Abstract
Cardiovascular disease (CVD) is a major cause of mortality in type 1 diabetes mellitus (T1D). However, evidence of its risks and management is often extrapolated from studies in type 2 diabetic (T2D) patients or the general population. This approach is unsatisfactory given that the underlying pathology, demographics and natural history of the disease differ between T1D and T2D. Furthermore, with a rising life expectancy, a greater number of T1D patients are exposed to the cardiovascular (CV) risk factors associated with an ageing population. The aim of this review is to examine the existing literature around CVD in T1D. We pay particular attention to CVD prevalence, how well we manage risk, potential biomarkers, and whether the studies included the older aged patients (defined as aged over 65). We also discuss approaches to the management of CV risk in the older aged. The available data suggest a significant CVD burden in patients with T1D and poor management of CV risk factors. This is underpinned by a poor evidence base for therapeutic management of CV risk specifically for patients with T1D, and in the most relevant population – the older aged patients. We would suggest that important areas remain to be addressed, particularly exploring the risks and benefits of therapeutic approaches to CVD management in the older aged.
Keywords: Type 1 diabetes, older aged, older adults, elderly, ageing, cardiovascular disease, prevalence, risk factor, management, treatment
Introduction
Cardiovascular disease (CVD) is a major cause of mortality in type 1 diabetes mellitus (T1D) [Morrish et al. 2001]. The management of CVD in patients with T1D is, however, based on evidence that is at best sparse and often nonexistent. Frequently, management has been based on evidence extrapolated from studies in type 2 diabetes mellitus (T2D) or the general population [The National Collaborating Centre for Chronic Conditions, 2004]. This approach is unsatisfactory for a number of reasons. Firstly, there is emerging evidence that the pathogenesis of atherosclerosis in CVD differs between T1D and T2D and the nondiabetic population [Pajunen et al. 2000; Moreno et al. 2000]. Secondly, the age at which CVD becomes evident differs between T1D and T2D, compromising a reliance on therapies validated in older T2D patients. Thirdly, differences seen in the duration and natural history of CVD in patients with T1D and T2D raise the prospect of a need to initiate cardiovascular (CV) protective therapy earlier in T1D.
These considerations gain increasing importance in the context of a rising life expectancy in T1D [Miller et al. 2012; Lung et al. 2014]. As mortality from renal disease and acute metabolic complications fall (though not in all countries) [Pambianco et al. 2006], a greater number of T1D patients are exposed to the CV risk that associates with an ageing population. A recent cross-sectional survey undertaken by our group using data generated within a UK primary care setting identified a CVD prevalence of 40% in T1D patients aged over 65 years [Chapman et al. 2013].
This narrative review describes existing literature relating to CVD in T1D. Particular focus is applied to CVD prevalence, how well we manage risk, potential biomarkers in monitoring CVD, and the extent to which studies have included older aged participants (defined as aged over 65). We also discuss approaches to the management of CV risk in the older aged. Areas in which there is a paucity of available evidence are identified and a number of strategies suggested for improved research in to, and management of, T1D in older patients.
Methods
A literature search was undertaken using Medline (Ovid) and Embase (Ovid), with respective temporal limits of 1946 to March 2014 and 1974 to 1 April 2014. CVD, as defined by the British Heart Foundation, includes all diseases of the heart and circulation, including coronary artery disease (CAD), heart failure, congenital heart disease and stroke [British Heart Foundation, undated]. The search terms ‘cardiovascular disease’ (disease) and ‘type 1 diabetes’ (population) were used in the search. A total of 1765 papers were initially identified, 461 of which were duplicates.
The remaining 1304 manuscripts were subsequently screened by title. Review articles, conference abstracts and manuscripts with titles that were not relevant to CVD and T1D were removed. A total of 335 articles were subsequently reviewed and each was classified into 1 or more of 31 broad categories. We focused on 11 of these categories which were pertinent to the objective of this review (Table 1). A total of 99 papers from the 11 pertinent categories were included, in addition to 7 additional articles identified from the original search which were considered relevant and 30 papers identified through lateral searches. Flow chart 1 and Table 1 outline the search process of paper selection and the categories manuscripts were assigned to.
Table 1.
Inclusion and exclusion criteria and the broad categories.
| Inclusion criteria | |
| |
| Exclusion criteria | |
| |
| All categories | Included categories |
|
|
CACTI, Coronary Artery Calcification in Type 1 Diabetes; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial; T1D, type 1 diabetes.
Flowchart 1.
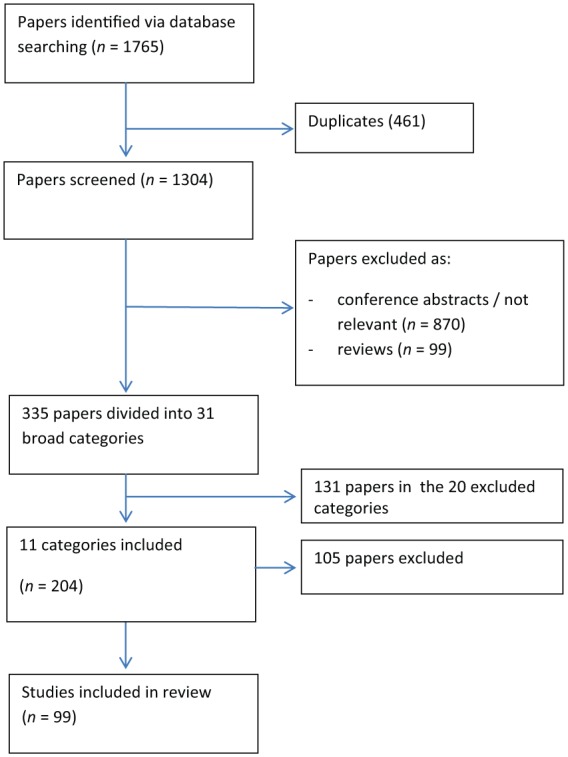
Search strategy flow chart with 99 studies included in this review paper.
Prevalence of CVD in T1D
For clarity, we have described prevalence for CVD disease, CVD mortality and CVD risk factors in separate sections. Incidence rate ratio (IRR) describes the incidence rate (incident cases over the follow-up length) of the study population as a proportion of the incidence rate of the controls [Sedgwick, 2010]. Hazard ratio (HR) compares the rate of death or event in the study population with that of the controls across the follow-up period [Sedgwick, 2011]. The standardized mortality ratio (SMR) is a comparison of the number of observed death in the study population with the number of expected deaths based on age specific rates in a standard population [Public Health England]. The cohorts of commonly referenced studies are abbreviated and listed in Table 2.
Table 2.
Abbreviations of the cohorts of commonly referenced studies.
| Acronym | Short title of the study cohort | Reference |
|---|---|---|
| CLM | Castilla-La Mancha Multicentre Study | Sastre et al. [2012] |
| DCCT/EDIC | Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications | Nathan et al. [2005 |
| DPV | German Surveillance Database | Schwab et al. [2006] |
| EDC | The Pittsburgh Epidemiology of Diabetes Complication Study | Pambianco et al. [2006] |
| EURODIAB | EURODIAB Prospective Complication Study | Soedamah-Muthu et al. [2008] |
| FinnDiane | Finnish Diabetic Nephropathy Study | Gordin et al. [2011] |
| GPRD | UK General Practice Research Database | Soedamah-Muthu et al. [2006b] |
| IEMR | Cross-sectional study of cardiovascular disease risk factors in patients with type 1 diabetes at the Isfahan Endocrine & Metabolism Research Centre | Kalantari et al. [2007] |
| JMRH | Jamaica Major Referral Hospitals Study | Tulloch-Reid et al. [2009] |
| LAHDCA | Liverpool Aintree Hospital Diabetes Clinic Audit | Wallymahmed et al. [2005] |
| LDRDS | Lithuanian Type 1 Diabetes Register Database Study | Dobrovolskienė et al. [2013] |
| NCDQ | Norwegian Childhood Diabetes and Quality Project | Margeirsdottir et al. [2008] |
| NCDR | Norwegian Childhood Diabetes Registry | Skrivarhaug et al. [2006] |
| SRLS | Scottish Registry Linkage Study | Livingstone et al. [2012] |
| UHVGPD | University Hospitals Vienna and Graz Paediatric Department Study | Steigleder-Schweiger et al. [2012] |
CVD events are increased in T1D
There are a few established large T1D cohorts that provide valuable epidemiological data of CVD prevalence. Amongst these include the Pittsburgh Epidemiology of Diabetes Complication Study (EDC), the Finnish Diabetic Nephropathy Study (FinnDiane), the European Diabetes Prospective Complication Study (EURODIAB) and the Epidemiology of Diabetes Interventions and Complications study (EDIC, 1994) which is a long-term follow up of the Diabetes Control and Complications Trial cohort (DCCT, 1983–1993).
The EDC 1950–1980 cohort reported a CAD incidence density of 0.36 per 100 person-years (n = 906, baseline mean age 28, follow up censored in 2000) [Pambianco et al. 2006]. The FinnDiane followed 3110 T1D (baseline mean age 39) for a median of 5 years and found 269 (9%) patients had an incident CVD [Gordin et al. 2011]. At year 11 of the EDIC study, the CVD event rate was 0.38 and 0.80 per 100 patient-years in the intensive and conventional diabetes treatment group, respectively [follow up 17 years (mean), n = 593 and 589, mean age 45 at follow up] [Nathan et al. 2005].
Two large observational studies show higher rates of CVD in T1D compared with the general population. In the Scottish Registry Linkage Study (SRLS), data for T1D patients aged 20 and above were compared with the nondiabetic populations from the Scottish national surveys. This showed that the age adjusted IRR for first CVD event was 2.3 for men and 3.0 for women [Livingstone et al. 2012]. A separate study using the UK General Practice Research Database (GPRD) compared T1D patients with aged and sex-matched nondiabetic controls between 1992 and 1999. This study reported a HR for major CVD of 3.6 for T1D men and 7.7 for T1D women, with a mean age of 33 years in both groups [Soedamah-Muthu et al. 2006b].
The SRLS analysis found that the IRR of first CVD event for patients aged over 70 was 1.71 for men and 1.85 for women [Livingstone et al. 2012]. In the GPRD study, the major CVD HR for the age group 65–75 was 2.3 for men and 8.3 for women [Soedamah-Muthu et al. 2006b]. In both studies, the figures were lower than the younger age groups, likely reflecting the increasing risk of CVD with age in the general population.
The prevalence of CVD in T1D has also been reported in smaller observational studies within other worldwide populations. In a cohort of 209 Chinese with young-onset T1D (defined as diagnosis before age of 40; T1D participants’ mean age was 27.8 years) in Hong Kong, the incidence of CVD was 0.6 per 1000 person years [Luk et al. 2014]. In comparison, the crude incidence rate of first CVD event for T1D aged 20–39 in the SRLS was 2.73 (men) and 1.76 (women) per 1000 person years [Livingstone et al. 2012]. In a small sample of 100 Saudi Arabian T1D patients, 4% (4/100) developed CV complications [Ammari, 2004].
In summary, CVD prevalence appears to be higher in T1D than the general population, particularly in younger women but this effect was not so pronounced in the older aged group.
CVD mortality is increased in T1D
Two large population based observational studies reported the SMR for CVD in T1D. The Allegheny County childhood onset T1D registry (onset age <18 years) reported a SMR of 12.9 [Secrest et al. 2010] in a cohort of 1075 T1D patients diagnosed between 1965 and 1979, with a mean age of 42.9 years old. Data for childhood-onset T1D from the Norwegian Childhood Diabetes Registry (NCDR) (n = 1906, onset age <15 years, diagnosed between 1973 and 1982, follow up till 2002) reported SMRs of 11 for men and 10 for women with T1D [Skrivarhaug et al. 2006]. A smaller Swiss study assessed mortality of patients with T1D and T2D compared with the general Swiss population between 1974 and 2005. There were 225 Swiss T1D patients with a mean age of 43 years old. This study reported a CVD SMR of 6.6; CVD SMR did not differ significantly between T1D and T2D [Allemann et al. 2009]. Finally, a New Zealand paper of 995 insulin-treated diabetics (including T2D) showed a CVD SMR of 4.48 in T1D diagnosed before the age of 30; the lower SMR in this paper could be due to a dilution effect of T1D with an older age of onset. In fact in the same paper, the CVD SMR was halved in T1D with onset age >30 years compared with <30 years [Florkowski et al. 2003].
CVD related deaths have also been reported as IRR, HR and annual mortality rate. In the SRLS, the IRR for CVD mortality related to T1D was 3.4 and 3.5 for men and women, respectively. The HR for CVD deaths in T1D was 7.4 in the GPRD study [Soedamah-Muthu et al. 2006b]. The annual mortality rate for CVD was 1.4 per 1000 person-years (n = 2787, baseline mean age 33, 7 years follow up) [Soedamah-Muthu et al. 2008].
CVD appears to be the predominant cause of death in adults with T1D. In the World Health Organization (WHO) multinational cohort, CVD accounted for 44% of T1D deaths [Morrish et al. 2001]. In a Danish study with 4821 T1D patients, CVD was the main cause of death [31% (125/402) and 30% (81/271) of all death for men and women, respectively] [Jørgensen et al. 2013]. This was also the case in the GPRD study [Soedamah-Muthu et al. 2006b].
Studies show that acute diabetic complications, such as ketosis and hypoglycaemia, are more likely to be the cause of death in the young, and CVD begins to predominate as patients become older. This was observed in a Japanese nationwide population-based cohort of 1385 T1D patients diagnosed between 1965 and 1979, where a lower mortality from acute diabetic complications and greater mortality from CVD was seen with increasing follow up. Here CVD was described as the main cause of death in those with more than 20 years’ disease duration [Morimoto et al. 2013]. Similarly in the SRLS, the most common cause of death was diabetes [41% (51/123)] for the under 40s but circulatory disease [38% (349/907)] for those aged over 40 [Livingstone et al. 2012]. This trend is supported by the Allegheny study where acute diabetic complication was the main cause of death (73%) within the first 10 years of diagnosis and CVD was the leading cause of death (40%) after 20 years of T1D [Secrest et al. 2010]. Finally, in the NCDR study, acute diabetic complication and violent death was the main cause of death for under 30s but CVD accounts for the most death [30% (11/37)] for over 30s [Skrivarhaug et al. 2006].
Focusing on ischemic heart disease (IHD), the Diabetes UK cohort (23,000 T1D patients followed up till 2000) reported a IHD mortality rate that was higher than the general population across all age groups: the overall SMR for IHD was 4.5 (men) and 8.8 (women). Within this cohort, the SMR for IHD was exceptionally high in young women: female T1D patients at ages 20–29 had a SMR of 44.8 [Laing et al. 2003]. This gender difference for mortality was also seen in Huxley and colleagues’ meta-analysis of 26 studies in T1D (n = 214114); the pooled women-to-men ratio of the SMR for fatal CVD and incident coronary heart disease was 1·86 and 2.54, respectively [Huxley et al. 2015].
Only the Diabetes UK cohort study showed data for older T1D, but this was for IHD: those aged 70–84 had IHD SMR of 2.2 for men and 5.3 for women. These figures are lower than the younger age bands and again likely reflect rising CVD risk with age in the general population, and the possibility that those with CVD are no longer alive to contribute to analyses.
One observational study from a tertiary centre in Australia found that there were more CVD death and risk factors in 354 young onset T2D (age of onset between 15 and 30 years) than the 470 young onset T1D observed [Constantino et al. 2013]. An 18-year observational study conducted in Finland involving 173 T1D and 834 T2D (aged 45–64 years at baseline) found that both types of diabetes had similar CVD mortality, although there was a 3–4 fold increase of risk in men and 10–13 fold increase for women. However, the impact of glycaemic control on CVD mortality was higher in T1D than in T2D: an increment of 1 unit (%) of glycated haemoglobin increased the risk of CV mortality by 52.5% [95% confidence interval (CI) 28.4–81.3] in T1D and 7.5% (95% CI 4.3–10.8) in T2D [Juutilainen et al. 2008].
CV risk factors are increased in T1D
Whilst diabetes itself is a risk factor for CVD, a majority of T1D patient will have at least one further risk factor. The proportion who do so ranges from 69% in 27,358 T1D patients aged 0.25–26 years in a cross-sectional study from a German surveillance database (DPV) to 89% of 177 T1D patients with end stage renal failure and a mean age of 37 in a Spanish study [Schwab et al. 2006; Rueda et al. 2009]. The percentage of T1D patients with 3 or more CVD risk factors ranged from 2% in the DPV study to 15% in a Norwegian Childhood Diabetes and Quality Project (NCDQ) cohort (2658 T1D patients with mean age of 13) [Schwab et al. 2006; Margeirsdottir et al. 2008].
Managing CVD risk
Guidelines for CVD risk management have been proposed by major diabetes associations [American Diabetic Association, 2014; The National Collaborating Centre for Chronic Conditions, 2004; European Society of Cardiology (ESC) et al. 2013]. The American Diabetes Association (ADA) guidelines are outlined in Table 3. These do not recommend routine screening for CVD in patients with diabetes and suggest this does not provide any greater benefit than screening for and actively managing CVD risk factors. The approach to managing CV risk in patients with T1D is consequently comparable with that of the nondiabetic population.
Table 3.
American Diabetes Association 2014 guidelines for type 1 diabetes management.
| HbA1c | 6.5–7.0% |
| Blood pressure | <140/80 mmHg |
| may be appropriate to aim for lower 130/80 in individual cases | |
| Lipids | LDL < 1.8–2.6 mmol/l. |
| Triglyceride < 1.7 mmol/l. | |
| HDL cholesterol > 1.0 mmol/l (men), >1.3 mmol/l (women) | |
| Where there is pre-existing CVD, or multiple CVD risk factors, or when the patient is over 40 years of age, lipid lowering therapy should be considered. | |
| Antiplatelet | Consider where there are multiple CVD risk factors, or where there has been previous CVD. |
| Smoking | Smoking cessation |
| Albuminuria | ACE inhibitors or ARBs if urinary albumin excretion >30 mg/24 hours |
| Other medical management | Known CVD: consider ACE inhibitor, aspirin and statin |
| Prior myocardial infarct: beta blocker |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; HbA1c, glycated haemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein.
Assessment therefore consists of measuring clinical risk factors, calculating risk from appropriate risk engines and actively enquiring about symptoms of CVD. Current risk engines for CVD risk in diabetes are largely based on data from studies of the general population. These risk engines include PROCAM (a cohort of working people) (http://www.chd-taskforce.com/procam_interactive.html) and QRISK®2 (a primary care population) (http://www.qrisk.org/) [Assmann et al. 2002; Hippisley-Cox et al. 2008]. The latter has the option to include T1D.
Achieving target glycated haemoglobin (HbA1c) in T1D
Target HbA1c achievement is generally low (Table 4). The percentage of T1D patients achieving HbA1c < 7% ranged from 13% (SRLS) to 26% in a multicentre outpatient based T1D study in Castilla-La Mancha, Spain (CLM; n = 1465, mean age 39) [Livingstone et al. 2012; Wallymahmed et al. 2005; Sastre et al. 2012]. A recent large observational study using regional and national T1D registries across 19 countries (n = 324,501) showed that only 28% of the people in the whole dataset had HbA1c < 7.5% [McKnight et al. 2014].The overall prevalence of poor glycaemic control has been determined for paediatric patients in a number of cross-sectional studies. This is reported to range from 60.6% (HbA1c > 7.5%) in a paediatric department in Austria (UHVGPD study; n = 264, mean age 13) to 91% (HbA1c > 7%) of a small cohort of Caribbean youth with T1D in Jamaican major referral hospitals (JMRH; n = 36, mean age 18), respectively [Steigleder-Schweiger et al. 2012; Margeirsdottir et al. 2008; Tulloch-Reid et al. 2009].
Table 4.
Achieving CVD risk targets in T1D.
| Achieving target glycated haemoglobin (HbA1c) in T1D | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target HbA1c defined in the study | Mean BMI (SD), kg/m2 | Results | Reference |
| LAHDCA | 34 (11.9) | 14 (9.0) | 218 | 215 | <7.5% | n/a | Mean (SD) HbA1c was 9.7% (1.9) 17 (8%) had HbA1c <7.5% 126 (59%) had an HbA1c > 9% |
Wallymahmed et al. [2005] |
| CLM | 39.4 (13.5) | 19.4 (10.6) | 1465 | n/a | n/a | n/a | Mean HbA1c was 7.8% | Sastre et al. [2012] |
| 26% had HbA1c ⩽7% | ||||||||
| SRLS | n/a | Median (IQR) 17.5 (9.3–27.0) | 21,789 | 21,290 | <7% | Median BMI 27 | Median HbA1c was 8.5% 13% achieved the target 37% had Hb1Ac ⩾9% |
Livingstone et al. [2012] |
| 19 countries in Australasia, Europe and North America | n/a | n/a | 324,501 | 324,501 | n/a | n/a | 7.1% had HbA1c < 6.5% 8.7% had HbA1c 6.5–6.9% 12.3% had HbA1c 7.0–7.4% |
McKnight et al. [2014] |
| Paediatric cohort | ||||||||
| UHVGPD | 12.5 (3.5) | 4.6 (3.7) | 264 | n/a | <7.5% | 20.4 (3.9) | Mean HbA1c = 7.85% 160 (60.6%) had HbA1c > 7.5%. |
Steigleder-Schweiger et al. [2012] |
| JMRH | 20 (8) | 2.6 (2) | 36 | 36 | n/a | n/a | 33 (91%) had HbA1c > 7% | Tulloch-Reid et al. 2009 |
| NCDQ | 13.1 | 5.7 | 1658 | 1658 | <8.5% in 12 year-olds | 20.2 (3.8) | Mean HbA1c was 8.2% | Margeirsdottir et al. [2008] |
| <8.0% in 6–12 year-olds | 1149 (71.4%) above target level | |||||||
| <7.5% in >12 year-olds | ||||||||
| Older aged cohort | ||||||||
| SRLS | n/a | n/a | 1537 males1427 females | 2964 | <7% | Median BMI 27 in over 60s | Median HbA1c 8.1% (men) and 8.3% (women) in over 60s | Livingstone et al. [2012] |
| Achieving target blood pressure (BP) in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target blood pressure defined in the study, mmHg | Mean BMI (SD), kg/m2 | Results | Reference |
| Paediatric cohort | ||||||||
| NCDQ | 13.1 | 5.7 | 1658 | n/a | n/a | 20.2 (3.8) | 152 (6.9%) had BP above the 90th centile | Margeirsdottir et al. [2008] |
| 4% had BP above the 95th percentile | ||||||||
| 0.3% on antihypertensives | ||||||||
| DPV | Range 0.5–26 years | n/a | 27,358 | n/a | n/a | See weight section | 8.1% had systolic hypertension and 2.5% diastolic hypertension | Schwab et al. [2006] |
| 2.1% on anti-hypertensive therapy113 (21%) had arterial hypertension | ||||||||
| LDRDS | n/a | n/a | 539 | n/a | n/a | 13.4% overweight | Dobrovolskien et al. [2013] | |
| Adult cohort | ||||||||
| IEMR | 22.5 (10.3) | n/a | 219 | n/a | <120/80 | n/a | 17 (7.7%) had hypertension | Kalantari et al. [2007] |
| LAHDCA | 34 (11.9) | 14 (9.0) | 218 | 213 | SBP < 135DBP < 85 | n/a | 28 (13%) above target SBP8 (3.8%) above target DBP52 (24%) were taking antihypertensives | Wallymahmed et al. [2005] |
| CLM | 39.4 (13.5) | 19.4 (10.6) | 1465 | n/a | n/a | n/a | 23% were hypertensive | Sastre et al. [2012] |
| SRLS | n/a | 17 | 21,789 | n/a | BP < 130/80 | Median BMI 27 | 60% (men) and 53% (women) were above target BP | Livingstone et al. [2012] |
| 37% aged over 40 had BP ⩾ 140/90 | ||||||||
| Median SBP: 130 mmHg (men) 132 mmHg (women) in age group 40–59 | ||||||||
| EDIC | Year 11 BMI | Nathan et al. [2005] | ||||||
| - Intensive treatment | 45 (7) | 24 (5) | 593 | n/a | 28.4 (6.9) | 38% hypertensive | ||
| - Conventional treatment | 45 (7) | 23 (5) | 589 | n/a | Hypertension defined as: >140/90 | 27.6 (4.5) | 41% hypertensive | |
| EURODIAB | Baseline | |||||||
| - Deceased | 41 (11) | 22 (12) | 102 | n/a | Men: 24.0 (2.9); women:23.5 (3.6) | 56 (55%) hypertensive, 36 (35%) on antihypertensives | Soedamah-Muthu et al. [2008] | |
| - Survived | 32 (10) | 14 (9) | 2685 | n/a | Hypertension defined as: >140/90 mmHg, or on antihypertensives | Men: 23.6 (2.6); women: 23.5 (3.0) | 595 (22%) hypertensive, 225 (8%) on antihypertensives | |
| Older aged cohort | ||||||||
| SRLS | n/a | 17 | 21,789 | n/a | BP < 130/80 | Median BMI 27 for over 60 | Median SBP: 137 mmHg (men) and 138 mmHg (women) for over 60s | Livingstone et al. [2012] |
| 79.5% (men) and 79.4% (women) aged over 60 were on antihypertensives | ||||||||
| Achieving target lipids in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target lipids defined in the study | Mean BMI (SD), kg/m2 | Results | Reference |
| CLM | 39.4 (13.5) | 19.4 (10.6) | 1465 | n/a | n/a | n/a | 35% had dyslipidaemia. | Sastre et al. [2012] |
| DPV | n/apaediatric | n/a | 27,358 | 19,359 | n/a | See weight section | 29% had dyslipidaemia 0.4% on lipid lowering treatment |
Schwab et al. [2006] |
| USA | Median 14.3 | 6.4 (3.8) | 46 | n/a | n/a | Baseline BMI 22.8 (3.7) | 50% had dyslipidaemia | Reh et al. [2011] |
| EDIC | Year 11 BMI | Nathan et al. [2005] | ||||||
| - Intensive treatment | 45 (7) | 24 (5) | 593 | n/a | 28.4 (6.9) | 52% hyperlipidaemia, 34% on statin | ||
| - Conventional treatment | 45 (7) | 23 (5) | 589 | n/a | Hyperlipidaemia: defined as LDL >3.4 mmol/l or the use of lipid lowering agent | 27.6 (4.5) | 48% hyperlipidaemia, 33% on statin | |
| Cholesterol | ||||||||
| LDRDS | n/a | n/a | 539 | n/a | n/a | 13.4% overweight | Hypercholesterolemia was diagnosed in 120 (22.3%) | Dobrovolskienė et al. [2013] |
| IEMR | 22.5 (10.3) | n/a | 219 | n/a | Serum cholesterol <170 mg/dl | n/a | Hypercholesterolemia in 104 (47.4%) | Kalantari et al. [2007] |
| LAHDCA | 34 | 14 | 218 | Cholesterol <4.8 mmol/l | n/a | 112 (54.6%) had a total cholesterol above target | Wallymahmed et al. [2005] | |
| SRLS | n/a | Median (IQR) 17.5 (9.3–27.0) | 21,789 | 21,290 | n/a | Median BMI 27 | 41.7% were on a statinMedian cholesterol was 4.4 mmol/l (men) and 4.8 mmol/l (women) in the 40–59 age group | Livingstone et al. [2012] |
| USA | 13.6 (4.1) | 4.5 (0.3) | 360 | 360 | Total cholesterol <200 mg/dl | BMI Z-score 0.62 (1.00) | 16.9% had sustained raised total cholesterol ⩾ 200 mg/dl at follow up | Maahs et al. [2007] |
| LDL | ||||||||
| LDRDS | n/a | n/a | 539 | n/a | n/a | 13.4% overweight | High LDL in 79 (14.7%) | Dobrovolskienė et al. [2013] |
| JMRH | 18 (5) | 3 (2) | 36 | n/a | n/a | n/a | 24 (67%) had high LDL > 2.5 mmol/l | Tulloch-Reid et al. [2009] |
| 12 (33%) had low HDL <1.1 mmol/l | ||||||||
| NCDQ | 13.1 | 5.7 | 1,658 | 1,658 | n/a | 20.2 (3.8) | 453 (34.5%) had LDL > 2.6 mmol/l | Margeirsdottir et al. [2008] |
| Only 0.2% of all the patients or 3% of those who should have been were receiving lipid lowering treatment | ||||||||
| Spain | 37.4 (14.9) | 24.7 (12.2) | 270 | n/a | n/a | 23.2 (3.7) | Mean LDL was 105.06 mg/dl. | Amor et al. [2011] |
| LDL < 100 mg/dl increased from 26.3% in 1999–2000 to 65.9% in 2009–2010. | ||||||||
| HDL | ||||||||
| LDRDS | n/a | n/a | 539 | n/a | n/a | 13.4% overweight | Decreased HDL in 22 (4.1%) | Dobrovolskienė et al. [2013] |
| NCDQ | 13.1 | 5.7 | 1,658 | 1,658 | >1.1 mmol/l | 20.2 (3.8) | 94 (6.9%) had HDL <1.1 mmol/l | Margeirsdottir et al. [2008] |
| IEMR | 22.5 (10.3) | n/a | 219 | n/a | HDL > 35 mg/dl | n/a | HDL<35 mg/dl 22.8% (n = 50) | Kalantari et al. [2007] |
| JMRH | 18 (5) | 3 (2) | 36 | n/a | n/a | n/a | 12 (33%) had low HDL <1.1 mmol/l | Tulloch-Reid et al. [2009] |
| USA | n/a | n/a | 360 | 360 | HDL > 35 mg/dl | BMI Z-score 0.62 (1.00) | 3.3% had HDL <35 mg/dl | Maahs et al. [2007] |
| TG | ||||||||
| LDRDS | n/a | n/a | 539 | n/a | n/a | 13.4% overweight | High TG in 96 (17.8%) | Dobrovolskienė et al. [2013] |
| IEMR | 22.5 (10.3) | n/a | 219 | n/a | TG<150 mg/dl | n/a | Hypertriglyceridemia in 18.3% (n = 40) | Kalantari et al. [2007] |
| UHVGPD | 12.5 (3.5) | 4.6 (3.7) | 264 | n/a | Dyslipidaemia was defined as TG above 95th percentile | 20.4 (3.9) | 60 (22.7%) had raised triglycerides above target. | Steigleder-Schweiger et al. [2012] |
| Older aged cohort | ||||||||
| SRLS | n/a | n/a | Male: 1537Female: 1427 | 2964 | Median BMI 27 | Median cholesterol of 4.0 mmol/l (men) and 4.4 mmol/l (women) aged over 60 Median HDL of 1.4 mmol/l (male) and 1.7 mmol/l (female) aged over 60 Median triglyceride levels of 1.2 mmol/l (male) and 1.1 mmol/l (female) aged over 6072.8% male and 73.6% female over 60 on statins |
Livingstone et al. [2012] | |
| Smoking status in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Mean BMI (SD), kg/m2 | Results | Reference | |
| IEMR | 22.5 (10.3) | n/a | 219 | n/a | n/a | 15 (6.9%) smoke | Kalantari et al. [2007] | |
| SRLS | n/a | Median (IQR) | 21,789 | 21,290 | Median BMI 27 | 27.6% smoke overall | Livingstone et al. [2012] | |
| 17.5 (9.3–27.0) | ||||||||
| CLM | 39.4 (13.5) | 19.4 (10.6) | 1465 | n/a | n/a | 26% smoke | Sastre et al. [2012] | |
| DPV | 7.5 (2.5) | 2.5 (2.3) | n/a | n/a | 16% BMI > 90th centile | 0.24% smoke | Schwab et al. [2006] | |
| 13.7 (1.4) | 4.9 (3.6) | n/a | 20% BMI > 90th centile | 10.5% smoke | ||||
| 18.5 (2.3) | 8.2 (4.8) | n/a | 25% BMI > 90th centile | 34.8% smoke | ||||
| NCDQ | 13.1 | 5.7 | 1658 | n/a | 20.2 (3.8) | 2% smokeThe mean age of the smokers was 17.4 years. | Margeirsdottir et al. [2008] | |
| FinnDiane | n/a | Gordin et al. [2011] | ||||||
| - With incident CVD event | 39 (12) | n/a | 269 | n/a | 60% had history of smoking | |||
| - No incident CVD event | 38 (13) | n/a | 2698 | n/a | 40% had history of smoking | |||
| EDIC | Year 11 BMI | Nathan et al. [2005] | ||||||
| - Intensive treatment | 45 (7) | 24 (5) | 593 | n/a | 28.4 (6.9) | 14% current smoker at year 11 of EDIC study | ||
| - Conventional treatment | 45 (7) | 23 (5) | 589 | n/a | 27.6 (4.5) | 11% current smoker at year 11 of EDIC study | ||
| EURODIAB | Baseline | Soedamah-Muthu et al. [2008] | ||||||
| - Deceased | 41 (11) | 22 (12) | 102 | n/a | Men: 24.0 (2.9); women:23.5 (3.6) | 32 (31%) current smokers | ||
| - Survived | 32 (10) | 14 (9) | 2685 | n/a | Men: 23.6 (2.6); women: 23.5 (3.0) | 835 (31%) current smokers | ||
| Older aged cohorts | ||||||||
| SRLS | n/a | n/a | Male: 1537Female: 1427 | 2964 | Median BMI 27 for over 60 | 19.1% males and 15.4% females over 60 smoked | Livingstone et al. [2012] | |
| Achieving a healthy diet in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target health diet defined in the study | Mean BMI (SD), kg/m2 | Results | Reference |
| NCDQ | 13.1 | 5.7 | 1,658 | Variable – see results | Moderate physical activity >1 hour/dayFat <30% of energyFruit and vegetables >500 g/day | 20.2 (3.8) | 299/576 (51.9%) did moderate physical activity <1 hour/day | Margeirsdottir et al. [2008] |
| 423/518 (82%) had fat >30% of energy intake | ||||||||
| 471/518 (91%) consumed <500 g fruit and vegetables /day | ||||||||
| European | 33 (10) | 15 (9) | 533 | n/a | n/a | Baseline BMI 23.6 (2.7) | European T1D patients consumed a high atherogenic diet.2% achieved the recommended intake of dietary fibre13% achieved the recommended intake of saturated fatThe mean intake of natural dietary fibre was 17.3 g/day | Soedamah-Muthu et al. [2013] |
| 7 year follow up BMI 24.7 (3.2) | ||||||||
| European | n/a | n/a | 3250 | n/a | n/a | n/a | Toeller [2002] | |
| Fibre consumption was lowest in patients from Eastern European centres compared with patients from centres in southern and north-western Europe. The fibre density was highest in patients from southern Europe. |
||||||||
| Achieving target physical activity (PA) levels in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target physical activity levels defined in the study | Mean BMI (SD), kg/m2 | Results | Reference |
| European | 32.7 (10.2) | n/a | 3250 | 2185 | n/a | None / mild PA group: 23.4 (2.8) | 786 had none or mild PA once a week or more | Tielemans et al. [2013] |
| Moderate/vigorous PA group: 23.7 (2.8) | 1399 had moderate or vigorous PA once a week or more | |||||||
| Finland | 38.5 (12.3) | 23.4 (12.8) | 1945 | 1108 patients with normoalbuminuria | n/a | 25.1 (3.5) | 23% were sedentary | Wadén et al. [2008] |
| 20.6% less than one session of exercise per week | ||||||||
| Weight, body mass index (BMI) and the ‘metabolic syndrome’ in T1D | ||||||||
| Study cohort / country of study cohort | Mean age of participants in years (SD) | Mean diabetes duration in years (SD) | Overall number of participants in the study | Number of participants contributing to the risk factor | Target BMI defined in the study, kg/m2 | Results | Reference | |
| LDRDS | n/a | n/a | 539 | n/a | n/a | 72 (13.4%) were overweight | Dobrovolskienė et al. [2013] | |
| JMRH | 18 (5) | 3 (2) | 36 | 36 | n/a | 8 (22%) were overweight and 3 (8%) were obese in T1D. | Tulloch-Reid et al. [2009] | |
| NCDQ | 13.1 | 5.7 | 1,658 | 1658 | BMI > 95th percentile defined as obese | 71 (4.4%) were obese | Margeirsdottir et al. [2008] | |
| CLM | 39.4 (13.5) | 19.4 (10.6) | 1465 | n/a | n/a | 15% were obese | Sastre et al. [2012] | |
| UHVGPD | 12.5 (3.5) | 4.6 (3.7) | 264 | n/a | BMI > 90th percentiles = overweight | Mean BMI was 20.4 (3.9)53 (20.1%) had BMI > 90th centile. | Steigleder-Schweiger et al. [2012[ | |
| DPV | 7.5 (2.5) | 2.5 (2.3) | n/a | n/a | n/a | 16.4% had BMI above 90th percentile | Schwab et al. [2006] | |
| 13.7 (1.4) | 4.9 (3.6) | 20.0% had BMI above 90th percentile | ||||||
| 18.5 (2.3) | 8.2 (4.8) | 25.0% had BMI above 90th percentile | ||||||
| EURODIAB | baseline | BMI: mean (SD) | Soedamah-Muthu et al. [2008] | |||||
| - Deceased | 41 (11) | 22 (12) | 102 | n/a | Men: 24.0 (2.9); women:23.5 (3.6) | |||
| - Survived | 32 (10) | 14 (9) | 2685 | n/a | Men: 23.6 (2.6); women: 23.5 (3.0) | |||
| Paediatric Diabetes Consortium | 3 months after diagnosis: 9.7 (3.7) | n/a | 530 | 530 | Baseline median BMI percentile 50%, increasing to 67% at 1 month | Gregg et al. [2015] | ||
| DCCT | Mean (SD) BMI percentile (%) | Baskaran et al. [2015] | ||||||
| - 1999 | 12.2 (2.2) | 2.8 (1.5) | 94 | 94 | 71 (21) | |||
| - 2002 | 12.8 (2.3) | 6.5 (3.5) | 144 | 144 | 72 (21) | |||
| - 2006 | 12.1 (1.9) | 5.7 (3.3) | 133 | 133 | 70 (22) | |||
| - 2009 | 12.7 (2.5) | 6.4 (3.2) | 136 | 136 | 70 (23) | |||
| Ethiopia | 29.1 (12) | n/a | 778 | 778 | Mean BMI increased from 15.9 to 18.3 from 2000 to 2009 | Abebe et al. [2013] | ||
| EDC | Baseline 29.1 | n/a | n/a | 629 | Prevalence at baseline versus at 18years follow up | Conway et al. [2010] | ||
| Obesity: 3.4% versus 22.7% | ||||||||
| Overweight: 28.6% versus 46.0% | ||||||||
| Spain | 39.7 (13.2) | 16.7 (12.9) | 91 | n/a | n/a | 29 (32%) had metabolic syndrome according to the NCEP-ATP III modified criteria | Chillarón et al. [2010] | |
| FinnDiane | 37 (12) | 23 (12) | 3783 | n/a | n/a | Prevalence of metabolic syndrome at baseline was 44% from the FinnDiane study | Thorn et al. [2009] | |
| England | 46 | 21 | 1282 | n/a | n/a | CVD risk factor targets were poorly achieved with only 0.7% of patients achieving all minimal dataset targets. | Syed et al. [2007] | |
| HbA1c and TC targets were those most poorly achieved | ||||||||
| SRLS | n/a | n/a | Male: 1537 | 2964 | n/a | Median BMI in over 60s was 27 | Livingstone et al. [2012] | |
| Female: 1427 | ||||||||
BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; HDL, high density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; n/a, not available; NCEP-ATP III, National Cholesterol Education Program-Adult Treatment Panel III; PA, physical activity; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
In the older aged patient, the SRLS observed that the median HbA1c level for those aged over 60 was 8.1% (male) and 8.3% (female) [Livingstone et al. 2012].
Achieving target blood pressure in T1D
Estimates for the prevalence of hypertension vary between studies (Table 4). In paediatric T1D, the prevalence ranged from 7% in the NCDQ cohort (0.3% on antihypertensives) to 8% in the DPV study (2% on antihypertensives) and 21% in a Lithuanian T1D register database study (LDRDS; n = 539) [Margeirsdottir et al. 2008; Schwab et al. 2006; Dobrovolskienė et al. 2013]. For adults, this ranged from 8% in a group of Iranian T1D patients at the IEMR (n = 219; mean age 23), 13% in a UK consultant led T1D clinic (LAHDCA, n = 218, mean age 34) and 23% in the CLM study to 37% in over 40s in the SRLS [Kalantari et al. 2007; Wallymahmed et al. 2005; Sastre et al. 2012; Livingstone et al. 2012]. In the EDIC year 11 study, 38% and 41% of patients from the intensive treatment and conventional treatment group were hypertensive (>140/90 mmHg) [Nathan et al. 2005]. At baseline, 55% of the deceased versus 22% survivors in the EURODIAB study were hypertensive (>140/90 mmHg); 36 (35%) and 225 (8%) were on antihypertensives, respectively [Soedamah-Muthu et al. 2008].
In the SRLS, a greater proportion of older aged patients were prescribed antihypertensive medications (80% and 79% in over 60s compared with 50% and 44% in those aged 40–59, men and women, respectively). Despite this, median blood pressure remained elevated in the older aged population when compared with younger persons. [Livingstone et al. 2012].
Achieving target lipids in T1D
Four studies have measured overall dyslipidaemia, two of which observed paediatric T1D patients. The CLM study of adult T1D patients found 35% had dyslipidaemia [Sastre et al. 2012]. In the EDIC year 11 study, 52% and 48% of patients from the intensive treatment and conventional treatment group had hyperlipidaemia; 34% and 33% were on a statin, respectively [Nathan et al. 2005]. From the DPV, 29% of paediatric T1D patients had dyslipidaemia, of whom only 0.4% were prescribed lipid lowering therapy [Schwab et al. 2006]. In a small US observational study, 50% of paediatric T1D patients were diagnosed with dyslipidaemia [Reh et al. 2011].
Prevalence of raised total cholesterol (>4.8 mmol/l) ranged from 22.3% in the LDRDS paediatric cohort to 55% of the LAHDCA cohort [Dobrovolskienė et al. 2013; Kalantari et al. 2007; Wallymahmed et al. 2005]. In a cohort of T1D patients aged over 60 years, the SRLS identified a median cholesterol level of 4.0 and 4.4 mmol/l in men and women, respectively, which was slightly lower than that of younger patients (4.4 and 4.8 mmol/l in men and women, respectively, aged 40–59) [Livingstone et al. 2012].
In the paediatric cohort, prevalence of high low density lipoprotein (LDL) (>2.5 mmol/l) ranged from 14.7% in the LDRDS to 67% in the small JMRH cohort [Dobrovolskienė et al. 2013; Tulloch-Reid et al. 2009; Margeirsdottir et al. 2008; Kalantari et al. 2007]. Amor and colleagues identified an improvement in the prevalence of target LDL (<2.5 mmol/l) from 26.3% in 1999 to 65.9% in 2009 in patients undergoing assessment for kidney–pancreas transplant [Amor et al. 2011].
The existing literature relating to high density lipoprotein (HDL) in patients with T1D is poor. The proportion of patients with HDL <1.1 mmol/l or <35 mg/dl (undesirable) ranged from 3.3% in a Colorado cohort studied by Maahs and colleagues [Maahs et al. 2007] and 4.1% in the LDRDS paediatric cohort [Dobrovolskienė et al. 2013] to 7% in the NCDQ cohort [Margeirsdottir et al. 2008] versus 23% in the IEMR cohort to 33% in the JMRH cohort [Kalantari et al. 2007; Tulloch-Reid et al. 2009]. Over 60s in the SRLS had median HDL of 1.4 mmol/l (men) and 1.7 mmol/l (women), which was similar to the younger age group [Livingstone et al. 2012].
Prevalence for high triglycerides (TG) ranged from 18% in the LDRDS and IEMR cohort to 23% in the UHVGPD cohort [Dobrovolskienė et al. 2013; Kalantari et al. 2007; Steigleder-Schweiger et al. 2012]. In the SRLS over 60s cohort, the median triglyceride levels were 1.2 mmol/l (male) and 1.1 mmol/l (female), which was again similar to the younger age group [Livingstone et al. 2012].
The use of statins varied across age groups. In the NCDQ cohort, 0.2% T1D adolescents were prescribed statin therapy, contrasting with 6.4% (23/360) of paediatric T1D patients in Colorado, USA studied by Maahs and colleagues [Margeirsdottir et al. 2008; Maahs et al. 2007]. Within the SRLS, 41% of T1D patients were prescribed statins, although this figure was higher for older patients aged over 60 at 73% for males and 74% for females [Livingstone et al. 2012]. Data are summarized in Table 4.
Smoking status in T1D
The prevalence of smoking in T1D adult patients ranged from 7% in the IEMR study to 35% in the DPV study (Table 4) [Kalantari et al. 2007; Livingstone et al. 2012; Sastre et al. 2012; Soedamah-Muthu et al. 2008; Schwab et al. 2006]. In the FinnDiane cohort, 60% and 40% of T1D patients with incident CVD events and no CVD events smoked [Gordin et al. 2011]. A total of 14% and 11% of those who were in the intensive treatment and conventional treatment groups, respectively, were a current smoker at year 11 of the EDIC study [Nathan et al. 2005]. In the paediatric cohort, the NCDQ cohort identified that 3% of those ⩾12 years old reported smoking [Margeirsdottir et al. 2008]. For older aged patients, 19% male and 15% female T1D in the SRLS smoked, this was lower than the younger patients [Livingstone et al. 2012].
Achieving a healthy diet in T1D
In studies to date, there appear to be an overconsumption of fat and poor fibre consumption by patients with T1D (Table 4). In the NCDQ cohort, almost all study subjects had higher fat intake and lower fibre intake than recommended [Margeirsdottir et al. 2008]. Similar dietary pattern was observed in children aged under seven with T1D (n=24) in a small Swedish study [Sundberg et al. 2014]. In the EURODIAB study, European T1D patients consumed a high atherogenic diet, and very few patients achieved the recommended intake of dietary fibre (2%) and saturated fat (13%) [Soedamah-Muthu et al. 2013]. When comparing dietary patterns geographically, fibre intake was lowest in eastern Europe and highest in southern Europe [Toeller, 2002]. No study specifically observed the older age group.
Achieving target physical activity levels in T1D
Whilst studies have yet formally and objectively measured exercise and physical activity in T1D, a number of studies have analysed this subjectively (Table 4). The EURODIAB study quantified exercise through the use of questionnaires sent to over 2000 patients and showed that about a third undertook no or only mild physical activity [Tielemans et al. 2013]. Similarly, the Finnish Diabetic Neuropathy Study (FinnDiane) showed that 23% of people with T1DM were sedentary with a further 21% doing less than 1 session of exercise per week [Wadén et al. 2008]. A significant proportion of patients with T1D may therefore be considered physically inactive.
Weight, body mass index (BMI) and the ‘metabolic syndrome’ in T1D
A total of 13.4% of the children in the LDRDS and 22% of the JMRH adolescent cohort have been described as overweight [Dobrovolskienė et al. 2013; Tulloch-Reid et al. 2009]. The percentage of T1D patients classified as obese ranged from 4% of the NCDQ paediatric cohort to 15% of the CLM cohort [Margeirsdottir et al. 2008; Tulloch-Reid et al. 2009; Sastre et al. 2012]. A total of 20% of the UHVGPD cohort to 25% of young adults in the DPV study had a BMI > 90th centile [Steigleder-Schweiger et al. 2012; Schwab et al. 2006]. In the EURODIAB cohort, the baseline BMI for the deceased and the survivors were virtually the same; in those who survived the mean BMI was 24 [Soedamah-Muthu et al. 2008].
The Paediatric Diabetes Consortium’s study of 520 T1D youth (mean age 10, median BMI percentile 50%) found that the largest increase in BMI was in the first 3 months post diagnosis of T1D and thereafter remained stable at 12 months, thus reflecting gain of weight lost before diagnosis [Gregg et al. 2015]. The DCCT group examined the temporal trends of overweight/obesity across 4 cohorts representing different time point over a decade (1999, 2002, 2006, 2009; n = 507, mean age 12.0–12.8, mean BMI percentile 70–72) and found that the prevalence of overweight/obesity was similar, ranging from 27% to 36% [Baskaran et al. 2015]. However, at a cohort level, the Pittsburgh EDC group followed 589 T1D from 1986 to 1988 for 18 years and found that the prevalence of overweight and obesity increased by 47% and 7 fold, respectively (mean age 29, baseline prevalence of overweight and obesity 29% and 3%). Simultaneously, the use of intensive insulin regime increased from 7% to 82% and was quoted as a predictor of weight change [Conway et al. 2010].Similarly, a 10-year observational study at an Ethiopian hospital diabetes clinic reported that BMI increased from 16 to 18, although this remains in the underweight category (2000–2009, n = 778, mean age 29) [Abebe et al. 2013].
For the over 60s T1D patients, the median BMI was 27 in the SRLS; this was similar to the younger age groups [Livingstone et al. 2012] (see Table 4).
A Spanish hospital study showed that 32% of T1D outpatients had metabolic syndrome [Chillarón et al. 2010]. From the FinnDiane study, the prevalence of metabolic syndrome (by WHO definitions) at baseline was 44% [Thorn et al. 2009]. There were no data targeting the older aged group.
Whilst we accept that striving for prespecified targets may be inappropriate for some patients, these studies suggest that risk factors for CVD are suboptimally controlled in patients with T1D. We have previously shown in a UK single city multihospital study that targets of CVD risk factors were suboptimally recorded and only 0.7% of patients were achieving all minimal dataset target (total cholesterol, smoking, HbA1c) [Syed et al. 2007].
Biomarkers for CVD in T1D
Whilst the approach of managing CV risk through clinical assessment is simple, relatively straightforward and can be used in the clinical situation, there are a number of potential biomarkers for CVD in T1D that may prove to be useful. These are outlined in Table 5.
Table 5.
Potential biomarkers for CVD risk in T1D that has been investigated.
| Name of biomarker | Function of biomarker | Studies |
|---|---|---|
| Asymmetric dimethylarginine (ADMA) | Competitive inhibitor of nitric oxide synthase and linked with endothelial dysfunction and insulin resistance. | ADMA levels above the median predicted fatal and nonfatal cardiovascular events in T1D with overt nephropathy adjusted HR 2.05, 95% CI 1.31 - 3.20, p=0.002) (p
<0.001) [Lajer et al. 2008]. ADMA is marginally elevated in T1D with overt nephropathy compared with T1D with persistent normoalbuminuria (p <0.001) and significantly higher in patients with major cardiovascular events (p =0.05) [Tarnow et al. 2004]. Plasma ADMA concentrations were higher in T1D without any vascular complications than healthy controls (p <0.01) and higher ADMA levels are associated with CVD risk factors [Altinova et al. 2007]. In young T1D, there is no association between ADMA and endothelial dysfunction and levels are similar to healthy controls [Głowińska-Olszewska et al. 2010]. There is an inverse association between ADMA and HbA1c in T1D (p <0.001) in a longitudinal study [Marcovecchio et al. 2011] |
| Advanced glycation end product (AGE) and soluble receptor for advanced glycation end product (sRAGE) | Triggers inflammation and atheroma formation | T1D patients with CVD had higher levels of sRAGE than those without CVD (β=0.15, 95% CI 0.04–0.27) [Nin et al. 2009]. The incidence of fatal and nonfatal CVD increased with higher baseline levels of AGEs in T1D (HR=1.30, 95% CI 1.03–1.66) [Nin et al. 2011]. The AGE tetrahydropyrimidine was higher in T1D compared with healthy controls (p = 0.03) but had no association with either micro or macro vascular complications [Van Eupen et al. 2013]. Baseline soluble RAGE was independently associated with CV mortality in T1D (Fine–Gray competing risks model: HR 1.06) [Thomas et al. 2011]The incident of fatal and nonfatal CVD increased with higher baseline levels of log-transformed sRAGE in T1D (HR 1.90, 95% CI 1.13–3.21 and 2.12, 95% CI1.26–3.57) [Nin et al. 2010] |
| Immune complexes of oxidized-LDL (oxLDL-IC) and advanced glycation end products-LDL (AGE-LDL-IC) | Taken up by macrophages leading to transformation into foam cells, the hallmark of artherosclerosis | oxLDL-IC and AGE-LDL-IC predicts progression of carotid intima -medial thickness progression [Hunt et al. 2013] |
| High-mobility group box 1 protein (HMGB1) | Released extracellularly from necrotic and immune cells and acts as a pro-inflammatory cytokine | In T1D with nephropathy and persistent normoalbuminuria, higher levels of loge plasma HMGB1 were associated with a higher incidence of fatal and nonfatal CVD mortality (HR 1.55,95% CI 0.94–2.48 and HR1.86, 95% CI 1.18-2.9 respectively) in a 12 year follow up study [Nin et al. 2012a]. In T1D, higher serum HMGB1 are associated with greater prevalence and severity of albuminuria but not with cardiovascular disease [Nin et al. 2012b] |
| Osteoprotegerin (OPG) | Glycoprotein member of the TNF receptor family with a role in vascular calcification | In T1D with and without diabetic nephropathy, plasma OPG concentrations were increased in patients with CVD and correlated with HbA1c, systolic blood pressure and age. Plasma OPG was also significantly higher in T1D with nephropathy than without nephropathy (p<0.001) [Rasmussen et al. 2006]. |
| High OPG levels predicted CV mortality in T1D with diabetic nephropathy [HR 4.88 95% CI 1.57–15.14] [Jorsal et al. 2008]. | ||
| In the Finnish Diabetic Nephropathy T1D cohort of 1939 patients, OPG levels predicted incident CV events [HR 1.21, 95% CI 1.01–1.45, p = 0.035] [Gordin et al. 2013]. | ||
| Soluble CD40L (sCD40L) | Transmembrane portion of the TNF-alpha cytokine family that contributes to the atherosclerotic lesion progression | T1D with nephropathy had higher plasma sCD40L levels compared with T1D with normoalbuminuric (p
=
0.004). However sCD40L does not predict CVD [Lajer et al. 2010]. T1D is associated with increased serum CD40L levels (p = 0.006), increased CD40L expression on platelets (p < 0.001) and platelet–monocyte aggregation (p = 0.005) compared with healthy controls [Harding et al. 2004]. |
| T1D and T2D were found to have elevated sCD40L compared with healthy controls. SCD40L was also associated with in vitro adhesion molecules and monocyte chemo-attractant protein-1 release, impaired endothelial cell migration, more oxygen generation in monocytes and high levels correlated with HbA1C [Cipollone et al. 2005]. | ||
| High sensitivity C-reactive protein (hsCRP) | An acute phase protein and marker of inflammation, predictive of coronary events and prognostic of myocardial infarction. | In young adolescent T1D patients, hsCRP was significantly associated with triglycerides, apolipoprotein B and both systolic and diastolic blood pressure [Karantza et al. 2008]. hsCRP is significantly higher in T1D patients compared with healthy controls (p < 0.001). Uncontrolled T1D had higher levels of hsCRP compared with controlled T1D (p < 0.000). Hs-CRP correlated positively with total cholesterol (p < 0.0001), LDL (p < 0.001) and triglycerides (p < 0.0001), whereas HDL showed a negative correlation (p < 0.0001) [Fawaz et al. 2009]. |
| Tumor necrosis factor α (TNF-α) | Cytokine regulating vascular adhesion molecules causing beta cell damage, insulin resistance and atherosclerotic lesions. | TNF-α is significantly higher in T1D than in healthy controls (p
<
0.023) with a significant positive correlation with HbA1c (p
<
0.004) and fructosamine (p
<
0.049) and a negative correlation with HDL cholesterol (p
<
0.018) and apolipoprotein A1 levels (p
<
0.015) [Lechleitner et al. 2000]. Significant positive correlation of TNF-α with plasma levels of thiobarbituric acid reacting substances found in oxidative stress (p < 0.001), which showed a positive correlation with the duration of diabetes (p < 0.008) [Lechleitner et al. 2000]. In normotensive T1D, TNF-α correlated significantly with pulse pressure [González-Clemente et al. 2005]. Plasma TNF-α correlated with soluble vascular cell adhesion molecule 1 (sVCAM-1) (p = 0.008), triglycerides (p = 0.021) and diastolic blood pressure (p = 0.024) in T1D [Mohamed-Ali et al. 2001]. |
| Interleukin-6 (IL-6) | Inflammatory cytokine linked with myocardial injury, viral antigen presentation and cardiac hypertrophy. | Young T1D patients had significantly higher IL-6 levels compared with healthy controls (p
<
0.05) [Fawaz et al. 2009]. Plasma concentrations of IL-6 were elevated in T1D compared with healthy controls (p = 0.016), and in these patients IL-6 and soluble IL-6 receptor (sIL-6R) levels correlated with concentrations of soluble intracellular adhesions molecules 1 (sICAM-1), with p = 0.012 and p = 0.04, respectively [Mohamed-Ali et al. 2001]. |
| Homocysteine (Hcy) | Amino acid stimulating atherosclerosis through endothelial damage. | The median for total Hcy level was greater in T1D children than healthy controls (p < 0.05) [Dinleyici et al. 2006]. |
| Amongst T1D, total Hcy was significantly related to macroalbuminuria (adjusted OR=1.66, 95% CI 1.24–2.24), hypertension (adjusted OR=1.57, 95% CI 1.19–2.07) and decreased renal function [Soedamah-Muthu et al. 2005]. | ||
| There was no difference in total Hcy concentrations between T1D patients and controls [Atabek et al. 2006; Rossi et al. 2002; Pavia et al. 2000]. | ||
| Hcy levels were significantly lower among the diabetic male subjects than nondiabetic controls (p = 0.03) [Al-Attas et al. 2009]. | ||
| Endothelial progenitor cells (EPCs) | Produced in the bone marrow, expressing cell surface markers with the ability to differentiate and protect the endothelium | EPCs is significantly reduced in T1D children compared with healthy controls (p < 0.001) [Hörtenhuber et al. 2013]. |
| Adiponectin | Plasma protein that has anti-inflammatory and cardio-protective functions | Adiponectin concentrations were found to be higher in T1D children and adolescents compared with normal ranges [Galler et al. 2010]. Adiponectin-mediated release of IL-6, CCL2 and CXCL8 is disturbed in T1D patients [Abke et al. 2006]. Adiponectin Inversely predicted the incidence of coronary artery disease in T1D (HR=0.37, 95% CI 0.19–0.73, p = 0.004) [Costacou et al. 2005]. |
| Vascular progenitor cells | Involved in vascular repair with the number of circulating progenitor cells inversely related to CVD | Circulating vascular progenitor cell number was reduced (p <0.006) and function impaired in 22 T1D with microalbuminuria compared with T1D without microalbuminuria [Dessapt et al. 2010]. |
| Lipoprotein-associated phospholipase A2 (Lp-PLA2) | Macrophage derived pro-atherogenic enzyme linked with inflammation and oxidation | Lp-PLA2 activity was significantly lower in T1D patients than in healthy controls (p < 0.0001). High Lp-PLA2 activity was also associated with progression of coronary calcification (OR=1.77 95%CI 1.08–2.91, p = 0.02) [Kinney et al. 2011]. |
| Nitrous oxide | Free radical with a protective function on the endothelial lining | Serum nitric oxide was significantly lower and IL-8 was significantly higher in T1D children compared with their healthy siblings [Lo et al. 2004] |
| Mannose-binding lectin (MBL) | Activates the complement system and may aggravate inflammation | T1D patients with cardiovascular disease had significantly elevated MBL levels (p=0.02). (p < 0.0001) [Hansen et al. 2004]. |
| Sialic acid (SA) | A monosaccharide reflecting atherosclerotic activity and may predict coronary heart disease | No significant difference between mean serum total SA of T1D children and healthy controls. However, a significant correlation was found between serum total SA and total cholesterol, triglyceride and apolipoprotein B [Moussa et al. 2004] |
| Soluble intracellular adhesion molecules (sICAM) | Play a key inflammatory role in early stages of atherosclerosis | sICAM-1 concentration was higher in T1D children than in healthy controls (p = 0.04). High sICAM correlated with worse metabolic compensation and a family history of CVD [Głowińska et al. 2003]. |
| Soluble vascular cell adhesion molecule-1 (sVCAM-1) and solubleE-selectin | Soluble adhesion molecules | sVCAM-1 and sE-selectin has a positive association with CVD [Soedamah-Muthu et al. 2006a]. |
| Albuminuria | Indicates proteinuria which is a risk factor for CVD | Triglyceride (p <0.01) and LDL cholesterol (p <0.01) levels were higher in macroalbuminuric T1D subjects compared with normoalbuminuric T1D subjects [Sibley et al. 1999]. |
| Cystatin-C | Estimates renal function (renal disease is a CVD risk factor) | Increasing levels of cystatin C was associated with coronary atherosclerosis progression in T1D [Maahs et al. 2010b]. |
| Heat shock protein (HSP) | HSP60 may have a role as an autoantigen in atherosclerosis; HSP70 protects against CVD. | Anti-HSP70 antibody levels were significantly greater in T1D with no micro/macro complications compared with T1D patients with complications, whereas anti-HSP60 antibody levels were smilar in both these groups. Anti-HSP70 levels were also associated with a 47% reduced odds ratio of micro/macrovascular complications [Gruden et al. 2009] |
| Bilirubin | Anti-atherogenic functions by preventing the formation of reactive oxygen species | Bilirubin level did not correlate with predictors of CVD in the diabetic population [Yeh et al. 2009]. |
| YKL-40 | A marker of inflammation and endothelial dysfunction | Median levels of serum YKL-40 were significantly higher in T1D with normoalbuminuria compared with healthy controls (p < 0.01). Higher albuminuria was independently associated with increasing YKL-40 levels (p < 0.001) [Rathcke et al. 2009]. |
| Plasma alpha defensins | Antimicrobial peptides that have been shown to be proatherogenic | Baseline plasma alpha defensin was higher in T1D patients with nephropathy than without (p < 0.0001). A baseline level of alpha defensins within the upper tertile compared with lower tertile significantly increased the CVD related morbidity and mortality to an adjusted HR of 2.8, 95% CI 1.3–5.9, p = 0.006 [Joseph et al. 2008]. |
| Hyaluronan | Hyperglycaemia-induced perturbation of hyaluronan metabolism, characterized by increased hyaluronidase | Plasma hyaluronan and hyaluronidase were significantly increased in T1D patients without micro/macrovascular complications compared with healthy controls . In univariate |
| activity with subsequent increased plasma hyaluronan levels, may indicate increased vascular vulnerability. | analysis, mean carotid intima-media thickness (surrogate marker for CVD) was associated with plasma hyaluronan. [Nieuwdorp et al. 2007]. | |
| N-terminal pro brain natriuretic peptide (NT-proBNP) | Traditionally been described as a marker of heart failure and left ventricular dysfunction. | Higher NT-proBNP concentrations (4th versus 1st quartile) were associated with macrovascular disease in 208 T1D patients in Denmark (OR 5.84, 95% CI 1.65–20.74) [Grauslund et al. 2010]. |
| Combinations of inflammatory markers | Mean Z score calculated for:1. C-reactive protein, IL-6, soluble intercellular adhesion molecule (sICAM-1) and secreted phospholipase A22. C-reactive protein, IL-6 and TNF-αlevels | 1. The mean Z-score for inflammatory biomarkers was associated with the combined endpoint of CV mortality and morbidity with borderline significance after adjustment (HR 1.5, 95% CI 1.0- 2.3, p=0.051; 391 T1D patients; 199 had diabetic nephropathy, 192 had normoalbuminuria; mean age 41 and 43) [Astrup et al. 2008]. |
| 2. The mean Z-score for the combined inflammatory markers are associated with CVD in T1D (p for trend <0.001) [Schram et al. 2005]. | ||
| Combinations of endothelial dysfunction markers | Mean Z score calculated for a combination of endothelial dysfunction biomarkers: soluble vascular cell adhesion molecule 1, plasminogen activator inhibitor-1 and sICAM-1 | The mean Z-score for endothelial dysfunction was associated with the combined endpoint of CV mortality and morbidity in unadjusted Cox regression (HR 1.7, 95% CI 1.2–2.3, p = 0.001) [Astrup et al. 2008]. |
CI, confidence interval; CVD, cardiovascular disease; HbA1c, glycated haemoglobin; HDL, high density lipoprotein; HR, hazard ratio; LDL, low density lipoprotein; OR, odds ratio; T1D, type 1 diabetes; T2D, type 2 diabetes.
Management of CV risk in the older aged patient with T1D
There is a paucity of literature concerning effective strategies for the management of CV risk in the older aged patient with T1D. It appears on the basis of current evidence, however, that strategies should include tight control of both diabetes specific factors, such as blood glucose regulation, and the more general modifiable CV risk factors. The relative benefit afforded by targeting each of these risk factors remains unclear, with no single dominant factor predicting CV morbidity in patients with T1D and evidence to implicate the metabolic syndrome in its pathogenesis [Mäkinen et al. 2009; Thorn et al. 2009].
Interestingly, the Joslin 50-year medallist study provides evidence to suggest that there is a limit to the extent to which risk management strategies are effective in ageing patients with T1D [Sun et al. 2011]. Substantiating this, the authors provide evidence for a greater prevalence of CVD amongst patients with lower systolic blood pressure, mean arterial pressure, heart rate, total cholesterol and LDL, likely reflecting the use of pharmacological agents amongst these patients. Despite this, there is a clear link in the study between deranged lipids and CV risk, emphasizing a need for effective lipid management in aged patients with T1D. There is, in addition, further evidence within the Golden Years Cohort for genetically determined elevated HDL-cholesterol affording protection from large vessel disease in long-lived subsets of patients with T1D [Bain et al. 2003].
Taken together, these analyses of long-lived patients appear to suggest that HDL control may afford a significant therapeutic target for preventing CVD in ageing patients with T1D. There is, nevertheless, wider evidence amongst nonaged populations for a multifactorial approach to CV risk reduction in patients with T1D. Given that a number of these studies report extended follow up, albeit in younger patients than focused on in this review, their results are likely generalizable to an aged cohort.
Wallymahmed and colleagues provide evidence to suggest that lifestyle modifications may improve CV health within their randomized controlled trial comparing nurse-led CV risk factor intervention to routine care in patients with a mean age of 34.6 years [Wallymahmed et al. 2011]. In identifying positive impacts stemming from nurse-led intervention they do, however, note that much of the improvement seen was likely secondary to greater use of lipid-lowering or antihypertensive agents. It is additionally difficult to translate many of the lifestyle findings relating to young patients with T1D to their older aged counterparts. Chen and colleagues previously identified low physical activity to equate to decreased heart rate variability in children with T1D, for example, suggesting that strategies to improve exercise are important for preventing CVD [Chen et al. 2008].It is not clear whether this is possible in an aged cohort with multiple comorbidities, many of which are likely to be musculoskeletal.
The putative impact of pharmacological agents in older aged patients is again unclear. This is further compounded by the lack of trials evaluating the impact of optimal blood pressure control or use of antihypertensive medications on CVD in T1D. The major clinical trials (UKPDS, HOT, ADVANCE) were conducted in the T2D cohort [UKPDS group 1998; Hansson et al. 1998; Patel et al. 2007]. Nevertheless, observations from the EDC cohort showed that higher blood pressure was associated with higher relative risks of CAD [Orchard et al. 2001]. Amongst the general population, there is an additional recognition that effective management of widened pulse pressure in older aged persons reduces CVD. Created by a concomitant rise in systolic blood pressure and fall in diastolic blood pressure, increases in pulse pressure are recognized to occur earlier in patients with T1D, indicating accelerated arterial stiffness and ageing [Rönnback et al. 2004; Gordin et al. 2012]. The additional recognition that pulse pressure predicts a first ever CVD event in patients with T1D, identified in a cohort with a mean age of 37 years, seems to support the need for effective blood pressure control in older aged patients [Gordin et al. 2011].
There were a few small studies from the literature search that evaluated nonconventional pharmacological treatment in T1D. Cavallo and colleagues evaluated the use of melatonin in lowering nocturnal diastolic blood pressure in 11 T1D and 10 healthy controls using a randomized placebo-controlled double-blind crossover study design and found a significant but marginal reduction in nocturnal diastolic blood pressure (17.8 mmHg versus 16.0 mmHg) [Cavallo et al. 2004]. Djurhuus and colleagues found that magnesium repletion lowered atherogenic lipid fraction in 10 magnesium depleted T1D patient, there was no randomization or control group [Djurhuus et al. 2001]. These studies did not target the older aged T1D patients, have small sample sizes and lack long-term data to support the efficacy in improving CVD risk or mortality.
Poor glycaemic control is predictive of CVD events in patients with T1D, as highlighted by the FinnDiane prospective multicentre study that demonstrated a strong association between HbA1c variability and CVD events [Wadén et al. 2009]. The relationship between glycaemic control and CV health is, however, complex. In their 2010 analysis of 652 patients with T1D followed up over a period of 6 years, Maahs and colleagues identified that whilst good HbA1c control affords changes in fasting lipids, dyslipidaemia medications are nevertheless still required even in patients with well controlled diabetes in order to optimize CV health [Maahs et al. 2010a]. There is also some evidence to suggest that attempting to control blood glucose within too regimented a range might lead to adverse effects, though this is contested. Gruden and colleagues, for instance, argue that their analysis of 2181 T1D patients taken from the EURODIAB Prospective Complications Study suggests that severe hypoglycaemia does not increase the risk of CVD [Gruden et al. 2012]. Similarly, Eeg-Olofsson and colleagues highlight in their observational study of 7454 patients that, whilst CV risks increase with HbA1c levels, there is no J-shaped curve to indicate an increase risk resulting from hypoglycaemia [Eeg-Olofsson et al. 2010]. This linear relationship between HbA1c and CV health is further supported by a number of other authors reporting both observational studies and a meta-analysis [Wadén et al. 2009; Shankar et al. 2007; Selvin et al. 2004]. Somewhat conflictingly, an analysis published by Giménez and colleagues reported the opposite, with repeated severe hypoglycaemia increasing CV risk [Giménez et al. 2012]. This latter study is however a retrospective study and smaller than the EURODIAB studies.
Strategies to ameliorate the potential for CVD in older patients may additionally focus on oxidative stress, exposure to which arguably increases significantly with age. Costacou and colleagues have, for instance, identified that the anti-oxidant alpha-tocopherol provides protection against CAD in patients with T1D [Costacou et al. 2006]. Whilst this research was undertaken in a population with a mean age of 28 years, results were taken at a follow up of 10 years, suggesting an extended advantage to targeted antioxidant therapy which might extend into older age.
In their respective analyses of patients undergoing pancreas transplant alone, both Boggi and colleagues and Larsen and colleagues highlighted a number of improvements to independent CV risk factors, in addition to evidence to suggest directly improved left ventricular ejection fraction resulting from pancreatic transplantation [Boggi et al. 2012; Larsen et al. 2004]. Furthermore, combined pancreas and kidney transplantation for patients with T1D and end stage kidney disease (ESKD) has been associated with significantly lower mean arterial pressure, lower pulse pressure, lower LDL cholesterol and fewer required lipid-moderating medications which is likely secondary to a resultant lower atherosclerotic risk profile [Luan et al. 2007; Fiorina et al. 2001].
There are, finally, numerous reports within the literature concerning the proinflammatory state considered to accompany ageing. Inflammation in this context relates to a chronic overresponse that results in the accrual of cytokines and immune cells predisposing to atherosclerotic disease.
Whilst there is no direct evidence to link this state, often referred to under the umbrella term of ‘inflammaging’, to adverse events amongst aged patients with T1D, it is arguably implicated by work conducted within younger patient populations. González-Clemente and colleagues have, for instance, identified an association between interleukin (IL) 6 levels and lower heart rate variability, implying adverse outcomes stemming from raised cytokine levels [González-Clemente et al. 2007]. Although an untested hypothesis, it is possible that strategies to moderate inflammation amongst ageing patients may positively impact on CV morbidity and mortality.
Discussion
The available data suggest a significant CV burden in patients with T1D and poor management of CV risk factors. This is underpinned by a poor evidence base for therapeutic management of CV risk specifically for patients with T1D and in the most relevant population – the older aged patients. Whilst recent years have seen a decrease in CVD related mortality in patients with T1D [Miller et al. 2012], it still remains the leading cause of mortality and therefore significant further effort is required.
We would suggest that important areas remain to be addressed, particularly exploring the risks and benefits of therapeutic approaches to CVD management in the older aged. Thought will be required around the design of these studies. Clinical CVD outcomes (myocardial infarction, heart failure) may appear sooner than in a younger population because the older aged patients are more at risk and therefore studies could potentially be shorter and/or smaller. We also see greater risk of side effects associated with polypharmacy in the older aged patients and so dropout rates may be higher. The use of surrogate endpoints such as carotid intima thickness and cardiac magnetic resonance imaging may provide useful information more quickly in the interim.
An important and urgent question relates to the benefits of blood pressure, lipid and glucose control in patients with T1D and at what age these benefits become significant. This is particularly relevant in the older aged patients where we risk committing them to many years of therapy against the risk of side effects and potentially minimal benefit.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Siang Ing Lee, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, UK.
Mitesh Patel, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, UK.
Christopher M. Jones, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, UK
Parth Narendran, Institute of Biomedical Research, The Medical School, University of Birmingham, Edgbaston B15 2TT, UK.
References
- Abebe S., Berhane Y., Worku A., Alemu S. (2013) Increasing trends of diabetes mellitus and body weight: a ten year observation at Gondar University teaching referral hospital, northwest Ethiopia. PLoS One 8: e60081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abke S., Neumeier M., Weigert J., Wehrwein G., Eggenhofer E., Schäffler A., et al. (2006) Adiponectin-induced secretion of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1, CCL2) andinterleukin-8 (IL-8, CXCL8) is impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attas O., Al-Daghri N., Appiedu G. (2009). Fasting homocysteine levels in a cross-section of Saudi adults with type 1 diabetes mellitus. Diabetes Metab Syndr 3: 45–49. [Google Scholar]
- Allemann S., Saner C., Zwahlen M., Christ E., Diem P., Stettler C. (2009) Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. Swiss Med Wkly 139: 576–583. [DOI] [PubMed] [Google Scholar]
- Altinova A., Arslan M., Sepici-Dincel A., Akturk M., Altan N., Toruner F. (2007) Uncomplicated type 1 diabetes is associated with increased asymmetric dimethylarginine concentrations. J Clin Endocrinol Metab 92: 1881–1885. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2014) Standards of medical care in diabetes – 2014. Diabetes Care 37(Suppl. 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- Ammari F. (2004) Long-term complications of type 1 diabetes mellitus in the western area of Saudi Arabia. Diabetol Croat 33: 59–63. [Google Scholar]
- Amor A., Ricart M., Torres F., De Hollanda A., Yago G., Ara P., et al. (2011) Prevalence and control of the cardiovascular disease risk factors in patients with type 1 diabetes mellitus candidates for kidney-pancreas transplant from 1999 to 2010. Av Diabetol 27: 137–142. [Google Scholar]
- Assmann G., Cullen P., Schulte H. (2002) Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 105: 310–315. [DOI] [PubMed] [Google Scholar]
- Astrup A., Tarnow L., Pietraszek L., Schalkwijk C., Stehouwer C., Parving H., et al. (2008) Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: association with mortality and decline of glomerular filtration rate. Diabetes Care 31: 1170–1176. [DOI] [PubMed] [Google Scholar]
- Atabek M., Pirgon O., Karagozoglu E. (2006) Plasma homocysteine levels in children and adolescents with type 1 diabetes. Indian Pediatr 43: 401–407. [PubMed] [Google Scholar]
- Bain S., Gill G., Dyer P., Jones A., Murphy M., Jones K., et al. (2003) Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 20: 808–811. [DOI] [PubMed] [Google Scholar]
- Baskaran C., Volkening L., Diaz M., Laffel L. (2015), A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr Diabetes 16: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggi U., Vistoli F., Amorese G., Giannarelli R., Coppelli A., Mariotti R., et al. (2012) Long-term (5 years) efficacy and safety of pancreas transplantation alone in type 1 diabetic patients. Transplantation 93: 842–846. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation (no date) Cardiovascular disease. Available at: http://www.bhf.org.uk/heart-health/conditions/cardiovascular-disease.aspx (accessed 20 October 2014).
- Cavallo A., Daniels S., Dolan L., Khoury J., Bean J. (2004) Blood pressure response to melatonin in type 1 diabetes. Pediatr Diabetes 5: 26–31. [DOI] [PubMed] [Google Scholar]
- Chapman M., Crockett S., Purvis T., Anderson M., Whittaker P., Bhattacharjee R., et al. (2013) Macrovascular disease in the elderly with type 1 diabetes. J Diabetes Metab 4: 299. [Google Scholar]
- Chen S., Lee Y., Chiu H., Jeng C. (2008) Impact of physical activity on heart rate variability in children with type 1 diabetes. Childs Nerv Syst 24: 741–747. [DOI] [PubMed] [Google Scholar]
- Chillarón J., Flores-Le-Roux J., Goday A., Benaiges D., Carrera M., Puig J., et al. (2010) [Metabolic syndrome and type-1 diabetes mellitus: prevalence and associated factors]. Rev Esp Cardiol 63: 423–429. [PubMed] [Google Scholar]
- Cipollone F., Chiarelli F., Davi G., Ferri C., Desideri G., Fazia M., et al. (2005) Enhanced soluble CD40 ligand contributes to endothelial cell dysfunction in vitro and monocyte activation in patients with diabetes mellitus: effect of improved metabolic control. Diabetologia 48: 1216–1224. [DOI] [PubMed] [Google Scholar]
- Constantino M., Molyneaux L., Limacher-Gisler F., Al-Saeed A., Luo C., Wu T., et al. (2013) Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 36: 3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B., Miller R., Costacou T., Fried L., Kelsey S., Evans R., et al. (2010) Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med 27: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costacou T., Zgibor J., Evans R., Otvos J., Lopes-Virella M., Tracy R., et al. (2005) The prospective association between adiponectin and coronary artery disease among individuals with type 1diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 48: 41–48. [DOI] [PubMed] [Google Scholar]
- Costacou T., Zgibor J., Evans R., Tyurina Y., Kagan V., Orchard T. (2006) Antioxidants and coronary artery disease among individuals with type 1 diabetes: findings from the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications 20: 387–394. [DOI] [PubMed] [Google Scholar]
- Dessapt C., Karalliedde J., Hernandez-Fuentes M., Prieto Martin P., Maltese G., Dattani N., et al. (2010) Circulating vascular progenitor cells in patients with type 1 diabetes and microalbuminuria. Diabetes Care 33: 875–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinleyici E., Kirel B., Alatas O., Muslumanoglu H., Kilic Z., Dogruel N. (2006) Plasma total homocysteine levels in children with type 1 diabetes: relationship with vitamin status, methylene tetrahydrofolate reductase genotype, disease parameters and coronary risk factors. J Trop Pediatr 52: 260–266. [DOI] [PubMed] [Google Scholar]
- Dobrovolskienė R., Mockevičienė G., Urbonaitė B., Jurgevičienė N., Preikša R., Ostrauskas R. (2013) The risk of early cardiovascular disease in Lithuanian diabetic children and adolescents: a type 1 diabetes register database based study. Diabetes Res Clin Pract 100: 119–125. [DOI] [PubMed] [Google Scholar]
- Djurhuus M., Klitgaard N., Pedersen K., Blaabjerg O., Altura B., Altura B., et al. (2001) Magnesium reduces insulin-stimulated glucose uptake and serum lipid concentrations in type 1 diabetes. Metabolism 50: 1409–1417. [DOI] [PubMed] [Google Scholar]
- Eeg-Olofsson K., Cederholm J., Nilsson P., Zethelius B., Svensson A., Gudbjörnsdóttir S., et al. (2010) Glycemic control and cardiovascular disease in 7454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 33: 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESC Authors/Task Force Members, Rydén L., Grant P., Anker S., Berne C., Cosentino F., Danchin N., et al. (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34: 3035–3087. [DOI] [PubMed] [Google Scholar]
- Fawaz L., Elwan A., Kamel Y., Farid T., Kamel A., Mohamed W. (2009) Value of C-reactive protein and IL-6 measurements in type 1 diabetes mellitus. Arch Med Sci 5: 383–390. [Google Scholar]
- Fiorina P., La Rocca E., Venturini M., Minicucci F., Fermo I., Paroni R., et al. (2001) Effects of kidney-pancreas transplantation on atherosclerotic risk factors and endothelial function in patients with uremia and type 1 diabetes. Diabetes 50: 496–501. [DOI] [PubMed] [Google Scholar]
- Florkowski C., Scott R., Graham P., Han D., Moir C. (2003) Cause-specific and total mortality in the Canterbury (New Zealand) insulin-treated Diabetic Registry population: a 15-year follow-up study. Diabet Med 20: 191–197. [DOI] [PubMed] [Google Scholar]
- Galler A., Heitmann A., Siekmeyer W., Gelbrich G., Kapellen T., Kratzsch J., et al. (2010) Increased arterial stiffness in children and adolescents with type 1 diabetes: no association between arterial stiffness and serum levels of adiponectin. Pediatr Diabetes 11: 38–46. [DOI] [PubMed] [Google Scholar]
- Giménez M., López J., Castell C., Conget I. (2012) Hypoglycaemia and cardiovascular disease in type 1 diabetes. Results from the Catalan National Public Health registry on insulin pump therapy. Diabetes Res Clin Pract 96: e23–e25. [DOI] [PubMed] [Google Scholar]
- Głowińska B., Urban M., Peczyńska J., Szczepańska-Kostro J. (2003) [Selected adhesion molecules: sICAM-1 and sVCAM-1 as markers of endothelial dysfunction in diabetic children and adolescence]. Pol Merkur Lekarski 14: 205–209. [PubMed] [Google Scholar]
- Głowińska-Olszewska B., Luczyński W., Jabłońska J., Otocka A., Florys B., Bossowski A. (2010) [Asymmetric dimethylarginine (ADMA) in children with diabetes type 1]. Pediatr Endocrinol Diabetes Metab 16: 137–141. [PubMed] [Google Scholar]
- González-Clemente J., Gimenez-Perez G., Richart C., Broch M., Caixas A., Megia A., et al. (2005) The tumour necrosis factor (TNF)-alpha system is activated in accordance with pulse pressure in normotensive subjects with type 1 diabetes mellitus. Eur J Endocrinol 153: 687–691. [DOI] [PubMed] [Google Scholar]
- González-Clemente J., Vilardell C., Broch M., Megia A., Caixàs A., Giménez-Palop O., et al. (2007) Lower heart rate variability is associated with higher plasma concentrations of IL-6 in type 1 diabetes. Eur J Endocrinol 157: 31–38. [DOI] [PubMed] [Google Scholar]
- Gordin D., Soro-Paavonen A., Thomas M., Harjutsalo V., Saraheimo M., Bjerre M., et al. (2013) Osteoprotegerin is an independent predictor of vascular events in Finnish adults with type 1 diabetes. Diabetes Care 36: 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin D., Wadén J., Forsblom C., Thorn L., Rosengård-Bärlund M., Heikkilä O., et al. (2012) Arterial stiffness and vascular complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Ann Med 44: 196–204. [DOI] [PubMed] [Google Scholar]
- Gordin D., Wadén J., Forsblom C., Thorn L., Rosengård-Bärlund M., Tolonen N., et al. (2011) Pulse pressure predicts incident cardiovascular disease but not diabetic nephropathy in patients with type 1 diabetes (The FinnDiane Study). Diabetes Care 34: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauslund J., Nybo M., Green A., Sjølie A. (2010) N-terminal pro brain natriuretic peptide reflects long-term complications in type 1 diabetes. Scand J Clin Lab Invest 70: 392–398. [DOI] [PubMed] [Google Scholar]
- Gregg B., Connor C., Ruedy K., Beck R., Kollman C., Schatz D., et al. (2015) Body mass index changes in youth in the first year after type 1 diabetes diagnosis. J Pediatr 166: 1265.e1–1269.e1. [DOI] [PubMed] [Google Scholar]
- Gruden G., Barutta F., Chaturvedi N., Schalkwijk C., Stehouwer C., Witte D., et al. (2012) Severe hypoglycemia and cardiovascular disease incidence in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 35: 1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G., Bruno G., Chaturvedi N., Burt D., Pinach S., Schalkwijk C., et al. (2009) ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med 266: 527–536. [DOI] [PubMed] [Google Scholar]
- Hansen T., Tarnow L., Thiel S., Steffensen R., Stehouwer C., Schalkwijk C., et al. (2004) Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 53: 1570–1576. [DOI] [PubMed] [Google Scholar]
- Hansson L., Zanchetti A., Carruthers S., Dahlöf B., Elmfeldt D., Julius S., et al. (1998) Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 351: 1755–1762. [DOI] [PubMed] [Google Scholar]
- Harding S., Sommerfield A., Sarma J., Twomey P., Newby D., Frier B., et al. (2004) Increased CD40 ligand and platelet-monocyte aggregates in patients with type 1 diabetes mellitus. Atherosclerosis 176: 321–325. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., Minhas R., Sheikh A., et al. (2008) Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. Brit Med J 336: 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtenhuber T., Rami-Mehar B., Satler M., Nagl K., Höbaus C., Höllerl F., et al. (2013) Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes over time. Diabetes Care 36: 1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt K., Baker N., Cleary P., Backlund J., Lyons T., Jenkins A., et al. (2013) Oxidized LDL and AGE-LDL in circulating immune complexes strongly predict progression of carotid artery IMT in type 1 diabetes. Atherosclerosis 231: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley R., Peters S., Mishra G., Woodward M. (2015) Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 3: 198–206. [DOI] [PubMed] [Google Scholar]
- Jørgensen M., Almdal T., Carstensen B. (2013) Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia 56: 2401–2404. [DOI] [PubMed] [Google Scholar]
- Jorsal A., Tarnow L., Flyvbjerg A., Parving H., Rossing P., Rasmussen L. (2008) Plasma osteoprotegerin levels predict cardiovascular and all-cause mortality and deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetologia 51: 2100–2107. [DOI] [PubMed] [Google Scholar]
- Joseph G., Tarnow L., Astrup A., Hansen T., Parving H., Flyvbjerg A., et al. (2008) Plasma alpha-defensin is associated with cardiovascular morbidity and mortality in type 1 diabetic patients. J Clin Endocrinol Metab 93: 1470–1475. [DOI] [PubMed] [Google Scholar]
- Juutilainen A., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. (2008) Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 31: 714–719. [DOI] [PubMed] [Google Scholar]
- Kalantari F., Hovsepian S., Haghighi S., Amini M. (2007) The prevalence of cardiovascular risk factors in patients with type 1 diabetes in Isfahan, Iran. IJDLD 6: 255–262. [Google Scholar]
- Karantza M., Mittelman S., Dorey F., Samie S., Kaiserman K., Halvorson M., et al. (2008) Relationship of highly sensitive C-reactive protein and lipid levels in adolescents with type 1 diabetes mellitus. Pediatr Diabetes 9: 122–126. [DOI] [PubMed] [Google Scholar]
- Kinney G., Snell-Bergeon J., Maahs D., Eckel R., Ehrlich J., Rewers M., et al. (2011) Lipoprotein-associated phospholipase A2 activity predicts progression of subclinical coronary atherosclerosis. Diabetes Technol Ther 13: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing S., Swerdlow A., Slater S., Burden A., Morris A., Waugh N., et al. (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46: 760–765. [DOI] [PubMed] [Google Scholar]
- Lajer M., Tarnow L., Jorsal A., Teerlink T., Parving H., Rossing P. (2008) Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 31: 747–752. [DOI] [PubMed] [Google Scholar]
- Lajer M., Tarnow I., Michelson A., Jorsal A., Frelinger A., Parving H., et al. (2010) Soluble CD40 ligand is elevated in type 1 diabetic nephropathy but not predictive of mortality, cardiovascularevents or kidney function. Platelets 21: 525–532. [DOI] [PubMed] [Google Scholar]
- Larsen J., Colling C., Ratanasuwan T., Burkman T., Lynch T., Erickson J., et al. (2004) Pancreas transplantation improves vascular disease in patients with type 1 diabetes. Diabetes Care 27: 1706–1711. [DOI] [PubMed] [Google Scholar]
- Lechleitner M., Koch T., Herold M., Dzien A., Hoppichler F. (2000). Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med 248: 67–76. [DOI] [PubMed] [Google Scholar]
- Livingstone S., Looker H., Hothersall E., Wild S., Lindsay R., Chalmers J., et al. (2012) Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 9: e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H., Lin S., Wang Y. (2004) The relationship among serum cytokines, chemokine, nitric oxide, and leptin in children with type 1 diabetes mellitus. Clin Biochem 37: 666–672. [DOI] [PubMed] [Google Scholar]
- Luan F., Miles C., Cibrik D., Ojo A. (2007) Impact of simultaneous pancreas and kidney transplantation on cardiovascular risk factors in patients with type 1 diabetes mellitus. Transplantation 84: 541–544. [DOI] [PubMed] [Google Scholar]
- Luk A., Lau E., So W., Ma R., Kong A., Ozaki R., et al. (2014) Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care 37: 149–157. [DOI] [PubMed] [Google Scholar]
- Lung T., Hayes A., Herman W., Si L., Palmer A., Clarke P. (2014) A meta-analysis of the relative risk of mortality for type 1 diabetes patients compared to the general population: exploring temporal changes in relative mortality. PLoS One 9: e113635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maahs D., Ogden L., Dabelea D., Snell-Bergeon J., Daniels S., Hamman R., et al. (2010a) Association of glycaemia with lipids in adults with type 1 diabetes: modification by dyslipidaemia medication. Diabetologia 53: 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maahs D., Snell-Bergeon J., Hokanson J., Kinney G., Berl T., Rewers M., et al. (2010b) Relationship between cystatin C and coronary artery atherosclerosis progression differs by type 1 diabetes. Diabetes Technol Ther 12: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maahs D., Wadwa R., McFann K., Nadeau K., Williams M., Eckel R., et al. (2007) Longitudinal lipid screening and use of lipid-lowering medications in pediatric type 1 diabetes. J Pediatr 150: 146–150, 150.e1–2. [DOI] [PubMed] [Google Scholar]
- Mäkinen V., Forsblom C., Thorn L., Wadén J., Kaski K., Ala-Korpela M., et al. (2009) Network of vascular diseases, death and biochemical characteristics in a set of 4197 patients with type 1 diabetes (the FinnDiane Study). Cardiovasc Diabetol 8: 54. doi: 10.1186/1475-2840-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecha-Jȩdraszek A., Burska A., Donica H., Matuszek B., Nowakowski A. (2012) Serum adiponectin concentration in patients with type 1 diabetes. Curr Issues Pharm Med Sci 25: 360–366. [Google Scholar]
- Marcovecchio M., Widmer B., Turner C., Dunger D., Dalton R. (2011) Asymmetric dimethylarginine in young people with type 1 diabetes: a paradoxical association with HbA(1c). Diabet Med 28: 685–691. [DOI] [PubMed] [Google Scholar]
- Margeirsdottir H., Larsen J., Brunborg C., Overby N., Dahl-Jørgensen K. Norwegian Study Group for Childhood Diabetes. (2008) High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia 51: 554–561. [DOI] [PubMed] [Google Scholar]
- McKnight J., Wild S., Lamb M., Cooper M., Jones T., Davis E., et al. (2014) Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 32: 1036–1050. [DOI] [PubMed] [Google Scholar]
- Miller R., Secrest A., Sharma R., Songer T., Orchard T. (2012) Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 61: 2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V., Armstrong L., Clarke D., Bolton C., Pinkney J. (2001) Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors in type 1 diabetes. J Intern Med 250: 415–421. [DOI] [PubMed] [Google Scholar]
- Moreno P., Murcia A., Palacios I., Leon M., Bernardi V., Fuster V., et al. (2000) Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 102: 2180–2184. [DOI] [PubMed] [Google Scholar]
- Morimoto A., Onda Y., Nishimura R., Sano H., Utsunomiya K., Tajima N., et al. (2013) Cause-specific mortality trends in a nationwide population-based cohort of childhood-onset type 1 diabetes in Japan during 35 years of follow-up: the DERI Mortality Study. Diabetologia 56: 2171–2175. [DOI] [PubMed] [Google Scholar]
- Morrish N., Wang S., Stevens L., Fuller J., Keen H. (2001) Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44(Suppl. 2): S14–S21. [DOI] [PubMed] [Google Scholar]
- Moussa M., Alsaeid M., Refai T., Abdella N., Al-Sheikh N., Gomez J. (2004) Association of serum sialic acid with cardiovascular metabolic risk factors in Kuwaiti children and adolescents with type 1 diabetes. Metabolism 53: 638–643. [DOI] [PubMed] [Google Scholar]
- Nathan D., Cleary P., Backlund J., Genuth S., Lachin J., Orchard T., et al. (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwdorp M., Holleman F., de Groot E., Vink H., Gort J., Kontush A., et al. (2007) Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia 50: 1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin J., Ferreira I., Schalkwijk C., Jorsal A., Prins M., Parving H., et al. (2012a) Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12 year follow-up study. Diabetologia 55: 2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin J., Ferreira I., Schalkwijk C., Prins M., Chaturvedi N., Fuller J., et al. (2009) Levels of soluble receptor for AGE are cross-sectionally associated with cardiovascular disease in type 1 diabetes, and this association is partially mediated by endothelial and renal dysfunction and by low-grade inflammation: the EURODIAB Prospective Complications Study. Diabetologia 52: 705–714. [DOI] [PubMed] [Google Scholar]
- Nin J., Ferreira I., Schalkwijk C., Prins M., Chaturvedi N., Fuller J., et al. (2012b) Serum high-mobility group box-1 levels are positively associated with micro- and macroalbuminuria but not with cardiovascular disease in type 1 diabetes: the EURODIAB prospective complications study. Eur J Endocrinol 166: 325–332. [DOI] [PubMed] [Google Scholar]
- Nin J., Jorsal A., Ferreira I., Schalkwijk C., Prins M., Parving H., et al. (2010) Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes 59: 2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin J., Jorsal A., Ferreira I., Schalkwijk C., Prins M., Parving H., et al. (2011) Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care 34: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard T., Forrest K., Kuller L., Becker D. Pittsburgh Epidemiology of Diabetes Complications Study (2001) Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 24: 1053–1059. [DOI] [PubMed] [Google Scholar]
- Pajunen P., Taskinen M., Nieminen M., Syvänne M. (2000) Angiographic severity and extent of coronary artery disease in patients with type 1 diabetes mellitus. Am J Cardiol 86: 1080–1085. [DOI] [PubMed] [Google Scholar]
- Pambianco G., Costacou T., Ellis D., Becker D., Klein R., Orchard T. (2006) The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 55: 1463–1469. [DOI] [PubMed] [Google Scholar]
- Pavia C., Ferrer I., Valls C., Artuch R., Colome C., Vilaseca M. (2000) Total homocysteine in patients with type I diabetes. Diabetes Care 23: 84–87. [DOI] [PubMed] [Google Scholar]
- Patel A., ADVANCE Collaborative Group, MacMahon S., Chalmers J., Neal B., Woodward M., et al. (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370: 829–840. [DOI] [PubMed] [Google Scholar]
- Public Health England (no date) Age standardised rates. Available at: http://www.lho.org.uk/lHO_Topics/Data/Methodology_and_Sources/AgeStandardisedRates.aspx (accessed 20 October 2014).
- Rasmussen L., Tarnow L., Hansen T., Parving H., Flyvbjerg A. (2006) Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol 154: 75–81. [DOI] [PubMed] [Google Scholar]
- Rathcke C., Persson F., Tarnow L., Rossing P., Vestergaard H. (2009) YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes andincreases with levels of albuminuria. Diabetes Care 32: 323–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh C., Mittelman S., Wee C., Shah A., Kaufman F., Wood J. (2011) A longitudinal assessment of lipids in youth with type 1 diabetes. Pediatr Diabetes 12: 365–371. [DOI] [PubMed] [Google Scholar]
- Rönnback M., Fagerudd J., Forsblom C., Pettersson-Fernholm K., Reunanen A., Groop P., et al. (2004) Altered age-related blood pressure pattern in type 1 diabetes. Circulation 110: 1076–1082. [DOI] [PubMed] [Google Scholar]
- Rossi L., Lucchetti A., Palla A., De Marco S., Carrai M., Paci A., et al. (2002) Homocysteine levels in patients with cardiovascular diseases and type 1 diabetes and in normal subjects: a preliminary survey and suggestions for screening. Intern Med Clin Lab 10: 22–25. [Google Scholar]
- Rueda S., Fernández C., Nicolau J., Ricart M., Esmatjes E. (2009) Prevalence of cardiovascular risk factors in patients with type 1 diabetes in end-stage renal disease: changes in the trend from 1999 to 2006. J Diabetes Complications 23: 317–322. [DOI] [PubMed] [Google Scholar]
- Sastre J., Pinés P., Moreno J., Aguirre M., Blanco B., Calderón D., et al. (2012) Metabolic control and treatment patterns in patients with type 1 diabetes in Castilla-La Mancha: the DIAbetes tipo 1 in Castilla-La Mancha study. Endocrinol Nutr 59: 539–546. [DOI] [PubMed] [Google Scholar]
- Schram M., Chaturvedi N., Schalkwijk C., Fuller J., Stehouwer C. EURODIAB Prospective Complications Study Group. (2005) Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes – the EURODIAB Prospective Complications Study. Diabetologia 48: 370–378. [DOI] [PubMed] [Google Scholar]
- Schwab K., Doerfer J., Hecker W., Grulich-Henn J., Wiemann D., Kordonouri O., et al. (2006) Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care 29: 218–225. [DOI] [PubMed] [Google Scholar]
- Secrest A., Becker D., Kelsey S., Laporte R., Orchard T. (2010) Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1diabetes. Diabetes 59: 3216–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick P. (2010) Incidence rate ratio. Brit Med J 341: c4804. [Google Scholar]
- Sedgwick P. (2011) Hazard ratios. Brit Med J 343: d5918. [Google Scholar]
- Selvin E., Marinopoulos S., Berkenblit G., Rami T., Brancati F., Powe N., et al. (2004) Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 141: 421–431. [DOI] [PubMed] [Google Scholar]
- Shankar A., Klein R., Klein B., Moss S. (2007) Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 166: 393–402. [DOI] [PubMed] [Google Scholar]
- Sibley S., Hokanson J., Steffes M., Purnell J., Marcovina S., Cleary P., et al. (1999) Increased small dense LDL and intermediate-density lipoprotein with albuminuria in type 1 diabetes. Diabetes Care 22: 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrivarhaug T., Bangstad H., Stene L., Sandvik L., Hanssen K., Joner G. (2006) Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 49: 298–305. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu S., Chaturvedi N., Fuller J., Toeller M. EURODIAB Prospective Complications Study Group (2013) Do European people with type 1 diabetes consume a high atherogenic diet? 7-year follow-up of the EURODIAB Prospective Complications Study. Eur J Nutr 52: 1701–1710. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu S., Chaturvedi N., Schalkwijk C., Stehouwer C., Ebeling P., Fuller J., et al. (2006a) Soluble vascular cell adhesion molecule-1 and soluble E-selectin are associated with micro- and macrovascular complications in type 1 diabetic patients. J Diabetes Complications 20: 188–195. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu S., Chaturvedi N., Teerlink T., Idzior-Walus B., Fuller J., Stehouwer C. (2005) Plasma homocysteine and microvascular and macrovascular complications in type 1 diabetes: a cross-sectional nested case-control study. J Intern Med 258: 450–459. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu S., Chaturvedi N., Witte D., Stevens L., Porta M., Fuller J., et al. (2008) Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB prospective complications study (PCS). Diabetes Care 31: 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soedamah-Muthu S., Fuller J., Mulnier H., Raleigh V., Lawrenson R., Colhoun H. (2006b) High risk of cardiovascular disease in patients with type 1 diabetes in the UK: a cohort study using the general practice research database. Diabetes Care 29: 798–804. [DOI] [PubMed] [Google Scholar]
- Steigleder-Schweiger C., Rami-Merhar B., Waldhor T., Frohlich-Reiterer E., Schwarz I., Fritsch M., et al. (2012) Prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes in Austria. Eur J Pediatr 171: 1193–1202. [DOI] [PubMed] [Google Scholar]
- Sun J., Keenan H., Cavallerano J., Asztalos B., Schaefer E., Sell D., et al. (2011) Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-year medalist study. Diabetes Care 34: 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg F., Augustsson M., Forsander G., Cederholm U., Axelsen M. (2014) Children under the age of seven with diabetes are increasing their cardiovascular risk by their food choices. Acta Paediatr 103: 404–410. [DOI] [PubMed] [Google Scholar]
- Syed A., Hussain S., Nightingale P., De P., Charlton M., Gangopadhyay K., et al. (2007) Cardiovascular risk factors and their management in 1282 adult people with type 1 diabetes. Curr Med Res Opin 23: 2921–2927. [DOI] [PubMed] [Google Scholar]
- Tarnow L., Hovind P., Teerlink T., Stehouwer C., Parving H. (2004) Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care 27: 765–769. [DOI] [PubMed] [Google Scholar]
- The National Collaborating Centre for Chronic Conditions (2004) Chapter 8 Arterial risk control. In: Type 1 Diabetes in Adults: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians, pp. 73–87. [Google Scholar]
- Thomas M., Soderlund J., Lehto M., Makinen V., Moran J., Cooper M., et al. (2011) Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia 54: 2669–2677. [DOI] [PubMed] [Google Scholar]
- Thorn L., Forsblom C., Wadén J., Saraheimo M., Tolonen N., Hietala K., et al. (2009) Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 32: 950–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielemans S., Soedamah-Muthu S., De Neve M., Toeller M., Chaturvedi N., Fuller J., et al. (2013) Association of physical activity with all-cause mortality and incident and prevalent cardiovascular disease among patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetologia 56: 82–89. [DOI] [PubMed] [Google Scholar]
- Toeller M. (2002) Fibre consumption, metabolic effects and prevention of complications in diabetic patients: epidemiological evidence. Dig Liver Dis 34(Suppl. 2): S145–S149. [DOI] [PubMed] [Google Scholar]
- Tulloch-Reid M., Boyne M., Smikle M., Choo-Kang E., Parkes R., Wright-Pascoe R., et al. (2009) Cardiovascular risk profile in Caribbean youth with diabetes mellitus. West Indian Med J 58: 219–226. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Brit Med J 317: 703–713. [PMC free article] [PubMed] [Google Scholar]
- Van Eupen M., Schram M., Colhoun H., Hanssen N., Niessen H., Tarnow L., et al. (2013) The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia 56: 1845–1855. [DOI] [PubMed] [Google Scholar]
- Wadén J., Forsblom C., Thorn L., Gordin D., Saraheimo M., Groop P. Finnish Diabetic Nephropathy Study Group (2009) A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 58: 2649–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadén J., Forsblom C., Thorn L., Saraheimo M., Rosengård-Bärlund M., Heikkilä O., et al. (2008) Physical activity and diabetes complication in patients with type 1 diabetes and complication. Diabetes Care 31: 230–232. [DOI] [PubMed] [Google Scholar]
- Wallymahmed M., Morgan C., Gill G., Macfarlane I. (2011) Nurse-led cardiovascular risk factor intervention leads to improvements in cardiovascular risk targets and glycaemic control in people with Type 1 diabetes when compared with routine diabetes clinic attendance. Diabet Med 28: 373–379. [DOI] [PubMed] [Google Scholar]
- Wallymahmed M., Pinkney J., Saunders S., MacFarlane I. (2005) Vascular risk factors in patients with type 1 diabetes. Pract Diab Int 22: 81–85. [Google Scholar]
- Yeh S., Doupis J., Rahangdale S., Horr S., Malhotra A., Veves A. (2009) Total serum bilirubin does not affect vascular reactivity in patients with diabetes. Vasc Med 14: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]


