Abstract
Chronic urticaria is a debilitating disease characterized by itching and hives with or without angioedema lasting for more than 6 weeks. The disease carries a significant emotional and economic burden for the patient and often results in an odyssey between doctors of different specialities. Patients suffering from chronic urticaria are considered more difficult to satisfy, treat and to have a bigger emotional burden than the average patient in dermatology, paediatric and general practice settings. A joint initiative under the Dermatology section of the European Academy of Allergy and Clinical immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF) and the World Allergy Organization (WAO) has resulted in recently published guidelines for the diagnosis, classification and treatment of chronic urticarial: these guidelines are clinically useful and have a high success rate when followed in daily clinical practice. The treatment of choice for chronic urticaria is still nonsedating antihistamines although other treatments are available, with omalizumab (humanized IgG anti IgE antibodies) as the newest therapy. The pathogenesis of urticaria is poorly understood but autoimmunity is considered as one of the major underlying causes for this disease, although other theories exist.
Keywords: Chronic Urticaria, Diagnosis, treatment
Diagnosis and classification of urticaria
Urticaria is per definition the appearance of wheals and/or angioedema. However, as described in the following, there are many other diseases presenting with wheals and angioedema that are not chronic urticaria, e.g. allergic anaphylaxis. Wheals are characterized by three features: (1) swelling and erythema, (2) itching/burning sensation and (3) transient nature with the skin returning to normal within 1–24 hours.
According to the European Academy of Allergy and Clinical immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF) and the World Allergy Organization (WAO) guidelines [Zuberbier et al. 2014], which are the internationally accepted guidelines, urticaria can be classified according to duration and cause. Acute urticaria is defined as the occurrence of spontaneous wheals with or without angioedema for less than 6 weeks, whereas chronic urticaria is occurrence of hives with or without angioedema for 6 weeks or more.
Chronic urticaria is classified according to whether it is inducible or not into chronic spontaneous urticaria or chronic inducible urticaria. Chronic spontaneous urticaria may be due to known (e.g. autoantibodies) or unknown causes. The inducible urticarias include cold urticaria, delayed pressure urticaria, solar urticaria, heat urticaria, vibratory urticaria, cholinergic urticaria, contact urticaria, and aquagenic urticaria. A patient may well have more than one type of urticaria. Most of the conditions mentioned are mainly mediated by histamine (Table 1).
Table 1.
Subtypes of chronic urticaria according to European Academy of Allergy and Clinical immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF) and the World Allergy Organization (WAO) [Zuberbier et al. 2014].
| Chronic urticaria subtypes | |
| Chronic spontaneous urticaria | Chronic inducible urticaria |
| Spontaneous urticaria and/or angioedema for more than 6 weeks without known cause | Symptomatic urticarial dermographism, cold urticaria, pressure urticaria, solar urticaria, cholinergic urticaria, contact urticaria, aquagenic urticaria |
Wheals and angioedema are not always urticaria
Type I allergies should be suspected from a thorough history that reveals consistent appearance of wheals or angioedema after ingestion/inhalation, etc., of a specific allergen. To further elucidate whether a type I allergy is the cause of the disease allergen specific skin-prick test and radioallergosorbent test should be performed and finally, if possible, provocation with the suspected allergen will determine whether an allergy is present [Muraro et al. 2014].
Auto-inflammatory conditions characterized by urticarial rashes, fever, increased C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and increased neutrophils should be considered when a patient presents with severe treatment refractory urticarial rashes [Krause et al. 2012]. Auto-inflammatory diseases masquerading as chronic urticaria includes cryopurin-associated periodic syndromes (CAPS), caused by a mutation in the NLRP3 gene that encodes part of the NLRP3 inflammasome. These conditions where previously referred to as Familial cold auto-inflammatory syndrome, Muckle–Wells syndrome or Neonatal Onset Multisystem Inflammatory Disease [Aksentijevich et al. 2007; Petrilli et al. 2007; Krause et al. 2012]. Mutations in NLRP12 may also be associated with cold-induced auto-inflammatory syndrome [Borghini et al. 2011]. Schnitzlers syndrome is characterized by monoclonal gammopathy (IgM or IgG), symptoms of systemic inflammation, and by urticarial rashes. Schnitzlers syndrome may be a prodrome for mb. Waldenström and this should always be considered [Lipsker, 2010]. Other auto-inflammatory diseases presenting with urticarial rashes include adult-onset Still’s disease, systemic-onset juvenile idiopathic arthritis, and Mevalonate kinase deficiency/hyper IgD and periodic fever syndrome [Krause et al. 2012].
Aetiology and work-up
The standard workup varies among different parts of the world. One thing that is common is that a very thorough history should be taken. These are from the EACCI guidelines [Zuberbier et al. 2014], but the general purpose is to discover whether there are any eliciting factors of urticaria since the simplest treatment of course is elimination of any such factors including food allergies, physical provocation factors, etc. If an eliciting factor is suspected, provocations should be performed, e.g. double-blinded placebo-controlled food provocation, pressure, cold, heat, etc., to determine whether it is indeed an eliciting factor. If no symptom-inducing factor can be identified, only differential blood count and CRP or ESR is recommended since chronic or recurrent infections are known to induce urticaria [Zuberbier et al. 2014]. Any other tests should only be performed if the history of the patient’s symptoms and signs indicate this. Autoimmunity in the pathogenesis of urticaria is still a highly debated topic [Stitt and Dreskin, 2013]. The autologous skin serum test (ASST) and the histamine-release assay are at the moment the only available tests for autoimmunity and even though they correlate with both each other and activity in urticaria symptoms they do not always yield identical results and are not diagnostic for autoimmune urticaria [Platzer et al. 2005]. Although not validated, the basophil activation test (BAT) may also turn out to have some diagnostic value in the diagnosis of chronic spontaneous urticaria [Christensen et al. 2013].
Patients presenting with wheals lasting for more than 24 hours should be investigated thoroughly for urticarial vasculitis; however, biopsy should be considered even if the wheals are present for less than 24 hours. In a study of 312 patients, 47 histologically had urticarial vasculitis and of these 81% had extracutaneous symptoms [Tosoni et al. 2009].
Treatment guideline
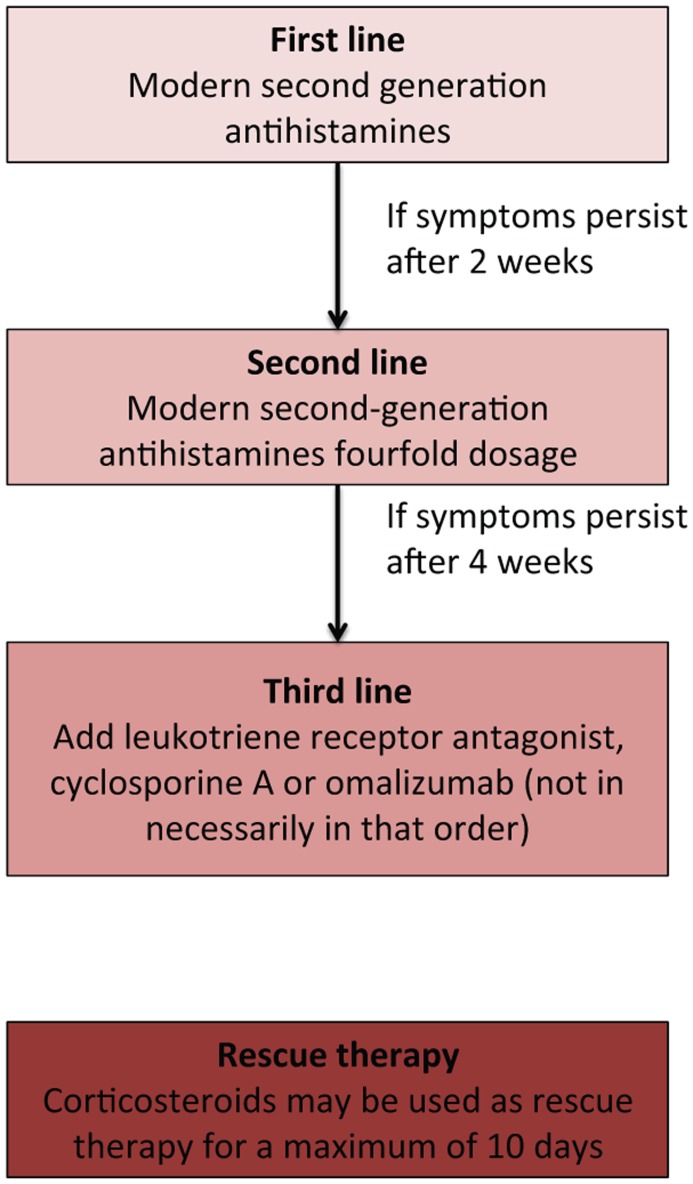
Several drugs have been implicated in the treatment of chronic urticaria with evidence ranging from randomized double-blind placebo-controlled clinical investigations to anecdotal case reports. In order to establish a firm treatment guide, an initiative under the EAACI/Ga2LEN/EDF/WAO, using a modified version of the Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology, led to a consensus conference in 2012 at which guidelines on the diagnosis and treatment of urticaria, first published in 2006, were updated for the third version [Zuberbier et al. 2014]. The newest guidelines recommend a stepwise approach to the pharmacological treatment of chronic urticaria. First-line therapy is nonsedating antihistamines and if symptoms persist for more than 2 weeks, updosing to fourfold the normal dose is recommend. If the symptoms persist for more than 4 weeks the guidelines recommend addition of omalizumab, cyclosporine A or montelukast (not in any preferred order) (Figure 1). Other therapeutic options, which have not been included in the guideline, are dapsone, anticoagulants, methotrexate, azathioprine, mycophenolate mofetil and biologics including intravenous immunoglobulins (IVIGs), rituximab, and adalimumab.
Figure 1.
Chronic urticaria treatment guideline according to the European Academy of Allergy and Clinical immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF) and the World Allergy Organization (WAO) [Zuberbier et al. 2014].
Nonsedating antihistamines
The majority of the symptoms seen in chronic urticaria are mediated by the H1-histamine receptor located on the endothelial cells of the vessels and nerve fibres. Antihistamines are inverse receptor agonists that stabilize the inactive conformation of the H1-receptor and interfere with the action of histamine [Church et al. 2010].
The effect of second-generation antihistamines in the treatment of chronic spontaneous urticaria has been examined in several double-blind placebo-controlled randomized trials and is unquestionably the best-documented effective treatment for urticaria. Currently, it is not possible to make a recommendation of which second-generation antihistamine to use. Only a few studies comparing antihistamines exist, and only for the relatively new antihistamine ebastine, which, when compared to fexofenadine and desloratidine has comparable effects and side effects [Barbanoj et al. 2003; Antonijoan et al. 2007]. If the effect of the recommended dose of the antihistamine is not sufficient, studies on bilastine, cetirizine, desloratidine, levocetirizine, rupatidine and fexofenadine have shown that updosing to fourfold dosage is an effective and safe strategy [Kontou-Fili et al. 1989; Zuberbier et al. 1996; Gimenez-Arnau et al. 2009; Siebenhaar et al. 2009; Staevska et al. 2010].
First-generation antihistamines are equally efficient in the relief of symptoms: it is however not recommended to use these due to their sedating effects which damage sleep quality by reduced and delayed rapid eye movement (REM) sleep, but also due to a ‘hangover’ effect with impaired cognitive functions up to 24 hours after use, with learning disabilities and loss of productivity as a consequence [Church et al. 2010].
Leukotriene receptor antagonists
If there is insufficient effect of treatment with nonsedating antihistamines in fourfold the recommended dose, one of the treatments recommended in the guidelines is addition of leukotriene receptor antagonists (LTRAs). These include montelukast, zafirlukast and pranlukast that block the leukotriene receptors for the cysteinyl leukotrienes, which are potent inflammatory mediators. The LTRAs are registered for the treatment of asthma and allergic rhinitis, yet their role in the treatment of chronic urticaria is still debated, as there are contradictory results on their use. One randomized placebo-controlled study including 160 adult patients showed that the LTRA, montelukast, was superior to placebo in controlling symptoms whereas there was no significant difference when comparing the results of treatment with desloratidine and desloratidine combined with montelukast. Treatment with desloratidine in this study was superior in effect to treatment with montelukast only [Di Lorenzo et al. 2004]. Another randomized double-blind placebo-controlled study included 81 patients treated with desloratidine, desloratidine plus montelukast or placebo. In this study, the desloratidine plus montelukast combination showed better effect in controlling symptoms than desloratidine alone [Nettis et al. 2004]. Several smaller studies [Erbagci, 2002; Reimers et al. 2002; Bagenstose et al. 2004; Godse, 2006; Agcaoili et al. 2011; Kosnik and Subic, 2011] have been performed and taken together they favour the use of LTRAs together with antihistamines rather than antihistamines alone, whereas antihistamines alone are better than LTRAs alone as reviewed by De Silva and colleagues [De Silva et al. 2014].
Cyclosporine A
Cyclosporine A inhibits calcineurin, which dephosphorylates the cytoplasmic form of NF-ATc (cytoplasmic form of nuclear factor of T cell) resulting in activation and relocation to the nucleus, NF-ATn, where it controls the transcription of, for example, inflammatory cytokines. In a randomized placebo-controlled study, 30 patients suffering from antihistamine-resistant chronic urticaria, and with a positive ASST were randomized 2:1 to either cyclosporine A (4 mg kg−1) or placebo both as add-on with antihistamines [Grattan et al. 2000]. In another randomized double-blind placebo-controlled study of 99 patients suffering from chronic spontaneous urticarial, patients were treated with cyclosporine A (3 mg kg−1) for 16 weeks, or for 8 weeks followed by 8 weeks of placebo or 16 weeks with placebo only. These studies showed a response rate between 53% and 70% compared with the placebo group [Vena et al. 2006].
Prospective open-label studies of cyclosporine A in doses ranging from 2.5 to 8 mg kg−1 with a total of 258 patients included showed a very variable response rate from 13% to 100%. Unfortunately outcome measures were not consistent, but taken together cyclosporine A does have a place in the treatment of urticaria although its nephrotoxicity should be given serious consideration before starting treatment [Toubi et al. 1997; Ilter et al. 1999; Loria et al. 2001; Di Gioacchino et al. 2003; Baskan et al. 2004; Serhat Inaloz et al. 2008; Ohtsuka, 2010; Boubouka et al. 2011].
Taken together there is good evidence for using cyclosporine A in the treatment of chronic spontaneous urticaria, although with proper surveillance of the potential side effects of this treatment with regular screening of haematological parameters and decreased kidney and liver function.
Omalizumab
Omalizumab is a humanized IgG–anti-IgE antibody, which binds human IgE and inhibits its binding to the FcεR-I. It was primarily registered for the use in the treatment of allergic asthma, but has been shown to be very effective in the treatment of chronic urticaria.
The effect of omalizumab on chronic spontaneous urticaria with or without angioedema has been demonstrated in several double-blinded randomized placebo-controlled studies, including almost 1200 patients [Maurer et al. 2011; Maurer 2011a; Saini et al. 2011; Kaplan et al. 2013; Maurer et al. 2013; Saini et al. 2015]. Dosages range between 75 and 600 mg with active treatment for up to 24 weeks. All dosages show a significant effect on urticaria symptoms, with 300 mg every 4 weeks as the most effective dose on several symptoms including itch, number of hives, angioedema and quality of life. Use of antihistamines was also reduced. Complete resolution of symptoms was achieved in 34–44% of the patients, and almost complete or complete resolution of the symptoms was seen in 52–66% of patients when treated with 300 mg every 4 weeks. Side effects of treatment with omalizumab were comparable with placebo, although a tendency to an increased number of cases with headaches, upper respiratory tract infections, arthralgia and irritation at the site of injection was observed.
Retrospective studies of the effect of omalizumab on chronic spontaneous urticaria also demonstrate the effectiveness of omalizumab. In one study of 51 patients, treatment with omalizumab (300 mg every 4 weeks) resulted in total resolution of the symptoms in 83% of the patients suffering from chronic spontaneous urticaria and in 73% of the patients suffering from chronic inducible urticaria [Metz et al. 2014] whereas a prospective cohort study of 61 patients showed complete resolution in 80% of the patients with chronic spontaneous urticaria. A retrospective case-series study on 17 patients has also showed that omalizumab is effective over a period of at least 4 years [Lefevre et al. 2013], whereas another case-series study has shown that omalizumab treatment may be paused and reinstated without loss of effect [Sussman et al. 2014].
Although omalizumab is registered at 300 mg every 4 weeks as the dosage for chronic spontaneous urticarial, there is also an effect at 150 mg every 4 weeks in some patients and in some cases dosage needs to be increased to 300 mg every 2 weeks in order to achieve effect. One paper has suggested an algorithm for updosing and tapering of omalizumab when used for the treatment of chronic spontaneous urticaria. According to this algorithm omalizumab is started at 150 mg, symptoms are assessed after 2 weeks and dosage is either increased or continued. Every 4th week symptoms are re-evaluated and if the patient is symptom free the interval between injections is increased up to 7 weeks after which treatment is stopped [Uysal et al. 2014].
Omalizumab is also reported to be effective in treatment of other urticaria forms such as cold urticaria, solar urticaria, cholinergic urticaria and symptomatic urticaria factitia. In addition, omalizumab has also shown effect on the symptoms of indolent systemic mastocytosis [Boyce, 2006; Guzelbey et al. 2008; Metz et al. 2008; Bindslev-Jensen and Skov, 2010; Krause et al. 2010; Kibsgaard et al. 2014; Kibsgaard b.
In summary, omalizumab is the latest development in the treatment of urticaria and offers an efficient and, from high quality studies, scientifically well-established and safe treatment, with relatively few side effects.
Methotrexate
Methotrexate is an anti-metabolite with anti-proliferative and anti-inflammatory properties. A few case reports have shown effect of this drug in the treatment of chronic urticaria [Weiner, 1989; Gach et al. 2001], and a small retrospective study of 16 patients showed effect in 12 cases [Perez et al. 2010] whereas a randomized double-blind placebo controlled study with 29 patients showed no effect of the use of methotrexate [Sharma et al. 2014]. Thus methotrexate is in general not recommended for the treatment of chronic spontaneous urticaria, although it may be useful in the treatment of urticarial vasculitis [Morgan and Khan, 2008].
Azathioprine and mycophenolate mofetil
Azathioprine is a prodrug which together with its metabolites inhibits the function of the lymphocytes through suppression of the purine nucleotide synthesis and through induction of apoptosis of T cells [Sanderson et al. 2004]. It is a widely used drug in inflammatory skin diseases, but reported use in chronic spontaneous urticaria has been limited to a few cases and a case-series study [Kibsgaard et al. 2014; Kibsgaard b; Tal et al. 2015], whereas in urticarial vasculitis it has been used more widely [Venzor et al. 2002; Carlson et al. 2006; Breda et al. 2013].
Mycophenolate mofetil is another purine synthesis inhibitor, which has been reported to be effective in chronic spontaneous urticaria in case reports [Kibsgaard et al. 2014; Kibsgaard b; Raghavendran et al. 2014], and in open-label studies [Shahar et al. 2006; Zimmerman et al. 2012]. In these reports, mycophenolate has resulted in complete resolution of symptoms in some but not all cases.
To summarise, azathioprine and mycophenolate mofetil are alternatives for the treatment of chronic spontaneous urticaria, but is not part of the EAACI guideline.
Dapsone
Dapsone (4,4’-diaminodiphenylsulfone) is an antibiotic with immunomodulatory properties often used in the treatment of inflammatory dermatological diseases. Dapsone has also been used in the treatment of urticaria although evidence for its efficacy has been scarce [Maurer et al. 2011; Maurer b]. In one nonblinded clinical study 65 chronic urticaria patients were treated with dapsone plus antihistamine or dapsone alone. Both groups showed significant reduction in urticaria activity score (UAS) and in a visual analogue score (VAS) of the symptoms compared with baseline but not compared to each other. However there was a significantly more-prolonged effect in the group treated with dapsone at 3 months after the end-of-treatment period [Engin and Ozdemir, 2008]. Another open-label study of 11 patients showed complete response in 9 of 11 patients in response to cetirizine 10 and 25 mg/day dapsone. The last two patients had complete response after increase of dapsone to 50 mg/day [Cassano et al. 2005]. Recently, a double-blind placebo-controlled crossover study of the effect of dapsone 100 mg in 22 patients with significant decrease in urticaria activity as measured by VAS, UAS and Itch score was published [Morgan and Khan, 2008]. These studies are limited by their methods and number of patients, but they suggest that dapsone may be an alternative in the treatment of chronic spontaneous urticaria especially if treatment is combined with antihistamines.
Other drugs
IVIG has been used in smaller case-series studies, and in case reports. The average dose of IVIG ranges from 0.15 to 2 g/kg/day for 1–3 consecutive days and repeated monthly or until remission, which could be sustained for up to 1 year [Kroiss et al. 2000; Klote et al. 2005; Pereira et al. 2007; Hrabak and Calabria, 2010; Mitzel-Kaoukhov et al. 2010]. IVIG has also been used for treatment of solar urticaria and urticarial vasculitis [Watkins et al. 2012].
Hydroxychloroquine, an antimalarial drug also used as anti-inflammatory drug in other diseases, has been used for the treatment of urticaria. The evidence for this drug is very low though and only one randomized double-blind placebo-controlled study has been performed. The study included 18 patients treated with 200 mg/day, and showed no significant effect [Reeves et al. 2004]. Colchicine has also been tested in a few case-series studies on urticarial vasculitis [Pho et al. 2011] and delayed pressure urticaria [Lawlor et al. 1989], but without convincing effect.
Sulfasalazine (5-aminosalicylic acid derivative) has anti-inflammatory effects through suppression of leukotrienes, mast cell degranulation and inhibition of B-lymphocyte proliferation. For these reasons sulfasalazine has been used in the treatment of urticaria. However only a case-series study and a few case reports have been published on the use of this drug, and its use in the treatment of chronic spontaneous urticaria is doubtful [Engler et al. 1995; Mcgirt et al. 2006].
Rituximab, a humanized anti CD-20 antibody has also been reported in a few cases to be effective in the treatment of chronic spontaneous urticaria [Arkwright, 2009; Chakravarty et al. 2011], whereas one other case did not show any effect [Mallipeddi and Grattan, 2007]. Antibodies against tumour necrosis factor alpha (TNF-α) have also been used in the treatment of chronic spontaneous urticaria but with variable success and only in small numbers [Cooke et al. 2015].
Monitoring treatment outcome
Both in clinical settings and in study settings, monitoring the outcome of the treatment is important for both the treating physician and the patient. When monitoring a chronic disease such as chronic spontaneous urticaria both disease activity and quality of life of the patient should be recorded, and done in a standardized way in order for studies to be compared. For disease activity, two different outcome measures are used, the UAS over 7 days (UAS7) and the urticaria control test (UCT). The UAS7 is a questionnaire filled in over 7 days where the patient scores the number of hives each day on a three-point scale and the intensity of the itch also on a three-point scale, making the maximum score over 7 days [Saini et al. 2011]. The scale is easy to use and has been validated and shown to have a minimal important difference (MID) of 9.5–10.5 [Mathias et al. 2012]. The UCT covers four areas over the last 4 weeks, physical symptoms, life quality, adequacy of treatment and total control of disease, all four scores on a scale from 1 to 4 where high score is better than low score and giving a maximum of 16 [Weller et al. 2014]. The score has been validated showing that a score of 12 or above signifies disease control. Quality of life can be measured by the CU-Q2oL (Chronic urticaria Quality of Life Questionnaire) which, through 23 questions scored on a five-point Likert scale [Baiardini et al. 2005], measures the impact of the disease on the patient’s quality of life. These outcome measure can demonstrate the impact of the treatment for the physician but, importantly, also for the patient.
Conclusions
Chronic spontaneous urticaria is still an enigmatic disease with regard to its aetiology. There is an efficient, simple an easy to use classification from the EAACI/GA2LEN/EDF/WAO guideline. This guideline also includes a simple algorithm for the treatment of chronic spontaneous urticaria. It should be kept in mind, however, that treatments not mentioned in the guideline may be of benefit for selected patients and should not be completely abolished although the evidence level for these is low. The group of patients suffering from chronic spontaneous urticaria is diverse, and the palette of treatments should be as well.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or notfor- profit sectors.
Declaration of conflict of interest statement: PVCV has received honoraria from Novartis, Abbvie and Leo Pharma for lectures, has served on advisory boards for Novartis and Abbvie, and has also been an investigator for the aforementioned.
Contributor Information
Christian Vestergaard, Department of Dermatology, Aarhus University Hospital, Aarhus, Denmark.
Mette Deleuran, Department of Dermatology, Aarhus University Hospital, Aarhus, Denmark.
References
- Agcaoili M., Sumpaico M., Aleta L., Recto M., Abong J. (2011) Montelukast in the treatment of chronic urticaria: a randomized double blind, placebo-controlled study. Ann Allergy Asthma Immunol 107: A17–A17. [Google Scholar]
- Aksentijevich I., Putnam C., Remmers E., Mueller J., Le J., Kolodner R., et al. (2007) The clinical continuum of cryopyrinopathies: novelcias1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum 56: 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonijoan R., Garcia-Gea C., Puntes M., Valle M., Esbri R., Fortea J., et al. (2007) A comparison of ebastine 10 mg fast-dissolving tablet with oral desloratadine and placebo in inhibiting the cutaneous reaction to histamine in healthy adults. Clin Drug Invest 27: 453–461. [DOI] [PubMed] [Google Scholar]
- Arkwright P. (2009) Anti-CD20 or anti-IgE therapy for severe chronic autoimmune urticaria. J Allergy Clin Immunol 123: 510–511; author reply 511. [DOI] [PubMed] [Google Scholar]
- Bagenstose S., Levin L., Bernstein J. (2004) The addition of zafirlukast to cetirizine improves the treatment of chronic urticaria in patients with positive autologous serum skin test results. J Allergy Clin Immunol 113: 134–140. [DOI] [PubMed] [Google Scholar]
- Baiardini I., Pasquali M., Braido F., Fumagalli F., Guerra L., Compalati E., et al. (2005) A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL). Allergy 60: 1073–1078. [DOI] [PubMed] [Google Scholar]
- Barbanoj M., Antonijoan R., Garcia-Gea C., Morte A., Gich I., Gispert J., et al. (2003) A study comparing the inhibitory effects of single and repeated oral doses of ebastine and fexofenadine against histamine-induced skin reactivity. Int Arch Allergy Immunol 132: 263–267. [DOI] [PubMed] [Google Scholar]
- Baskan E., Tunali S., Turker T., Saricaoglu H. (2004) Comparison of short- and long-term cyclosporine a therapy in chronic idiopathic urticaria. J Dermatol Treat 15: 164–168. [DOI] [PubMed] [Google Scholar]
- Bindslev-Jensen C., Skov P. (2010) Efficacy of omalizumab in delayed pressure urticaria: a case report. Allergy 65: 138–139. [DOI] [PubMed] [Google Scholar]
- Borghini S., Tassi S., Chiesa S., Caroli F., Carta S., Caorsi R., et al. (2011) Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an Nlrp12 mutation. Arthritis Rheum 63: 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubouka C., Charissi C., Kouimintzis D., Kalogeromitros D., Stavropoulos P., Katsarou A. (2011) Treatment of autoimmune urticaria with low-dose cyclosporin A: a one-year follow-up. Acta Derm Venereol 91: 50–54. [DOI] [PubMed] [Google Scholar]
- Boyce J. (2006) Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol 117: 1415–1418. [DOI] [PubMed] [Google Scholar]
- Breda L., Nozzi M., Harari S., Del Torto M., Lucantoni M., Scardapane A., et al. (2013) Hypocomplementemic urticarial vasculitis (HUVS) with precocious emphysema responsive to azathioprine. J Clin Immunol 33: 891–895. [DOI] [PubMed] [Google Scholar]
- Carlson J., Cavaliere L., Grant-Kels J. (2006) Cutaneous vasculitis: diagnosis and management. Clin Dermatol 24: 414–429. [DOI] [PubMed] [Google Scholar]
- Cassano N., D’argento V., Filotico R., Vena G. (2005) Low-dose dapsone in chronic idiopathic urticaria: preliminary results of an open study. Acta Derm Venereol 85: 254–255. [DOI] [PubMed] [Google Scholar]
- Chakravarty S., Yee A., Paget S. (2011) Rituximab successfully treats refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J Allergy Clin Immunol 128: 1354–1355. [DOI] [PubMed] [Google Scholar]
- Christensen C., Vestergaard C., Hoffmann H. (2013) Activation markers CD63 and CD203c are upregulated in chronic urticaria. Ann Dermatol 25: 522–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M., Maurer M., Simons F., Bindslev-Jensen C., Van Cauwenberge P., Bousquet J., et al. (2010) Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy 65: 459–466. [DOI] [PubMed] [Google Scholar]
- Cooke A., Bulkhi A., Casale T. (2015) Role of biologics in intractable urticaria. Biologics 9: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva N., Damayanthi H., Rajapakse A., Rodrigo C., Rajapakse S. (2014) Leukotriene receptor antagonists for chronic urticaria: a systematic review. Allergy Asthma Clin Immunol 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioacchino M., Di Stefano F., Cavallucci E., Verna N., Ramondo S., Paolini F., et al. (2003) Treatment of chronic idiopathic urticaria and positive autologous serum skin test with cyclosporine: clinical and immunological evaluation. Allergy Asthma Proc 24: 285–290. [PubMed] [Google Scholar]
- Di Lorenzo G., Pacor M., Mansueto P., Esposito Pellitteri M., Lo Bianco C., Ditta V., et al. (2004) Randomized placebo-controlled trial comparing desloratadine and montelukast in monotherapy and desloratadine plus montelukast in combined therapy for chronic idiopathic urticaria. J Allergy Clin Immunol 114: 619–625. [DOI] [PubMed] [Google Scholar]
- Engin B., Ozdemir M. (2008) Prospective randomized non-blinded clinical trial on the use of dapsone plus antihistamine vs. antihistamine in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol 22: 481–486. [DOI] [PubMed] [Google Scholar]
- Engler R., Squire E., Benson P. (1995) Chronic sulfasalazine therapy in the treatment of delayed pressure urticaria and angioedema. Ann Allergy Asthma Immunol 74: 155–159. [PubMed] [Google Scholar]
- Erbagci Z. (2002) The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: a single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol 110: 484–488. [DOI] [PubMed] [Google Scholar]
- Gach J., Sabroe R., Greaves M., Black A. (2001) Methotrexate-responsive chronic idiopathic urticaria: a report of two cases. Br J Dermatol 145: 340–343. [DOI] [PubMed] [Google Scholar]
- Gimenez-Arnau A., Izquierdo I., Maurer M. (2009) The use of a responder analysis to identify clinically meaningful differences in chronic urticaria patients following placebo-controlled treatment with rupatadine 10 and 20 mg. J Eur Acad Dermatol Venereol 23: 1088–1091. [DOI] [PubMed] [Google Scholar]
- Godse K. (2006) Oral montelukast monotherapy is ineffective in chronic idiopathic urticaria: a comparison with oral cetirizine. Indian J Dermatol Venereol Leprol 72: 312–314. [DOI] [PubMed] [Google Scholar]
- Grattan C., O’donnell B., Francis D., Niimi N., Barlow R., Seed P., et al. (2000) Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol 143: 365–372. [DOI] [PubMed] [Google Scholar]
- Guzelbey O., Ardelean E., Magerl M., Zuberbier T., Maurer M., Metz M. (2008) Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy 63: 1563–1565. [DOI] [PubMed] [Google Scholar]
- Hrabak T., Calabria C. (2010) Multiple treatment cycles of high-dose intravenous immunoglobulin for chronic spontaneous urticaria. Ann Allergy Asthma Immunol 105: 245; author reply 245–246. [DOI] [PubMed] [Google Scholar]
- Ilter N., Gurer M., Akkoca M. (1999) Short-term oral cyclosporine for chronic idiopathic urticaria. J Eur Acad Dermatol Venereol 12: 67–69. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Ledford D., Ashby M., Canvin J., Zazzali J., Conner E., et al. (2013) Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol 132: 101–109. [DOI] [PubMed] [Google Scholar]
- Kibsgaard L., Lefevre A., Deleuran M., Vestergaard C. (2014a) A case series study of eighty-five chronic spontaneous urticaria patients referred to a tertiary care center. Ann Dermatol 26: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibsgaard L., Skjold T., Deleuran M., Vestergaard C. (2014b) Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Derm Venereol 94: 363–364. [DOI] [PubMed] [Google Scholar]
- Klote M., Nelson M., Engler R. (2005) Autoimmune urticaria response to high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol 94: 307–308. [DOI] [PubMed] [Google Scholar]
- Kontou-Fili K., Paleologos G., Herakleous M. (1989) Suppression of histamine-induced skin reactions by loratadine and cetirizine dihcl. Eur J Clin Pharmacol 36: 617–619. [DOI] [PubMed] [Google Scholar]
- Kosnik M., Subic T. (2011) Add-on montelukast in antihistamine-resistant chronic idiopathic urticaria. Respir Med 105(Suppl. 1): S84–S88. [DOI] [PubMed] [Google Scholar]
- Krause K., Ardelean E., Kessler B., Magerl M., Metz M., Siebenhaar F., et al. (2010) Antihistamine-resistant urticaria factitia successfully treated with anti-immunoglobulin E therapy. Allergy 65:1494–1495. [DOI] [PubMed] [Google Scholar]
- Krause K., Grattan C., Bindslev-Jensen C., Gattorno M., Kallinich T., De Koning H., et al. (2012) How not to miss autoinflammatory diseases masquerading as urticaria. Allergy 67: 1465–1474. [DOI] [PubMed] [Google Scholar]
- Kroiss M., Vogt T., Landthaler M., Stolz W. (2000) The effectiveness of low-dose intravenous immunoglobulin in chronic urticaria. Acta Derm Venereol 80: 225. [DOI] [PubMed] [Google Scholar]
- Lawlor F., Black A., Ward A., Morris R., Greaves M. (1989) Delayed pressure urticaria, objective evaluation of a variable disease using a dermographometer and assessment of treatment using colchicine. Br J Dermatol 120: 403–408. [DOI] [PubMed] [Google Scholar]
- Lefevre A., Deleuran M., Vestergaard C. (2013) A long term case series study of the effect of omalizumab on chronic spontaneous urticaria. Ann Dermatol 25: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsker D. (2010) The Schnitzler syndrome. Orphanet J Rare Dis 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria M., Dambra P., D’oronzio L., Nettis E., Pannofino A., Cavallo E., et al. (2001) Cyclosporin a in patients affected by chronic idiopathic urticaria: a therapeutic alternative. Immunopharmacol Immunotoxicol 23: 205–213. [DOI] [PubMed] [Google Scholar]
- Mallipeddi R., Grattan C. (2007) Lack of response of severe steroid-dependent chronic urticaria to rituximab. Clin Exp Dermatol 32: 333–334. [DOI] [PubMed] [Google Scholar]
- Mathias S., Crosby R., Zazzali J., Maurer M., Saini S. (2012) Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 108: 20–24. [DOI] [PubMed] [Google Scholar]
- Maurer M., Altrichter S., Bieber T., Biedermann T., Brautigam M., Seyfried S., et al. (2011a) Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol 128: 202–209; e205. [DOI] [PubMed] [Google Scholar]
- Maurer M., Rosen K., Hsieh H., Saini S., Grattan C., Gimenez-Arnau A., et al. (2013) Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 368: 924–935. [DOI] [PubMed] [Google Scholar]
- Maurer M., Weller K., Bindslev-Jensen C., Gimenez-Arnau A., Bousquet P., Bousquet J., et al. (2011b) Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 66: 317–330. [DOI] [PubMed] [Google Scholar]
- Mcgirt L., Vasagar K., Gober L., Saini S., Beck L. (2006) Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine. Arch Dermatol 142: 1337–1342. [DOI] [PubMed] [Google Scholar]
- Metz M., Bergmann P., Zuberbier T., Maurer M. (2008) Successful treatment of cholinergic urticaria with anti-immunoglobulin E therapy. Allergy 63: 247–249. [DOI] [PubMed] [Google Scholar]
- Metz M., Ohanyan T., Church M., Maurer M. (2014) Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci 73: 57–62. [DOI] [PubMed] [Google Scholar]
- Mitzel-Kaoukhov H., Staubach P., Muller-Brenne T. (2010) Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol 104: 253–258. [DOI] [PubMed] [Google Scholar]
- Morgan M., Khan D. (2008) Therapeutic alternatives for chronic urticaria: an evidence-based review, part 2. Ann Allergy Asthma Immunol 100: 517–526; quiz 526–518, 544. [DOI] [PubMed] [Google Scholar]
- Muraro A., Werfel T., Hoffmann-Sommergruber K., Roberts G., Beyer K., Bindslev-Jensen C., et al. (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69: 1008–1025. [DOI] [PubMed] [Google Scholar]
- Nettis E., Colanardi M., Paradiso M., Ferrannini A. (2004) Desloratadine in combination with montelukast in the treatment of chronic urticaria: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy 34: 1401–1407. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T. (2010) Response to oral cyclosporine therapy and high sensitivity-CRP level in chronic idiopathic urticaria. Int J Dermatol 49: 579–584. [DOI] [PubMed] [Google Scholar]
- Pereira C., Tavares B., Carrapatoso I., Loureiro G., Faria E., Machado D., et al. (2007) Low-dose intravenous gammaglobulin in the treatment of severe autoimmune urticaria. Eur Ann Allergy Clin Immunol 39: 237–242. [PubMed] [Google Scholar]
- Perez A., Woods A., Grattan C. (2010) Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol 162: 191–194. [DOI] [PubMed] [Google Scholar]
- Petrilli V., Dostert C., Muruve D., Tschopp J. (2007) The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19: 615–622. [DOI] [PubMed] [Google Scholar]
- Pho L., Eliason M., Regruto M., Hull C., Powell D. (2011) Treatment of chronic urticaria with colchicine. J Drugs Dermatol 10: 1423–1428. [PubMed] [Google Scholar]
- Platzer M., Grattan C., Poulsen L., Skov P. (2005) Validation of basophil histamine release against the autologous serum skin test and outcome of serum-induced basophil histamine release studies in a large population of chronic urticaria patients. Allergy 60: 1152–1156. [DOI] [PubMed] [Google Scholar]
- Raghavendran R., Humphreys F., Kaur M. (2014) Successful use of mycophenolate mofetil to treat severe chronic urticaria in a patient intolerant to ciclosporin. Clin Exp Dermatol 39: 68–69. [DOI] [PubMed] [Google Scholar]
- Reeves G., Boyle M., Bonfield J., Dobson P., Loewenthal M. (2004) Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J 34: 182–186. [DOI] [PubMed] [Google Scholar]
- Reimers A., Pichler C., Helbling A., Pichler W., Yawalkar N. (2002) Zafirlukast has no beneficial effects in the treatment of chronic urticaria. Clin Exp Allergy 32: 1763–1768. [DOI] [PubMed] [Google Scholar]
- Saini S., Rosen K., Hsieh H., Wong D., Conner E., Kaplan A., et al. (2011) A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol 128: 567–573 e561. [DOI] [PubMed] [Google Scholar]
- Saini S., Bindslev-Jensen C., Maurer M., Grob J., Bulbul Baskan E., Bradley M., et al. (2015) Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol 135: 925. [DOI] [PubMed] [Google Scholar]
- Sanderson J., Ansari A., Marinaki T., Duley J. (2004) Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem 41: 294–302. [DOI] [PubMed] [Google Scholar]
- Serhat Inaloz H., Ozturk S., Akcali C., Kirtak N., Tarakcioglu M. (2008) Low-dose and short-term cyclosporine treatment in patients with chronic idiopathic urticaria: a clinical and immunological evaluation. J Dermatol 35: 276–282. [DOI] [PubMed] [Google Scholar]
- Shahar E., Bergman R., Guttman-Yassky E., Pollack S. (2006) Treatment of severe chronic idiopathic urticaria with oral mycophenolate mofetil in patients not responding to antihistamines and/or corticosteroids. Int J Dermatol 45: 1224–1227. [DOI] [PubMed] [Google Scholar]
- Sharma V., Singh S., Ramam M., Kumawat M., Kumar R. (2014) A randomized placebo-controlled double-blind pilot study of methotrexate in the treatment of H1 antihistamine-resistant chronic spontaneous urticaria. Indian J Dermatol Venereol Leprol 80: 122–128. [DOI] [PubMed] [Google Scholar]
- Siebenhaar F., Degener F., Zuberbier T., Martus P., Maurer M. (2009) High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol 123: 672–679. [DOI] [PubMed] [Google Scholar]
- Staevska M., Popov T., Kralimarkova T., Lazarova C., Kraeva S., Popova D., et al. (2010) The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol 125: 676–682. [DOI] [PubMed] [Google Scholar]
- Stitt J., Dreskin S. (2013) Urticaria and autoimmunity: where are we now? Curr Allergy Asthma Rep 13: 555–562. [DOI] [PubMed] [Google Scholar]
- Sussman G., Hebert J., Barron C., Bian J., Caron-Guay R., Laflamme S., et al. (2014) Real-life experiences with omalizumab for the treatment of chronic urticaria. Ann Allergy Asthma Immunol 112: 170–174. [DOI] [PubMed] [Google Scholar]
- Tal Y., Toker O., Agmon-Levin N., Shalit M. (2015) Azathioprine as a therapeutic alternative for refractory chronic urticaria. Int J Dermatol 54: 367–369. [DOI] [PubMed] [Google Scholar]
- Tosoni C., Lodi-Rizzini F., Cinquini M., Pasolini G., Venturini M., Sinico R., et al. (2009) A reassessment of diagnostic criteria and treatment of idiopathic urticarial vasculitis: a retrospective study of 47 patients. Clin Exp Dermatol 34: 166–170. [DOI] [PubMed] [Google Scholar]
- Toubi E., Blant A., Kessel A., Golan T.(1997) Low-dose cyclosporin a in the treatment of severe chronic idiopathic urticaria. Allergy 52: 312–316. [DOI] [PubMed] [Google Scholar]
- Uysal P., Eller E., Mortz C., Bindslev-Jensen C. (2014) An algorithm for treating chronic urticaria with omalizumab: dose interval should be individualized.J Allergy Clin Immunol 133: 914–915; e912. [DOI] [PubMed] [Google Scholar]
- Vena G., Cassano N., Colombo D., Peruzzi E., Pigatto P., Neo I. (2006) Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol 55: 705–709. [DOI] [PubMed] [Google Scholar]
- Venzor J., Lee W., Huston D. (2002)Urticarial vasculitis. Clin Rev Allergy Immunol 23: 201–216. [DOI] [PubMed] [Google Scholar]
- Watkins C., Peiris E., Saleh H., Krishnaswamy G. (2012) Intravenous immunoglobulin as a potential therapy for refractory urticaria – a review. Inflamm Allergy Drug Targets 11: 375–381. [DOI] [PubMed] [Google Scholar]
- Weiner M. (1989) Methotrexate in corticosteroid-resistant urticaria. Ann Intern Med 110: 848. [DOI] [PubMed] [Google Scholar]
- Weller K., Groffik A., Church M., Hawro T., Krause K., Metz M., et al. (2014) Development and validation of the urticaria control test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol 133: 1365–1372, 1372 e1361–1366. [DOI] [PubMed] [Google Scholar]
- Zimmerman A., Berger E., Elmariah S., Soter N. (2012) The use of mycophenolate mofetil for the treatment of autoimmune and chronic idiopathic urticaria: experience in 19 patients. J Am Acad Dermatol 66: 767–770. [DOI] [PubMed] [Google Scholar]
- Zuberbier T., Aberer W., Asero R., Bindslev-Jensen C., Brzoza Z., Canonica G., et al. (2014) The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy 69: 868–887. [DOI] [PubMed] [Google Scholar]
- Zuberbier T., Munzberger C., Haustein U., Trippas E., Burtin B., Mariz S., et al. (1996) Double-blind crossover study of high-dose cetirizine in cholinergic urticaria. Dermatology 193: 324–327. [DOI] [PubMed] [Google Scholar]