoud T., et al. (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson G. (1896) On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet 148: 104–107. [PMC free article] [PubMed] [Google Scholar]
- Bergh J., Johnsson P., Lidbrink E., Trudeu M., Eiermann W., Brattstrom D., et al. (2012) Fact: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30: 1919–1925. [DOI] [PubMed] [Google Scholar]
- Bines J., Dienstmann R., Obadia R., Branco L., Quintella D., Castro T., et al. (2014) Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial. Ann Oncol 25: 831–836. [DOI] [PubMed] [Google Scholar]
- Board R., Wardley A., Dixon J., Armstrong A., Howell S., Renshaw L., et al. (2010) Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat 120: 461–467. [DOI] [PubMed] [Google Scholar]
- Boccardo F., Rubagotti A., Perrotta A., Amoroso D., Balestero M., DeMatteis A., et al. (1994) Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol 5: 337–342. [DOI] [PubMed] [Google Scholar]
- Buchanan R., Blamey R., Durrant K., Howell A., Paterson G., Preece P., et al. (1986) A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer. J Clin Oncol 4: 1326–1330. [DOI] [PubMed] [Google Scholar]
- Buzdar A., Jonat W., Howell A., Jones S., Blomqvist C., Vogel C., et al. (1998) Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Arimidex Study Group. Cancer 83: 1142–1152. [PubMed] [Google Scholar]
- Buzdar A., Robertson J., Eiermann W., Nabholtz J-M. (2002) An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer 95: 2006–2016. [DOI] [PubMed] [Google Scholar]
- Campbell R., Bhat-Nakasharit P., Patel N., Constantinidou D., Ali S., Nakashatri H. (2001) Phosphatidylinositol3-kinase/AKT-mediated activation estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276: 9817–9824. [DOI] [PubMed] [Google Scholar]
- Cardoso. F., Costa A., Norton I., Senkus E., Aapro M., André F., et al. (2014) ESO-ESMO 2nd international consensus guideline for advanced breast cancer (ABC2). The Breast 23: 489–502. [DOI] [PubMed] [Google Scholar]
- Carlson R., Theriault R., Schurman C., Rivera E., Chung C., Phan S., et al. (2010) Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol 28: 3917–3921. [DOI] [PubMed] [Google Scholar]
- Cavazzoni A., Bonelli M., Fumarola C., LaMonica S., Airoud K., Bertoni R., et al. (2012) Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett 323: 77–87. [DOI] [PubMed] [Google Scholar]
- Chavarri-Guerra Y., Higgins M., Szymonifka J., Cigler T., Liedke P., Partridge A., et al. (2014) Drug withdrawal in women with progressive metastatic breast cancer while on aromatase inhibitor therapy. Br J Cancer 111: 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny N., Sullivan R., Dafni U., Kerst J., Sobrero A., Zielinski C., et al. (2015) A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol 26: 1547–1573. [DOI] [PubMed] [Google Scholar]
- Chia S., Gradishar W., Mauriac L., Bines J., Amant F., Federico M., et al. (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26: 1664–1670. [DOI] [PubMed] [Google Scholar]
- Ciruelos E., Pascual T., Vozmediano M., Blanco M., Manso L., Parilla L., et al. (2014) The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. The Breast 23: 201–208. [DOI] [PubMed] [Google Scholar]
- Cole M., Jones C., Todd I. (1971) A new anti-estrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer 25: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs R., Dearnley D., Humphreys J., Gaszet J., Ford H., Nash A., et al. (1980) Danazol treatment of advanced breast cancer. Cancer Treat Rep 64: 1073–1076. [PubMed] [Google Scholar]
- Coombs R., Hall E., Gibson L., Paridaens R., Jassem J., Delozier T., et al. (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast caner. N Engl J Med 350:1081–1092. [DOI] [PubMed] [Google Scholar]
- Creighton C., Fu X., Hennessy B., Casa A., Zhang Y., Gonzalez-Angulo A., et al. (2010) Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res 12: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder R., Phommaly C., Tao Y., Hoog J., Luo J., Perou C., et al. (2009) PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res 69: 3955–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degraffenried L., Friedrichs W., Russell D., Donzis E., Middleton A., Silva J., et al. (2004) Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res 10: 8059–8067. [DOI] [PubMed] [Google Scholar]
- Deng G., Krishanukumar S., Powell A., Zhang H., Mindrinos M., Telli M., et al. (2014) Single cell mutation analysis of PIK3CA in circulating tumors cells and metastases in breast cancer reveals heterogeneity, discordance, and mutation persistence in cultured disseminated tumor cells from bone marrow. BMC Cancer 14: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler M., Barry W., Cirrincione C., Ellis M., Moynaham M., Innocenti F., et al. (2015) Phase III trial evaluating the addition of bevacizumab to letrozole as first-line endocrine therapy for treatment of hormone-receptor positive advanced breast cancer: CALGB 40503 (alliance). J Clin Oncol 33: abstr 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leo A., Jerusalem G., Petruzelka L., Torres R., Bondarenko I., Khasanov R., et al. (2010) Results of the confirm phase III trial comparing fulvestrant 250mg with fulvestrant 500mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28: 4594–4600. [DOI] [PubMed] [Google Scholar]
- Di Leo A., Jerusalem G., Petruzelka L., Torres R., Bondarenko I., Khasanov R., et al. (2014) Final overal survival: fulvestrant 500mg versus 250mg in the randomized CONFIRM trial. J Nat Cancer Inst 106: djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmerj L. (2014) The impact of tumor stroma on drug response in breast cancer. Semin Cancer Biol 31: 3–15. [DOI] [PubMed] [Google Scholar]
- Dombernowsky P., Smith I., Falkson G., Leonard R., Panasci L., Bellmunt J., et al. (1998) Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol 16: 453–461. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Cuzick J., Ingle J., Coates A., Forbes J., Bliss J., et al. (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28: 509–518. [DOI] [PubMed] [Google Scholar]
- Ellis M. (2004) Overcoming endocrine therapy resistance by signal transduction inhibition. Oncologist 9: 20–26. [DOI] [PubMed] [Google Scholar]
- Ellis M., Ding L., Shen D., Luo J., Suman V., Wallis J., et al. (2012) Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M., Gao F., Dehdashti F., Jeffe D., Marcom P., Carey L., et al. (2009) Lower-dose versus high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA 302: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M., Lin L., Crowder R., Tao Y., Hooq J., Snider J., et al. (2010) Phosphatidylinositol-3-kinase alpha catalityc subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res Treat 119: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R., Crown J., Lang I., Boer K., Bondarenko I., Kulyk S., Ettl J., et al. (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 Study. Lancet Oncol 16: 25–35. [DOI] [PubMed] [Google Scholar]
- Fioretti F., Sita-Lumdsen A., Bevan C., Brooke G. (2014) Revising the role of the androgen receptor in breast cancer. J Mol Endocrinol 52: R257–R265. [DOI] [PubMed] [Google Scholar]
- Gershanovich M., Chaudri H., Campos D., Lurie H., Bonaventura A., Jeffrey M., et al. (1998) Letrozole, a new oral aromatase inhibitor: randomised trial comparing 2.5 mg daily, 0.5 mg daily and aminoglutethimide in postmenopausal women with advanced breast cancer. Letrozole International Trial Group (AR/BC3). Ann Oncol 9: 639–645. [DOI] [PubMed] [Google Scholar]
- Giordano S., Temin S., Kirshner J., Chandarlapaty S., Crews J., Davidson N., et al. (2014) Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32: 2078–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Angulo A., Ferrer-Lonzano J., Stemke-Hale K., Sahin A., Luis S., Barrera J., et al. (2011) PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 10: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson S., Rubinek T., Ryvo L., Wolf I. (2013) Endocrine resistance in breast cancer: focus on the phosphatidylinositol 3-kinase/akt/ mammalian target of rapamycin signaling pathway. Breast Care 8: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi G., Piccart-Gebhart M., Rugo H., Burris H., Campone M., Noguchi S., et al. (2013) Correlation of molecular alterations with efficacy of everolimus in hormone receptor-positive, HER2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 31(Suppl.): abstract LBA509. [Google Scholar]
- Howell A., Robertson J., Abram P., Lichinister M., Elledge R., Bajetta E., et al. (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blinded, randomized trial. J Clin Oncol 22: 1605–1613. [DOI] [PubMed] [Google Scholar]
- Huang H., Neven P., Drijkoningen M., Paridaens R., Wildieris H., Van Limbergen E., et al. (2005) Association between tumor characteristics and HER2/neu by immunohistochemestry in 1362 women with primary operable breast cancer. J Clin Pathol 58: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Ahmann D., Green S., Edmondson J., Bisel H., Kvols L., et al. (1981) Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med 304: 16–21. [DOI] [PubMed] [Google Scholar]
- Iwase H., Yamamoto Y., Yamamoto-Ibusuki M., Murakami K., Okomura Y., Tomita S., et al. (2013) Ethinylestradiol is beneficial for postmenopausal patients with heavily pre-treated metastatic breast cancer after prior aromatase inhibitor treatment: a prospective study. Br J Cancer 109: 1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeselsohn R., Yelensky R., Buchwalter G., Frampton G., Meric-Berstam F., Gonzalez-Angulo A., et al. (2014) Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 20: 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Kilburn L., Ellis P., Dodwell D., Cameron D., Hayward L., et al. (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14: 989–998. [DOI] [PubMed] [Google Scholar]
- Johnston S., Pippen J., Pivot X., Lichinister M., Sadeghi S., Dieras V., et al. (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27: 5538–5546. [DOI] [PubMed] [Google Scholar]
- Juric D., Argiles G., Burris H., Gonzalez-Angulo A., Saura C., Quadt C., et al. (2012) Phase I study of BYLl719, an alpha-specific PI3K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-Positive (ER+) metastatic breast cancer (MBC) Cancer Res 72(Suppl. 24): abstract P6–10–07. [Google Scholar]
- Kaufmann M., Bajetta E., Dirix L., Fein L., Jones S., Zilembo N., et al. (2000) Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol 18: 1399–1411. [DOI] [PubMed] [Google Scholar]
- Kaufmann B., Mackey J., Clemens M., Bapsy P., Vaid A., Wardley A., et al. (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27: 5529–5537. [DOI] [PubMed] [Google Scholar]
- Klijn J., Beex L., Mauriac L., van Zijl J., Veyret C., Wildiers J., et al. (2000) Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: a randomized study. J Natl Cancer Inst 92: 903–911. [DOI] [PubMed] [Google Scholar]
- Klijn J., Blamey R., Boccardo F., Tominaga T., Duchateau L., Sylvester R. (2001) Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol 19: 343–353. [DOI] [PubMed] [Google Scholar]
- Krop I., Johnston S., Mayer I., Dickler M., Ganju V., Forrero-Torres A., et al. (2015) The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant versus fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer – part I results. 2014 San Antonio Breast Cancer Symposium, abstract S2-02. [Google Scholar]
- Kümler I., Christiansen O., Nielsen D. (2014) A systematic review of bevacizumab efficacy in breast cancer. Cancer Treat Rev 40: 960–973. [DOI] [PubMed] [Google Scholar]
- Kuter I., Gee J., Hegg R., Singer C., Badwe R., Lowe E., et al. (2012) Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized phase II study. Breast Cancer Res Treat 133: 237–246. [DOI] [PubMed] [Google Scholar]
- Lee T., Isaacs C. (2014) Treatment of primary breast tumors in de novo metastatic breast cancer. Clin Adv Hem Oncol 12: 820–827. [PubMed] [Google Scholar]
- Li S., Shen D., Shao J., Crowder R., Liu W., Prat A., et al. (2013) Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep 4: 1116–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litherland S., Jackson I. (1988) Antiestrogens in the management of hormone-dependent cancer. Cancer Treat Rev 15: 183–194. [DOI] [PubMed] [Google Scholar]
- Loi S., Michels S., Baselga J., Bartlett J., Singhal S., Sabine V., et al. (2013) PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS One 8: e53292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonning P., Eeikesal H. (2013) Aromatase inhibition: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer 20: R183–R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Ellis M. (2013) The Cancer Genome Atlas: clinical applications for breast cancer. Oncology 27: 1263–1269. [PubMed] [Google Scholar]
- Ma C., Reinert T., Chmielewska I., Ellis M. (2015) Mechanisms of aromatase inhibitors resistance. Nat Rev Cancer 15: 261–275. [DOI] [PubMed] [Google Scholar]
- Manni A., Arafah B., Pearson O. (1981) Androgen-induced remissions after antiestrogen and hypophysectomy in stage IV breast cancer. Cancer 48: 2507–2509. [DOI] [PubMed] [Google Scholar]
- Martin L., Andre F., Campone M., Bachelot T., Jerusalem G. (2013) mTOR inhibitors in advanced breast cancer: ready for prime time? Cancer Treat Rev 39: 742–752. [DOI] [PubMed] [Google Scholar]
- Martín M., Loibl S., Von Minckwitz G., Morales S., Martinez N., Guerrero A., et al. (2015) Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol 33: 1045–1052. [DOI] [PubMed] [Google Scholar]
- Mauri D., Pavlidis N., Polyzos N., Ioannidis J. (2006) Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Nat Cancer Inst 98: 1285–1291. [DOI] [PubMed] [Google Scholar]
- Mehta R., Barlow W., Albain K., Vanderberg T., Dhakil S., Tirumali N., et al. (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenbakh-Lamin K., Ben-Baruch N., Yeheskel A., Dvir A., Soussan-Gutman L., Jeselsohn R., et al. (2013) D538G mutation in estrogen receptor-α: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 73: 6856–6864. [DOI] [PubMed] [Google Scholar]
- Migliaccio I., Malorni L., Hart C., Guarducci C., Di Leo A. (2015) Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancer. BMC Med 13: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W., Bartlett J., Brodie A., Brueggermeier R., diSalle E., Lonning P., et al. (2008) Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter? Oncologist 13: 829–837. [DOI] [PubMed] [Google Scholar]
- Mouridsen H., Gershanovic M., Sun Y., Perez-Carrion R., Boni C., Monnier A., et al. (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19: 2596–2606. [DOI] [PubMed] [Google Scholar]
- Nabholtz J., Buzdar A., Pollak K., Harwin W., Burton G., Mangalik A., et al. (2000) Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 18: 3758–3767. [DOI] [PubMed] [Google Scholar]
- Nagaraj G., Ma C. (2015) Revisiting the estrogen receptor pathway and its role in endocrine therapy for postmenopausal women with estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat 150: 231–242. [DOI] [PubMed] [Google Scholar]
- NCCN (2015) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, version 3.2015. Fort Washington, PA: National Comprehensive Cancer Network. [Google Scholar]
- Osborne C., Pippen J., Jones S., Parker L., Ellis M., Come S., et al. (2003) Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advance breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20: 3386–3395. [DOI] [PubMed] [Google Scholar]
- Pagani O., Regan M., Walley B., Fleming G., Colleoni M., Lang I., et al. (2014) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paridaens R., Dirix L., Beex L., Nooij M., Cameron D., Cufer T., et al. (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastastic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Breast Cancer Cooperative Group. J Clin Oncol 26: 4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Ro J., Lee K., Kim E., Kwon Y., Nam B., et al. (2010) Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol 28: 2705–2711. [DOI] [PubMed] [Google Scholar]
- Paul D., Vukejla S., Holmes F., Blum J., McIntyre K., Kumar A. (2013) Letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor-positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor (AI) therapy. Cancer Res 73: abstract S3-07. [Google Scholar]
- Piccart M., Hortobahyi G., Campone M., Pritchard K., Lebrun F., Ito Y., et al. (2014) Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 25: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Lindemann J., Garnett S., Anderson E., Nicholson R., Kuter I., et al. (2014a) A good drug made better: the fulvestrant dose-response story. Clin Breast Cancer 14: 381–389. [DOI] [PubMed] [Google Scholar]
- Robertson J., Lombart-Cussac A., Feltl D., Dewar J., Jasiówka M., Hewson N., et al. (2014b) Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II ‘FIRST’ study. 2014 San Antonio Breast Cancer Symposium, abstract S6-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Llombart-Cusac A., Rolski J., Feltl D., Dewar J., Macpherson E., et al. (2009) Activity of fulvestrant 500mg versus anastrozole 1mg as first-line treatment for advanced breast cancer: results from the FIRST Study. J Clin Oncol 27: 4530–4535. [DOI] [PubMed] [Google Scholar]
- Robertson J., Osborne C., Howell A., Jones S., Mauriac L., Ellis M., et al. (2003) Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer 98: 229–238. [DOI] [PubMed] [Google Scholar]
- Robinson D., Wu Y., Vats P., Su F., Lonigro R., Cao X., et al. (2013) Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45: 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C., Ma C., Crowder R., Guintoli T., Phommaly C., Gao F., et al. (2011) Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res 13: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon G., Smith I. (2013) Endocrine therapy for advanced/metastatic breast cancer. Hematol Oncol North Am 27: 715–736. [DOI] [PubMed] [Google Scholar]
- Schnipper L., Davidson N., Wollins D., Tyne C., Blayney D., Blum D., et al. (2015) American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol 33: 2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEER*Stat Database (2014) Incidence-SEER 17 Regs Limited-Use, N.S.-V.S., Epidemiology, and End Results (SEER) Program. Bethseda, MD: National Cancer Institute; Available at: www.seer.cancer.gov [Google Scholar]
- Signore M., Ricci-Vitian L., De Maria R. (2013) Targeting apoptosis pathways in cancer stem cells. Cancer Lett 332: 374–382. [DOI] [PubMed] [Google Scholar]
- Sledge G., Mamounas E., Hortobagyi G., Burstein H., Goodwin P., Wolff A. (2015) Past, present and future challenges in breast cancer treatment. J Clin Oncol 22: 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Dowsett M. (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348: 2431–2442. [DOI] [PubMed] [Google Scholar]
- Thangavel C., Dean J., Ertel A., Knudsen K., Aldaz C., Witkiewicz A., et al. (2011) Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 18: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. (2012) Comprehensive molecular portraits of human breast tumours. Nature 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- Thürlimann B., Robertson J., Nabholtz J., Buzdar A., Bonneterre J. (2003) Efficacy of tamoxifen following anastrozole compared with anastrozole following tamoxifen as firstline treatment for advanced breast cancer in postmenopausal women. Eur J Cancer 39: 2310–2317. [DOI] [PubMed] [Google Scholar]
- Toy W., Shen Y., Won H., Green B., Sakr R., Will M., et al. (2013) ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 45: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treilleux I., Arnedos M., Cropet C., Wang Q., Ferrero J., Abadie-Lacourtoisie S., et al. (2015) Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol 26: 120–125. [DOI] [PubMed] [Google Scholar]
- Turner N., Ro J., André F., Lois S., Verma S., Iwata H., et al. (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373: 209–219. [DOI] [PubMed] [Google Scholar]
- Wolff A., Lazar A., Bondarenko I., Garin A., Brincat S., Chow L., et al. (2013) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 31: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley D., Ismail-Khan R., Melichar B., Lichinitser M., Munster P., Klein P., et al. (2013) Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31: 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]