Abstract
Saliva plays a major role in maintaining oral health. Patients with salivary hypofunction exhibit difficulty in chewing and swallowing foods, tooth decay, periodontal disease, and microbial infections. At this time, treatments for hyposalivation are limited to medications (e.g., muscarinic receptor agonists: pilocarpine and cevimeline) that induce saliva secretion from residual acinar cells as well as artificial salivary substitutes. Therefore, advancement of restorative treatments is necessary to improve the quality of life in these patients. Our previous studies indicated that salivary cells are able to form polarized 3-dimensional structures when grown on growth factor–reduced Matrigel. This basement membrane is rich in laminin-III (L1), which plays a critical role in salivary gland formation. Mitotically inactive feeder layers have been used previously to support the growth of many different cell types, as they provide factors necessary for cell growth and organization. The goal of this study was to improve salivary gland cell differentiation in primary cultures by using a combination of L1 and a feeder layer of human hair follicle–derived mesenchymal stem cells (hHF-MSCs). Our results indicated that the direct contact of mouse submandibular (mSMG) cell clusters and hHF-MSCs was not required for mSMG cells to form acinar and ductal structures. However, the hHF-MSC conditioned medium enhanced cell organization and multilumen formation, indicating that soluble signals secreted by hHF-MSCs play a role in promoting these features.
Keywords: acinar cells, salivary glands, laminin, mesenchymal stromal cell, cyclic AMP, aquaporin 5
Introduction
Hyposalivation is the condition of having reduced saliva production, which decreases the quality of life for many patients. Hyposalivation leads to microbial infections as well as difficulty in chewing, speaking, and swallowing (Gupta et al. 2006). Reduced saliva output is commonly seen in the following circumstances: a) Sjögren syndrome, b) head and neck γ-irradiation, c) trauma of the salivary glands, and d) developmental disorders such as ectodermal dysplasias (Nordgarden et al. 2001; Konings et al. 2005; Gupta et al. 2006; Rogus-Pulia and Logemann 2011). Current treatments for hyposalivation are more palliative than curative. Therefore, it is necessary to develop treatments for salivary gland dysfunction using novel approaches, such as culturing transplantable salivary structures.
To successfully culture cells in vitro, a biologically relevant environment must be achieved. This can be accomplished via extracellular matrices (ECMs), media, and feeder layers that provide growth factors and nourishment to the cells. For instance, it has been demonstrated that mouse salivary gland cells form acinar structures on the basement membrane, growth factor–reduced Matrigel (GFR-MG; BD Biosciences, San Jose, CA, USA; Maria et al. 2011). However, GFR-MG is a tumor-derived basement membrane extract and exhibits variability between batches, making it unsuitable for human transplantation (Fridman et al. 1991; Janiak et al. 1994; Benton et al. 2011). In an effort to circumvent the obstacle of tumorigenicity, our previous study investigated a safer scaffold alternative, fibrin hydrogel (FH; McCall et al. 2013). We demonstrated that FH polymerized with epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1) served as a suitable scaffold for salivary cells to express α-amylase (McCall et al. 2013). However, salivary cell clusters grown on FH polymerized with EGF and IGF-1 were disorganized and lacked tight junctions (TJs; McCall et al. 2013). Therefore, a superior ECM was needed to promote viable salivary gland formation.
Laminin, a primary component of GFR-MG, is a basal lamina protein that plays a critical role in cell organization and differentiation (Kubota et al. 1988). Laminin-111 (L1) is one of the best-characterized isoforms, with the nomenclature 111 denoting the isoform’s chains, α1β1γ1 (Ekblom et al. 1990; Klein et al. 1990; Sorokin et al. 1997; Ekblom et al. 1998; Durbeej 2010). While L1 is predominately expressed in the embryonic epithelium, it is also present in adult tissues, including breast, kidney, liver, testis, and ovaries (Durbeej 2010). Laminins are involved in signaling between cells and basal lamina components by activating a variety of cell signaling pathways leading to cell survival (Ekblom et al. 1998). Laminins have been previously used in combination with feeder layers to support the growth and differentiation of induced pluripotent stem cells and primary plasmacytoma cells (Degrassi et al. 1993; Liu et al. 2012), indicating that this combination could also be used for growth and differentiation of salivary cells. In particular, the human hair follicle–derived mesenchymal stem cells (hHF-MSCs) used in this study have been beneficial in supporting the growth of skin and development of vascular tissues (Liu et al. 2008; Peng et al. 2011; Hill et al. 2013).
The goal of this study was to test whether highly purified L1 in combination with hHF-MSCs improved growth, organization, and differentiation of salivary cell clusters grown in vitro. Our results indicate that mouse submandibular gland (mSMG) cell clusters are unable to grow directly on hHF-MSCs when using our experimental setup. Interestingly, mSMG cell clusters plated on L1 and supplemented with hHF-MSC conditioned medium (without cells; denoted L1 + hHF-MSC CM) showed improved multilumen formation, organized growth, and cell differentiation. These results indicate that direct contact between mSMG and hHF-MSCs is not required for mSMG cell clusters to form acinar/ductal structures. Furthermore, the hHF-MSC CM contains secreted soluble signals that promote the formation of salivary epithelial structures with well-organized lumens.
Materials and Methods
Preparation of L1 Gel
L1 gel was plated, as described in the Appendix.
Preparation of GFR-MG
GFR-MG was plated, as described in the Appendix.
Preparation of hHF-MSC Cell Feeder Layer
hHF-MSCs were cultured, as described in the Appendix.
Preparation of L1 with an hHF-MSC Feeder Layer
L1 was plated on top of an hHF-MSC feeder layer, as described in the Appendix.
Preparation of hHF-MSC CM
Tissue culture medium from hHF-MSCs was collected, as described in the Appendix.
Experimental Animals
Female C57BL/6 mice were anesthetized and euthanized, as described in the Appendix.
Intracellular Free Ca2+ Concentration Measurements
Relative intracellular free Ca2+ concentrations were visualized, as described in the Appendix.
Confocal Microscopy
mSMG cell clusters were fixed and stained, as described in the Appendix.
Western Blot Analysis
mSMG cell clusters were lysed and immunoblotted, as described in the Appendix.
Cyclic Nucleotide Measurements
Cyclic adenosine monophosphate (cAMP) levels in mSMG cell clusters were measured, as described in the Appendix.
Secretory Granule Visualization
Secretory granules were visualized, as described in the Appendix.
Statistical Analysis
Data are means ± SEM of results from 3 or more experiments. P values <0.05 (indicated by 1 asterisk) and <0.01 (indicated by 2 asterisks) were calculated using unpaired 2-tailed t tests.
Results
mSMG Cell Clusters Grown on Different ECMs with hHF-MSCs or hHF-MSC CM
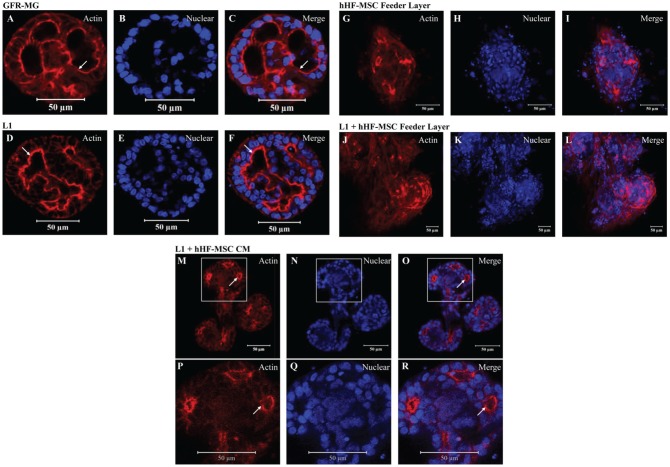
Previous studies have shown that primary salivary cells grown on GFR-MG form spheroids, whereas cells grown on FH form monolayers (McCall et al. 2013). However, primary salivary cells grown on a combination of GFR-MG + FH formed organized spheroids (McCall et al. 2013). Therefore, we investigated whether factors present in GFR-MG could help to improve spheroid acinar formation. Particularly, we wanted to determine if L1, a critical component of GFR-MG, enhanced acinar morphology. mSMG cell clusters grown on GFR-MG (Fig. 1A–C) and L1 (Fig. 1D–F) for 6 d formed organized round structures approximately 100 µm in diameter with developed lumens, as well as apical filamentous-actin (F-actin) rings. Since L1 proved to be optimal for the growth of mSMG cell clusters, we tested whether a combination of this ECM with different feeder layers enhanced cell differentiation and organization. As shown in Appendix Figure 1, hHF-MSCs were selected from a group of different feeder layers, given their ability to support lumen formation. However, further experiments showed that mSMG cell clusters grown on hHF-MSCs displayed areas of disorganization (Fig. 1G–I). Similarly, mSMG cell clusters grown on L1 with an hHF-MSC feeder layer formed disorganized structures showing very small central lumens (Fig. 1J–L). Interestingly, as shown in Figure 1M–O (and white-boxed insets, P–R), some cell clusters grown on L1 + hHF-MSC CM formed well-organized round structures that were interconnected by branches (where each end bud was approximately 50 µm in diameter and the complete structure measured approximately 200 µm in length). We observed that this branching pattern was not always evident, but cells developed multiple lumens and showed more consistent polarity compared to cells grown with L1 alone. Therefore, mSMG and hHF-MSC cell-cell contact was not necessary for cell growth; rather, the L1 and hHF-MSC CM were sufficient for cell cluster growth and differentiation.
Figure 1.
The effect of different extracellular matrices (ECMs) combined with human hair follicle–derived mesenchymal stem cells (hHF-MSCs) or hHF-MSC conditioned medium (hHF-MSC CM) on mouse submandibular gland (mSMG) cell cluster organization. mSMG cell clusters were grown for 6 d on the following combinations of ECM, feeder layer of hHF-MSCs, or hHF-MSC CM: growth factor–reduced Matrigel (GFR-MG; A–C), laminin-1 (L1; D–F), hHF-MSC feeder layer (G–I), L1 on top of an hHF-MSC feeder layer (J–L), or L1 + hHF-MSC CM (M–O; magnified insets P–R). Localization of filamentous-actin was determined using a phalloidin stain (A, D, G, J, M, P; red) and nuclei with a propidium iodide stain (B, E, H, K, N, Q; blue). mSMG cell clusters demonstrated organized lumen formation, as seen by the apical expression of filamentous-actin (white arrows) when grown on GFR-MG (A–C), L1 (D–F), and L1 + hHF-MSC CM (M–R). mSMG cell clusters grown on an hHF-MSC feeder layer (G–I) or on L1 with an hHF-MSC feeder layer (J–L) exhibited highly disorganized growth with little to no lumen formation.
Expression of Relevant Acinar, Ductal, Myoepithelial, and Proliferation Proteins in mSMG Cell Clusters Grown on L1 and L1 + hHF-MSC CM
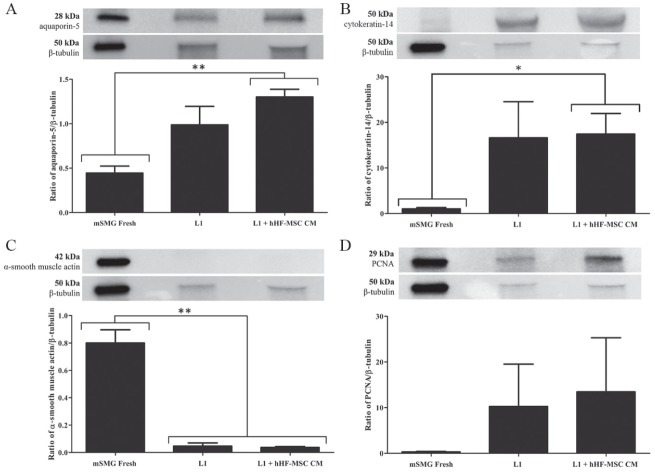
The combination of L1 + hHF-MSC CM supported optimal cell organization; for that reason, we decided to compare levels of relevant salivary acinar, ductal, myoepithelial, and proliferation proteins in cultures grown on L1 with and without hHF-MSC CM. We used aquaporin-5 as a marker of salivary acinar cells. As shown in Figure 2A, cells grown on L1 alone displayed similar expression levels of aquaporin-5 compared to cells grown on L1 + hHF-MSC CM. Expression levels of aquaporin-5 were significantly greater in mSMG cell clusters grown on L1 + hHF-MSC CM compared to freshly isolated mSMG cells (mSMG Fresh; P = 0.002; Fig. 2A).
Figure 2.
The effects of laminin-1 (L1) and L1 + human hair follicle–derived mesenchymal stem cell conditioned medium (hHF-MSC CM) on the expression of relevant acinar, ductal, myoepithelial, and proliferation proteins in mouse submandibular gland (mSMG) cell clusters. mSMG cell clusters were grown on L1 or L1 combined with hHF-MSC CM for 6 d and compared to freshly isolated mSMG cells (mSMG Fresh). Expression levels of relevant acinar, ductal, myoepithelial, and proliferation proteins were detected by Western blot analysis and normalized against β-tubulin expression. mSMG cell clusters grown on L1 with and without hHF-MSC CM maintained expression of aquaporin-5 (A), cytokeratin-14 (B), and proliferating cell nuclear antigen (PCNA) (D). A significant decrease in the myoepithelial cell marker, α–smooth muscle actin, was detected in mSMG cells cultured on L1 with and without hHF-MSC CM (C). Data are expressed as the means ± SEM of results from 3 or more experiments. Results from a representative experiment are shown.
We used cytokeratin-14 as a marker of salivary ductal and myoepithelial cells in culture. As shown in Figure 2B, mSMG cells grown on L1 alone as well as on L1 + hHF-MSC CM showed similar expression levels of cytokeratin-14. Additionally, we detected a significant increase in cytokeratin-14 expression in mSMG cell clusters grown on L1 + hHF-MSC CM compared to mSMG Fresh (P = 0.023; Fig. 2B). We also immunoblotted for the myoepithelial protein, α–smooth muscle actin. As shown in Figure 2C, mSMG cells displayed equal protein expression of α–smooth muscle actin when grown on L1 as well as L1 + hHF-MSC CM. Overall, we observed a significant loss of α–smooth muscle actin expression in both cultures compared to mSMG Fresh (mSMG Fresh compared to L1, P = 0.002; mSMG Fresh compared to L1 + hHF-MSC CM, P = 0.001; Fig. 2C). Finally, to elucidate any differences in proliferative activity, we immunoblotted for proliferating cell nuclear antigen (PCNA) and found that mSMG cells grown on L1 both with and without hHF-MSC CM showed statistically equivalent expression levels (Fig. 2D).
Localization of Relevant Structural and Proliferation Proteins in mSMG Cell Clusters Grown on L1 and L1 + hHF-MSC CM
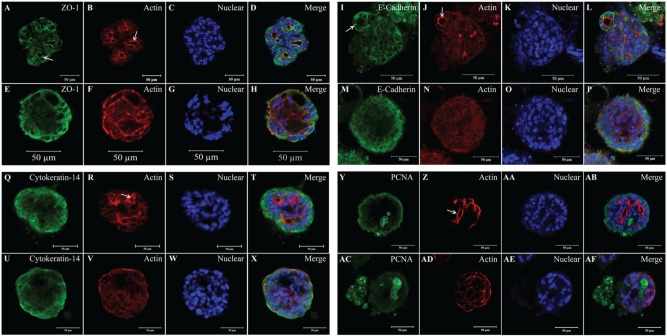
mSMG cell clusters grown on L1 with and without hHF-MSC CM were grown for 6 d and stained with an anti–ZO-1 antibody to visualize TJs and with an anti–E-cadherin antibody to visualize adherent junctions. As shown in Figure 3A, I, mSMG cell clusters grown on L1 + hHF-MSC CM displayed a strong apical expression of ZO-1 and basolateral expression of E-cadherin, respectively (see white arrows). In contrast, mSMG cell clusters grown on L1 alone (Fig. 3E, M) expressed ZO-1 and E-cadherin throughout the structure but with little specificity to the apical and basolateral margins; this finding was intensified by the apparent lack of lumens. Additionally, an anti–cytokeratin-14 antibody was used to localize ductal and myoepithelial cells. As illustrated in Figure 3Q, U, mSMG cell clusters grown on L1 and L1 + hHF-MSC CM maintained expression of cytokeratin-14 throughout the structure. Last, regions of proliferative activity were localized using an anti-PCNA antibody. mSMG cell clusters grown on L1 (Fig. 3AC) and L1 + hHF-MSC CM (Fig. 3Y) showed regions with high PCNA expression (regions outlined in white). Also, improved lumen formation can be seen via strong apical actin staining in all cultures of mSMG cell clusters grown on L1 + hHF-MSC CM compared to cell clusters grown without hHF-MSC CM (Fig. 3B, J, R, Z vs. 3F, N, V, AD; white arrows).
Figure 3.
The effects of laminin-1 (L1) and L1 + human hair follicle–derived mesenchymal stem cell conditioned medium (hHF-MSC CM) on the expression of zonula occludens-1 (ZO-1), E-cadherin, cytokeratin-14, and proliferating cell nuclear antigen (PCNA). Mouse submandibular gland (mSMG) cell clusters were grown on L1 (E–H, M–P, U–X, and AC–AF) or L1 combined with hHF-MSC CM (A–D, I–L, Q–T, and Y–AB) for 6 d and fixed. mSMG cell clusters were stained with anti–ZO-1 (A, E), anti–E-cadherin (I, M), anti–cytokeratin-14 (Q, U), and anti-PCNA (Y, AC) antibodies, followed by phalloidin (red; B, F, J, N, R, V, Z, AD) and propidium iodide (C, G) or TO-PRO-3 Iodide (K, O, S, W, AA, AE). mSMG cell clusters grown on L1 + hHF-MSC CM showed more well-defined lumens than cells grown without hHF-MSC CM, as indicated by a strong apical expression of F-actin (B, J, R, and Z vs. F, N, V, and AD; white arrows). Moreover, cells cultured on L1 + hHF-MSC CM demonstrated the correct localization of ZO-1 on the apical margin and basolateral expression of E-cadherin (A and I vs. E and M, white arrows). Additionally, mSMG cell clusters grown on L1 with and without hHF-MSC CM showed expression of cytokeratin-14 (Q, U) throughout the structure as well as regions of high proliferative activity (Y, AC; white outlined area). Results from a representative experiment are shown (n ≥ 3).
Agonist-Induced Intracellular Ca2+ Release
We tested whether mSMG cell clusters grown on different ECMs with hHF-MSCs or hHF-MSC CM activated intracellular calcium release (Ca2+) via the muscarinic secretory agonist, carbachol (1 µM). It is important to note that this type of calcium release assay is not quantitative but instead is a binary test indicative of whether the cells respond to a muscarinic agonist. Consequently, we cannot compare specific values of calcium responses between groups. In summary, a response was observed almost instantaneously in cells grown on all of the different experimental combinations of ECMs, feeder layer, and CM (Fig. 4A–E), demonstrating viability. Therefore, the combination of L1 + hHF-MSC CM maintained muscarinic receptor signaling while promoting formation of multilumenous salivary cell clusters.
Figure 4.
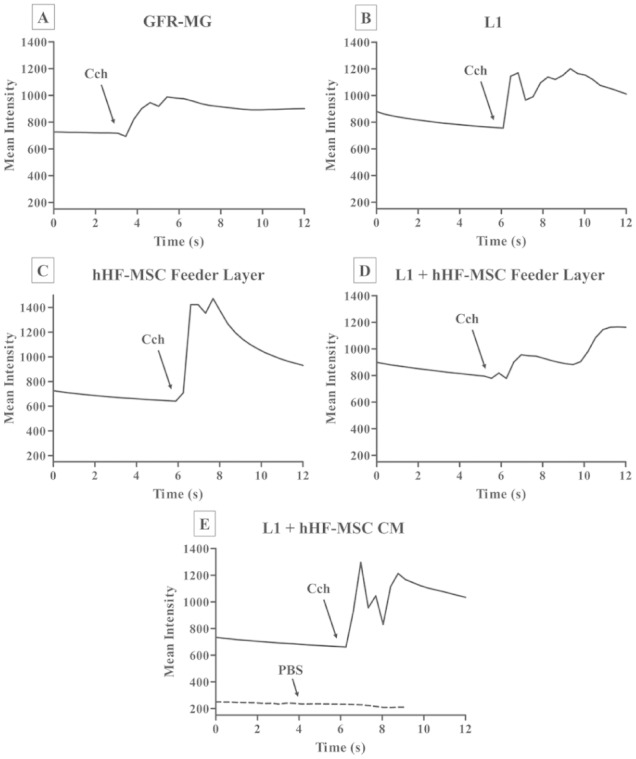
The effect of different extracellular matrices (ECMs) combined with human hair follicle–derived mesenchymal stem cells (hHF-MSCs) or hHF-MSC conditioned medium (hHF-MSC CM) on carbachol (Cch)–induced intracellular Ca2+ release. Mouse submandibular gland (mSMG) cell clusters were grown on different ECMs combined with a feeder layer of hHF-MSCs or hHF-MSC CM for 6 d (A–E). Then, cells were stimulated with Cch (1 µM; A–E) or phosphate-buffered saline (PBS; negative control, example of nonresponse overlaid on E). Changes in the Fluo-2 fluorescence intensity were recorded as described in the Appendix and analyzed using the Leica Application Suite software. mSMG cell clusters grown in all experimental conditions demonstrated an agonist-induced response (A–E). Results from a representative experiment are shown (n ≥ 3). GFR-MG, growth factor–reduced Matrigel; L1, Laminin-111.
Agonist-Induced Intracellular cAMP Synthesis and Secretory Granule Visualization
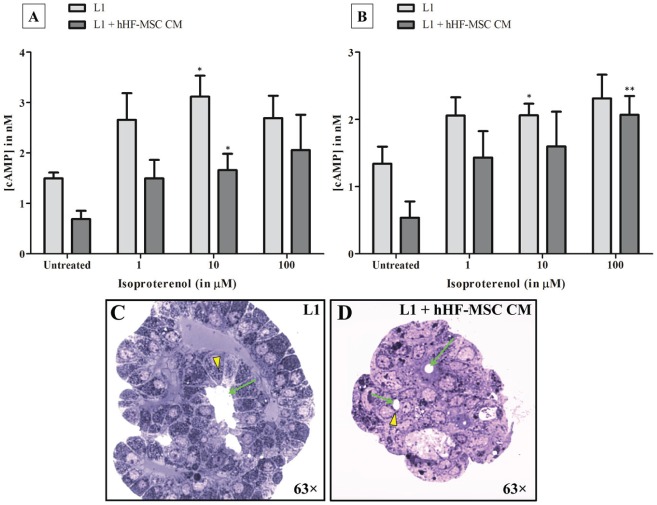
We tested whether mSMG cell clusters grown on L1 and L1 + hHF-MSC CM synthesized cAMP in response to the β-adrenergic secretory agonist, isoproterenol (1–100 µM). We detected a statistically equivalent dose-dependent response at 1 min (Fig. 5A) and 5 min (Fig. 5B) in cells grown on L1 and L1 + hHF-MSC CM (Fig. 5A, B; asterisks represent significant increases in cAMP concentration compared to untreated cells grown under the same conditions). Additionally, secretory granules can be seen in mSMG cell clusters grown on L1 (Fig. 5C) and L1 + hHF-MSC CM (Fig. 5D; see yellow arrowheads).
Figure 5.
The effect of laminin-1 (L1) + human hair follicle–derived mesenchymal stem cell conditioned medium (hHF-MSC CM) on isoproterenol-induced cyclic adenosine monophosphate (cAMP) synthesis and secretory granule visualization. Mouse submandibular gland (mSMG) cell clusters were grown on L1 or L1 combined with hHF-MSC CM for 6 d. Then, cells were stimulated with isoproterenol (1–100 µM) for 1 (A) and 5 (B) min. Changes in cAMP concentration were measured as described in the Appendix and analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). mSMG cell clusters grown on L1 both with and without hHF-MSC CM demonstrated a statistically equivalent dose response to the agonist after 1 and 5 min (A and B). Data are expressed as the means ± SEM of results from 4 or more experiments. Asterisks represent significant increases in cAMP concentration compared to untreated cell clusters. Secretory granules were visualized via toluidine blue staining in mSMG cell clusters grown on L1 (C; yellow arrowhead) and L1 + hHF-MSC CM (D; yellow arrowhead). Irregular lumens were observed in mSMG cell clusters grown on L1 (C; green arrow), while round lumens were observed in cell clusters grown on L1 + hHF-MSC CM (D; green arrows). This figure is available in color online at http://jdr.sagepub.com.
Discussion
Previous studies have shown that high densities of interfacing cells, such as feeder layers, could stop proliferation via contact inhibition and depletion of nutrients, leading cells to stay arrested for weeks (Leontieva et al. 2014). Furthermore, contact-inhibited cells are characterized by changes in morphology, similar to what we observed when mSMG cells were cultured on a feeder layer (Fig. 1G–I; Leontieva et al. 2014). In the case of mSMG cell clusters grown on L1 on top of an hHF-MSC feeder layer, we believe a similar interaction may have occurred. Specifically, hHF-MSCs could have outcompeted mSMG cells by binding to critical L1 peptides, thus inducing conformational changes in the ECM and inhibiting the organized growth of mSMG cells.
Interestingly, we found that the hHF-MSC CM was able to produce organized salivary cell clusters. A previous study showed that the administration of MSC CM has a long-lasting therapeutic rescue function, as shown by decreased progression of chronic kidney disease as well as reduced hypertension and glomerular injury (van Koppen et al. 2012). Other results demonstrated that MSC CM provides microenvironment cues that support long-term maintenance of pluripotent human embryonic stem cells. Our results are consistent with the preexisting literature, as we demonstrate that MSC CM is more important than cell-cell contact for salivary cell growth in vitro.
Isolated and cultured MSCs are known to secrete various cytokines, growth factors, ECM proteins, and chemokines into their culture medium that are important for cell survival, organization, and differentiation (Amable et al. 2014). MSCs differ in their secretome composition, and the source of derivation in the body dictates their proregenerative, proangiogenic, and anti-inflammatory capabilities (Amable et al. 2014). Currently, the exact composition of the hHF-MSC CM is unknown. Therefore, our system is suitable for in vitro studies until we can characterize the medium for future work in vivo. A previous study demonstrated that parotid gland cells cultured on FH supplemented with IGF-1 and EGF displayed an increase in amylase expression (McCall et al. 2013). Thus, we can hypothesize that IGF-1 and EGF may play an important role in maintaining the secretory function of mSMG cell clusters. Moreover, a study using the intravenous injection of bone marrow–derived cells (MSCs are likely present) to restore irradiation-damaged salivary glands showed increased levels of EGF in the saliva of treated mice, indicating that EGF may be vital to the repair of acinar cells (Sumita et al. 2011). Additionally, fibroblast growth factor (FGF)-1, -2, -7, and -10, as well as their respective receptors, are all known to be active in salivary gland morphogenesis (Ohuchi et al. 2000; Li et al. 2001; Steinberg et al. 2005; Wells et al. 2013; Yamamoto et al. 2008). Since hHF-MSCs are of connective tissue origin, it is likely that their CM is enriched with various FGFs. Other important factors regulating the growth of salivary glands include members of the ectodysplasin A receptor (EDAR) family and their ligands, which are important for branching morphogenesis (Häärä et al. 2011). We will try to determine which specific components are required for mSMG cell growth, organization, and differentiation (e.g., IGF-1, EGF, nerve growth factor [NGF], FGF, collagen I, heparan sulfate, transforming growth factor β [TGFβ], ectodysplasin A [EDA], interleukin [IL]–6, and IL-12), via an array of blocking antibody and inhibition experiments. Additionally, it has been determined that during salivary organogenesis, vasoactive intestinal peptide secreted by the innervating ganglia promotes the coalescence of microlumens to form contiguous lumens (Nedvetsky et al. 2014). We could apply this finding to our future work with postnatal cells to further improve glandular shape (specifically lumen formation) and organoid complexity in vitro. A fully defined formula of specific components and their respective concentrations will allow us to mimic optimal conditions in future transplantation studies.
In a previous attempt to create a safer scaffold alternative to GFR-MG, we used FH (water-swollen, cross-linked polymeric structures generated by mixing fibrinogen and thrombin) due to its known components and clinical viability (Ahmed et al. 2008; McCall et al. 2013). Although FH cannot support salivary cell organization and differentiation alone, it supports these processes when combined with GFR-MG (McCall et al. 2013). The current study determined that L1 + hHF-MSC CM yielded better results (i.e., improved cell organization) compared to GFR-MG, thus eliminating the need for GFR-MG. These results are important for our future goal of using 100% pure L1, which could be used in vivo without the limitations of GFR-MG.
Our results demonstrate that cells grown on L1 + hHF-MSC CM display multiple polarized lumens compared to cells grown on L1 alone (Fig. 3). In contrast, expression levels of acinar, ductal, and proliferation markers were not significantly different between the 2 groups (Fig. 2). An interesting finding in this study is that α–smooth muscle actin was expressed only in freshly isolated mSMG but not in the salivary cell clusters (Fig. 2). However, cytokeratin-14 was highly expressed in salivary cell clusters (Figs. 2, 3). Previous studies have shown the expression of both α–smooth muscle actin and cytokeratin-14 in salivary gland myoepithelial cells. The key difference is that α–smooth muscle actin is expressed in myoepithelial cells in early differentiation stages while cytokeratin-14 is expressed in well-differentiated myoepithelial cells. In short, these results indicate the presence of well-differentiated myoepithelial cells in our salivary cell clusters (Ogawa et al. 1999).
We demonstrated the ability of mSMG cell clusters to respond to the fluid secretory agonist, carbachol (Fig. 4A–E), as well as the protein secretory agonist, isoproterenol (Fig. 5A, B), indicating that the secretory machinery is intact, at least in terms of secondary messenger systems. Similarly, our previous studies showed that other types of salivary cells cultured on ECMs are able to respond to carbachol, as indicated by intracellular calcium release and changes in transepithelial potential difference (Baker et al. 2010; McCall et al. 2013; Leigh et al. 2014). Additionally, our salivary cell clusters maintained secretory granules (Fig. 5C, D). This is consistent with a previous transmission electron microscopy study showing that mSMG cells (grown on GFR-MG) maintain secretory granules (Leigh et al. 2014).
The present study used the commercially available ≥90% pure L1 (as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis; Trevigen, Gaithersburg, MD, USA), which is also murine tumor derived, since 100% pure L1 is not commercially available in a gel form and would require custom cross-linking of the peptides into a hydrogel. Thus, the L1 used in the present study could not be used in human transplantation studies but indicates that L1 peptides are beneficial for salivary cell growth in vitro, and exact functional domains could be identified in future studies. The ideal scaffold that we hope to achieve in the future will possess optimal bioactivity from native ECM peptides, as well as physical compliance (i.e., elastic modulus) for a robust cellular interface. Scaffold compliance is another critical variable in promoting cell differentiation and morphogenesis, since it could inhibit growth if not optimized (Peters et al. 2014). Ultimately, the cross-linking of pure L1 peptides with FH could be combined with nonbioactive materials, such as polyurethane and polyethylene glycol, to further improve compliance and support salivary cell growth, organization, and differentiation (Lee et al. 2005; Peled et al. 2007; Ahmed et al. 2008).
In summary, our results indicate that direct contact of mSMG cell clusters and MSC feeder cells disrupts organized growth of salivary gland structures, likely through contact inhibition. However, hHF-MSC CM improves multilumen formation and organization of mSMG cells when plated on an L1 ECM. These results demonstrate the importance of using stem cells for salivary gland bioengineering, given that soluble signals secreted by hHF-MSCs play a role in promoting glandular shape.
Author Contributions
C.L.M. Maruyama, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; N.J. Leigh, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J.W. Nelson, P. Lei, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; A.D. McCall, R.E. Mellas, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; S.T. Andreadis, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; O.J. Baker, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study is supported by the National Institutes of Health (NIH)–National Institute of Dental and Craniofacial Research (NIDCR) grants R01DE022971 (to O.J.B. and S.T.A.) and 1R01DE021697 (to O.J.B.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahmed TA, Dare EV, Hincke M. 2008. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 14(2):199–215. [DOI] [PubMed] [Google Scholar]
- Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. 2014. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther. 5(2):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker OJ, Schulz DJ, Camden JM, Liao Z, Peterson TS, Seye CI, Petris MJ, Weisman GA. 2010. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng Part C Methods. 16(5):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton G, Kleinman HK, George J, Arnaoutova I. 2011. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer. 128(8):1751–1757. [DOI] [PubMed] [Google Scholar]
- Degrassi A, Hilbert DM, Rudikoff S, Anderson AO, Potter M, Coon HG. 1993. In vitro culture of primary plasmacytomas requires stromal cell feeder layers. Proc Natl Acad Sci U S A. 90(5):2060–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M. 2010. Laminins. Cell Tissue Res. 339(1):259–268. [DOI] [PubMed] [Google Scholar]
- Ekblom M, Falk M, Salmivirta K, Durbeej M, Ekblom P. 1998. Laminin isoforms and epithelial development. Ann NY Acad Sci. 857:194–211. [DOI] [PubMed] [Google Scholar]
- Ekblom M, Klein G, Mugrauer G, Fecker L, Deutzmann R, Timpl R, Ekblom P. 1990. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 60(2):337–346. [DOI] [PubMed] [Google Scholar]
- Fridman R, Kibbey MC, Royce LS, Zain M, Sweeney TM, Jicha DL, Yannelli JR, Martin GR, Kleinman HK. 1991. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 83(11):769–774. [DOI] [PubMed] [Google Scholar]
- Gupta A, Epstein JB, Sroussi H. 2006. Hyposalivation in elderly patients. J Can Dent Assoc. 72(9):841–846. [PubMed] [Google Scholar]
- Häärä O, Fujimori S, Schmidt-Ullrich R, Hartmann C, Thesleff I, Mikkola ML. 2011. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development. 138(13):2681–2691. [DOI] [PubMed] [Google Scholar]
- Hill RP, Gardner A, Crawford HC, Richer R, Dodds A, Owens WA, Lawrence C, Rao S, Kara B, James SE, et al. 2013. Human hair follicle dermal sheath and papilla cells support keratinocyte growth in monolayer coculture. Exp Dermatol. 22(3):236–238. [DOI] [PubMed] [Google Scholar]
- Janiak M, Hashmi H, Janowska-Wieczorek A. 1994. Use of the Matrigel-based assay to measure the invasiveness of leukemic cells. Exp Hematol. 22(7):559–565. [PubMed] [Google Scholar]
- Klein G, Ekblom M, Fecker L, Timpl R, Ekblom P. 1990. Differential expression of laminin A and B chains during development of embryonic mouse organs. Development. 110(3):823–837. [DOI] [PubMed] [Google Scholar]
- Konings AW, Coppes RP, Vissink A. 2005. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 62(4):1187–1194. Published erratum in: Int J Radiat Oncol Biol Phys. 2006;64(1):330. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kleinman HK, Martin GR, Lawley TJ. 1988. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 107(4):1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Grad S, Gorna K, Gogolewski S, Goessl A, Alini M. 2005. Fibrin-polyurethane composites for articular cartilage tissue engineering: a preliminary analysis. Tissue Eng. 11(9–10):1562–1573. [DOI] [PubMed] [Google Scholar]
- Leigh NJ, Nelson JW, Mellas RE, McCall AD, Baker OJ. 2014. Three-dimensional cultures of mouse submandibular and parotid glands: a comparative study. J Tissue Eng. [epub ahead of print 4 Sep 2014].. doi: 10.1002/term.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Demidenko ZN, Blagosklonny MV. 2014. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc Natl Acad Sci U S A. 111(24):8832–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen Y, Schéele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. 2001. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 153(4):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Peng HF, Andreadis ST. 2008. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 79(1):24–33. [DOI] [PubMed] [Google Scholar]
- Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, et al. 2012. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 180(2):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria OM, Maria O, Liu Y, Komarova SV, Tran SD. 2011. Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol. 43(4):622–631. [DOI] [PubMed] [Google Scholar]
- McCall AD, Nelson JW, Leigh NJ, Duffey ME, Lei P, Andreadis ST, Baker OJ. 2013. Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng Part A. 19(20):2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, et al. 2014. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell. 30(4):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgarden H, Jensen JL, Storhaug K. 2001. Oligodontia is associated with extra-oral ectodermal symptoms and low whole salivary flow rates. Oral Dis. 7(4):226–232. [PubMed] [Google Scholar]
- Ogawa Y, Yamauchi S, Ohnishi A, Ito R, Ijuhin N. 1999. Immunohistochemistry of myoepithelial cells during development of the rat salivary glands. Anat Embryol (Berl). 200(2):215–228. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. 2000. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 277(3):643–649. [DOI] [PubMed] [Google Scholar]
- Peled E, Boss J, Bejar J, Zinman C, Seliktar D. 2007. A novel poly (ethylene glycol)–fibrinogen hydrogel for tibial segmental defect repair in a rat model. J Biomed Mater Res A. 80(4):874–884. [DOI] [PubMed] [Google Scholar]
- Peng HF, Liu JY, Andreadis ST, Swartz DD. 2011. Hair follicle–derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 17(7–8):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SB, Naim N, Nelson DA, Mosier AP, Cady NC, Larsen M. 2014. Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng Part A. 20(11–12):1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogus-Pulia NM, Logemann JA. 2011. Effects of reduced saliva production on swallowing in patients with Sjogren’s syndrome. Dysphagia. 26(3):295–303. [DOI] [PubMed] [Google Scholar]
- Sorokin LM, Pausch F, Durbeej M, Ekblom P. 1997. Differential expression of five laminin α (1–5) chains in developing and adult mouse kidney. Dev Dyn. 210(4):446–462. [DOI] [PubMed] [Google Scholar]
- Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. 2005. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 132(6):1223–1234. [DOI] [PubMed] [Google Scholar]
- Sumita Y, Liu Y, Khalili S, Maria OM, Xia D, Key S, Cotrim AP, Mezey E, Tran SD. 2011. Bone marrow–derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol. 43(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, Verhaar MC. 2012. Human embryonic mesenchymal stem cell–derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 7(6):e38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KL, Gaete M, Matalova E, Deutsch D, Rice D, Tucker AS. 2013. Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biol Open. 2(10):981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Fukumoto E, Yoshizaki K, Iwamoto T, Yamada A, Tanaka K, Suzuki H, Aizawa S, Arakaki M, Yuasa K. 2008. Platelet-derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression. J Biol Chem. 283(34):23139–23149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.