Abstract
Stem cells are remarkable, and stem cell–based tissue engineering is an emerging field of biomedical science aiming to restore damaged tissue or organs. In dentistry and reconstructive facial surgery, it is of great interest to restore lost teeth or craniofacial bone defects using stem cell–mediated therapy. In the craniofacial region, various stem cell populations have been identified with regeneration potential. In this review, we provide an overview of the current knowledge concerning the various types of tooth- and craniofacial bone–related stem cells and discuss their in vivo identities and regulating mechanisms.
Keywords: mesenchymal stromal cells, neural crest, hedgehogs, Gli1 protein, skull, stem cell niche
Introduction
Stem cells are characterized by their continuous self-renewal and multipotential differentiation capabilities. The existence of stem cells was first demonstrated in the hematopoietic system with studies showing that cells from the bone marrow can give rise to multilineage blood descendants while retaining their self-renewal ability (McCulloch and Till 1960). The same bone marrow tissue was subsequently shown to host a stromal type of multipotential cell—namely, mesenchymal stem cells (MSCs; Friedenstein et al. 1968). Since these pioneering studies, stem cells have become a popular topic for researchers and the general public, due to their remarkable ability to regenerate damaged tissue and treat certain diseases. Various tissue-specific stem cells have been identified in different organs.
Craniofacial tissue damage, including bone fractures and tooth loss, presents a major challenge for dentists and craniofacial surgeons. Craniofacial damage has a particularly negative effect for patients, because the face often represents our identity. For much of the history of these fields, defective bones or missing teeth could be replaced only with artificial prostheses, which can never completely restore the physiologic functions of natural organs. The concept of stem cell therapy provides a promising approach to designing new therapies for functional restoration. Although hematopoietic and MSCs were first identified >40 y ago, the study of stem cells in the dental and craniofacial field has lagged behind the study of stem cells in other tissues. The existence of dental stem cells was first demonstrated in the early 21st century (Gronthos et al. 2000). The major concepts and mechanisms in early dental and craniofacial stem cell studies were mostly borrowed from previous bone marrow MSC research. In the current review, we focus on the latest progress in studying bone marrow MSCs as well as dental and craniofacial MSCs. In addition, we briefly introduce research on dental epithelial stem cells due to their close association with dental MSCs.
Bone Marrow MSCs
MSCs were first identified in studies by Alexander Friedenstein and colleagues in the 1960s at the University of Moscow, which laid the foundation for subsequent MSC studies (Friedenstein et al. 1968). Friedenstein’s team discovered that bone marrow contains fibroblast-like stromal cells that are capable of forming clones and differentiating into osteocytes. Later studies indicated that similar populations are also capable of differentiating into adipocytes and chondrocytes (Friedenstein et al. 1982; Friedenstein et al. 1987; Mardon et al. 1987; Owen and Friedenstein 1988). The term mesenchymal stem cells was first adopted in the 1990s to define bone marrow stromal cells that can adhere to the culture plate and are capable of multipotential differentiation (Caplan 1991). Besides adipocytes, chondrocytes, and osteocytes, bone marrow MSCs are also able to differentiate into skeletal myocytes and tenocytes, among other cell types (Awad et al. 1999; Gang et al. 2004).
The study of MSCs has been profoundly influenced by earlier studies of hematopoietic stem cells. Because a number of surface markers were identified to aid in the identification and isolation of hematopoietic stem cells, similar approaches were adopted to identify MSCs. Many surface markers—including CD271, CD146, CD90, and CD105—have been used to identify MSCs (Cattoretti et al. 1993; Aslan et al. 2006; Sacchetti et al. 2007; Mabuchi et al. 2013; Kfoury and Scadden 2015). In 2006, the International Society for Cellular Society Therapy proposed a standard for defining MSCs as multipotential stromal cells that meet the following criteria:
they can attach to and grow on an uncoated culture dish;
they strongly express markers, including CD90, CD73, CD105, and CD44, but are negative for hematopoietic markers, such as CD34, CD45, CD11B, and CD19; and
they possess trilineage differentiation ability (osteogenic, adipogenic, and chondrogenic) in vitro, subject to appropriate conditions (Horwitz et al. 2005).
This definition is based on the in vitro properties of cultured cells, and it remains largely unknown whether the same criteria can be used to identify MSCs in vivo.
The use of transgenic mouse models has had a significant impact on the study of MSCs. Lineage tracing techniques enable the labeling of stem cells in a temporal and tissue-specific fashion. Animals with a fluorescent or LacZ reporter under the control of regulatory genetic elements critical for skeletal or mesenchymal development have been used for these studies (Table).
Table.
Genetic Mouse Models for Studying Adult Bone Marrow Mesenchymal Stem Cells.
| Marker | Labeling Method | In Vivo Contribution | Localization | Reference |
|---|---|---|---|---|
| Nestin | Nestin-CreERT | Osteoblasts, osteocytes upon injury repair | Perivascular | Mendez-Ferrer et al. (2010) |
| LeptinR | LeptinR-Cre | Bone, cartilage, adipocytes | Perivascular | Zhou et al. (2014) |
| Mx1 | Mx1-Cre | Osteoblasts, osteocytes, hematopoietic stem cells | Perivascular | Park et al. (2012) |
| Gremlin1 | Gremlin1-CreERT | Osteoblasts, osteocytes, chondrocytes | Perivascular adjacent to the growth plate and trabecular bone | Worthley et al. (2015) |
| Gli1 | Gli1-CreERT | Osteoblasts, osteocytes, chondrocytes, odontoblasts, fibroblasts | Perivascular | Kramann et al. (2015), Zhao et al. (2014) |
Nestin+ cells were first proposed to be the in vivo counterparts of bone marrow MSCs (Mendez-Ferrer et al. 2010). In the bone marrow, Nestin+ cells are a rare nonhematopoietic stromal population with a perivascular distribution. Isolated Nestin+ cells are capable of forming clones and have a robust self-renewal potential even after serial transplantation. Lineage tracing experiments based on the Nestin-CreERT model indicate the contribution of Nestin+ cells to osteochondral tissue during injury repair.
Mx1 was also proposed to label bone marrow MSCs (Park et al. 2012). Mx1+ cells are mostly perivascular and partially overlap with Nestin+ cells. They express typical MSC markers, such as CD105 and Sca1. Mx1+ cells exhibit enriched clonogenic ability and trilineage differentiation ability in vitro. Lineage tracing experiments indicate that Mx1+ cells contribute to bone formation during the repair of fracture injury. However, in addition to perivascular MSCs, Mx1 robustly labels hematopoietic cells, which makes it an inappropriate model for studying bone–hematopoietic stem cell interactions.
Leptin Receptor (LepR) was recently proposed to be an enriching marker for bone marrow MSCs (Zhou et al. 2014). Approximately 0.3% of bone marrow cells are LepR+, but they account for 94% of clonogenic ability in vitro. Lineage tracing experiments indicate that LepR+ cells can give rise to bone, cartilage, and adipocytes. They also make a significant contribution to new tissue formation during injury repair. Interestingly, LepR+ cells do not express Nestin. They are perivascular, arise postnatally, and give rise to most bone and adipocytes formed in adult bone marrow during postnatal development.
In addition, the BMP antagonist Gremlin1 was proposed to define MSCs in vivo (Worthley et al. 2015). Gremlin1+ cells are also perivascular stromal cells and do not express Nestin. They have a restricted ability to differentiate into osteoblasts, chondrocytes, and bone marrow stromal cells but do not differentiate into adipocytes. In vitro, Gremlin1+ cells possess robust clonogenic and trilineage differentiation ability.
Most recently, Gli1 was proposed to be a universal marker for MSCs in various organs, including the kidney, lung, liver, heart, tooth, and bone (Zhao et al. 2014; Kramann et al. 2015; Zhao et al. 2015). In vitro, Gli1+ cells highly express typical MSC markers, exhibit trilineage differentiation capacity, and possess colony-forming activity. Genetic lineage tracing analysis demonstrated that tissue-resident Gli1+ cells rapidly proliferate after kidney, lung, liver, or heart injury and give rise to myofibroblasts that contribute to organ fibrosis. Genetic ablation of Gli1+ cells significantly reduces fibrosis in multiple organs. In the bone marrow, Gli1+ cells line the CD31+ endothelium of bone marrow sinusoids as well as the endosteum of compact bones. Isolated stromal cells derived from Gli1+ MSCs express typical MSC markers, including CD44, CD29, CD105, and Sca1, with an absence of CD31, CD45, and CD34.
In summary, research on bone marrow MSCs is transitioning from in vitro analysis based on cell culture, differentiation assays, and surface marker profiling to a greater emphasis on in vivo identification and niche study. These new studies rely on transgenic models to trace and modify mesenchymal cells in mouse models (Table). Studies based on these models have indicated that bone marrow MSCs are perivascular cells in vivo and function as osteogenic stem cells to support bone turnover or injury repair (Shi and Gronthos 2003; Kfoury and Scadden 2015; Mendez-Ferrer et al. 2015). Besides perivascular MSCs, a recent study indicates that Sox9+ or Col2+ chondrocytes within the growth plate, which are not associated with any vasculature, can also contribute to the MSC population and long bone turnover (Ono et al. 2014). This suggests a novel function for chondrocytes, which not only provide the cartilaginous template but also participate in bone formation directly. It remains unclear how much osteogenesis occurs through the chondrocyte pathway.
The neural crest is a population of cells that originates from the dorsal margins of the closing neural folds. These cells then migrate extensively under the induction of signals to various locations in the embryo (Le Douarin and Dupin 2012). Neural crest cells contribute to remarkably diverse tissue types, including the peripheral nervous system, enteric ganglions, cardiac tissue, and the craniofacial skeleton (Le Douarin and Dupin 2012). Based on their origin, neural crest cells can be divided into 4 types: cranial, cardiac, vagal, and trunk (Betancur et al. 2010). The cranial neural crest cells give rise to the majority of the bone and cartilage of the craniofacial region, as well as the nerve ganglia, smooth muscle, connective tissue, and pigment cells. The variety of tissue types to which neural crest cells contribute demonstrates the multipotentiality and self-renewal capacities of these cells, which are cardinal features of stem cells (Stemple and Anderson 1992). Multipotential cranial neural crest cell–derived stem cells have been identified not only from the embryonic tissues but also in adults, a discovery that opens the door for applications of cranial neural crest cell–derived stem cells for regenerative medicine (Zhao et al. 2006; Chung et al. 2009).
Dental MSCs
The tooth is composed of enamel, dentin, and soft dental pulp tissue within. The teeth are connected to the alveolar bone through the periodontal ligament (PDL). Human teeth and mouse molars are both brachydont dentitions (low crown, long root). They do not undergo natural turnover and cannot be replaced if lost. Therefore, tissue engineering approaches based on tooth-related stem cells have become the focus of many recent studies.
Dental pulp stem cells (DPSCs) were the first adult stem cells identified from dental tissues (Gronthos et al. 2000). Cells obtained from adult third molars were shown to be highly proliferative and able to undergo osteogenic and chondrogenic differentiation. They also possess adipogenic ability under appropriate conditions, although with a reduced potential. They are positive for classical MSC markers, including CD44, CD73, CD90, CD105, Stro1, and CD146, but are negative for CD34, CD45, and CD14 in vitro. When transplanted into host mice, DPSCs can differentiate into odontoblast-like cells and form dentin-like structure, whereas bone marrow mesenchymal stem cells form distinct bone lamella structure under the same condition (Gronthos et al. 2002).
Subsequently, stem cells were isolated from human deciduous tooth pulp (i.e., SHED [stem cells from human exfoliated deciduous teeth]; Miura et al. 2003) and became more proliferative than bone marrow mesenchymal stem cells or DPSCs. They can undergo trilineage differentiation in vitro and also differentiate into neural cells. They highly express MSC markers, including CD105, CD146, Stro-1, and CD29, but are negative for CD31 and CD34. When transplanted, they form dentin-like structure.
The apical papilla is a transient tissue located at the apex of the root of a developing tooth. It has been proposed to be the cellular source of root formation. Stem cells isolated from the apical papilla (SCAP) have typical MSC properties (Sonoyama et al. 2006; Sonoyama et al. 2008). They can give rise to odontoblasts, osteoblasts, and adipocytes under the proper conditions. They strongly express CD73, CD44, CD105, CD146, and CD166 in vitro. SCAP present a distinct gene expression profile from that of DPSCs. Some markers, such as CD24 and survivin, are strongly expressed in SCAP but not in DPSCs.
The dental follicle is a mesenchymal condensation surrounding the tooth germ during tooth development. Dental follicle stem cells were isolated from dental follicles of the developing third molar (Morsczeck et al. 2005). They can differentiate into osteoblasts, chondrocytes, adipocytes, and neural-like cells in vitro and form cementum and PDL tissue after transplantation into host mice. They are highly positive for CD105, CD44, and CD29 but negative for hematopoietic markers CD34 and CD117 (Vollkommer et al. 2015).
The PDL contains MSCs known as PDLSCs (Seo et al. 2004; Sonoyama et al. 2006). PDLSCs show high expression levels of STRO-1, CD44, CD90, CD105, and CD146. They can differentiate into osteoblasts, chondrocytes, adipocytes, neurons, and even hepatocytes and are therefore multipotential. When transplanted into immunocompromised mice, PDLSCs are able to reconstruct PDL tissues in vivo (Yokoi et al. 2007).
Most of the studies listed above analyzed and defined dental MSCs based on their in vitro properties (Fig. 1). In vitro culture is variable and cannot mimic the stem cell niche. Indeed, some MSC populations defined according to in vitro culture are experimental artifacts (da Silva Meirelles et al. 2008). The identity and regulating mechanisms of dental MSCs in vivo remain largely unknown.
Figure 1.
The mesenchymal stem cell populations residing in the tooth–alveolar bone complex. Several mesenchymal stem cell populations have been identified from teeth of different developmental stages and associated alveolar bone, including dental pulp stem cells (DPSC), dental follicle stem cells (DFSC), stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSC), stem cells from the apical papilla (SCAP), and bone marrow mesenchymal stem cells (BMMSC).
The mouse incisor provides an excellent model for dental MSC study. It grows continuously throughout the lifetime of the animal at a rate of ~365 µm/d, as shown with a tritiated thymidine autoradiography technique (Smith and Warshawsky 1975). Both epithelial and mesenchymal compartments of the incisor rapidly turn over all their cells within 1 mo (Smith and Warshawsky 1975). The continuous turnover of incisor odontoblasts is supported by MSCs in the tooth. As in the long bone, pericytes were first proposed to be the stem cells for the mouse incisor mesenchyme. NG2 labels pericytes specifically. NG2+ pericytes are located surrounding all the vasculatures in the incisor and are immediately adjacent to the endothelium. Through lineage tracing analysis, NG2+ pericytes were shown to differentiate into odontoblasts during incisor growth. Upon injury, these NG2+ cells also contribute to the formation of the reparative dentin (Feng et al. 2011).
Recent studies identified Gli1+ cells surrounding the neurovascular bundle as the stem cells for the incisor dental mesenchyme (Kaukua et al. 2014; Zhao et al. 2014). Gli1+ cells are located in the apical region surrounding arterioles but not veins or capillaries. They are normally quiescent and can be activated into proliferation upon injury. Lineage tracing analysis indicated that Gli1+ cells give rise to the entire dental mesenchyme in vivo, including the undifferentiated dental mesenchyme, preodontoblasts, and odontoblasts. The majority of Gli1+ cells are negative for NG2, CD44, CD146, and other MSC markers in vivo. Cultured incisor mesenchymal cells are typical MSCs according to the classical definition and are almost entirely derived from Gli1+ cells. Lineage tracing analysis also indicated that NG2+ cells are a subpopulation of Gli1+ cells and are derived from them (Fig. 1; Zhao et al. 2014).
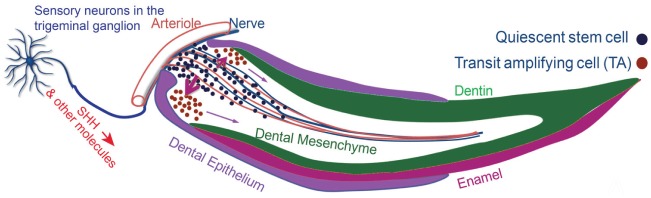
Identification of an in vivo marker for incisor MSCs provides the opportunity to study their regulating niche in vivo. Gli1 expression is an indicator of Hedgehog signaling activity (McMahon et al. 2003). Analysis based on transgenic models and immunohistochemical staining demonstrated that the sensory nerves within the incisor mesenchyme secrete SHH to regulate the incisor MSCs. Denervation abolished Gli1 activity and caused severe incisor phenotypes. Specific blockage of the Hedgehog signaling pathway also led to differentiation defects of the dentin, suggesting that Hedgehog signaling from the nerve regulates MSC differentiation. The nerves accompany the arterioles to form the neurovascular bundle. This relationship helps to define the location of the Gli+ MSCs near their neurovascular bundle niche (Fig. 2; Zhao et al. 2014).
Figure 2.
The neurovascular bundle niche and the in vivo origin of incisor mesenchymal stem cells. SHH is secreted by the sensory neurons in the trigeminal ganglion and transported through nerve axons into the incisor mesenchyme. SHH activates Gli1 expression in the stem cells surrounding the arterioles and regulates their differentiation. Gli1+ stem cells give rise to actively dividing transit amplifying cells and then differentiate into odontoblasts to support incisor mesenchyme turnover.
Strikingly, Gli1+ cells are absent from adult mouse molars. Mouse molars contain NG2+ cells surrounding all types of vasculature. These NG2+ cells make no contribution to the dentin under normal physiologic conditions but can contribute significantly to reparative dentin formation upon injury. Mouse molars are similar to human teeth in that neither organ undergoes self-renewal. The absence of Gli1+ cells might explain why mouse molars do not have natural turnover (Zhao et al. 2014).
Craniofacial Bone MSCs
Craniofacial bone development is a lengthy process initiated during early embryogenesis and completed during adulthood. Craniofacial bones originate from 2 sources: most are of cranial neural crest origin, but the parietal bones arise from the paraxial mesoderm. Unlike long bones that are connected by well-defined joints, craniofacial bones are connected by sutures. Sutures are the major sites of bone growth during craniofacial development. The expanding brain provides stimulus for the cranial vault to expand (Opperman 2000). The sutures respond by adding bone at the osteogenic front through intramembranous ossification. For sutures to function as growth sites, they need to remain in a patent and unossified state. Previously, it was proposed that the balance among cell proliferation, differentiation, and apoptosis within the sutures is critical for suture patency (Opperman 2000). Craniosynostosis, or the premature closure of ≥1 sutures, can occur when such a balance is disrupted. This condition can lead to craniofacial dysmorphology and other symptoms. Many signaling pathways, such as Fgf, Bmp, TGF-β, and ephrinB, are critical for the maintenance of suture patency (Slater et al. 2008; Grova et al. 2012; Levi et al. 2012).
Bone marrow MSCs have also been harvested from craniofacial bones, and they exhibit distinct properties from long bone MSCs. Most craniofacial bone marrow MSCs originate from the neural crest cells (Chung et al. 2009), and their gene expression profiles differ from that of long bone MSCs (Matsubara et al. 2005; Fig. 1). Some bone-related congenital diseases affect only the craniofacial bones, such as cherubism (Ueki et al. 2001), Treacher Collins syndrome (Kadakia et al. 2014), craniofacial fibrous dysplasia (Ricalde et al. 2012) and hyperparathyroid jaw tumor syndrome (Pepe et al. 2011), despite the fact that the genes involved with these diseases are expressed throughout the body. MSCs obtained from craniofacial and long bones also show distinct properties and behavior upon culture and transplantation (Chung et al. 2009). Compared with long bone MSCs, craniofacial bone marrow MSCs proliferate more rapidly, express higher levels of alkaline phosphatase, and form more compact bone and less bone marrow space upon transplantation or culture (Akintoye et al. 2006; Chung et al. 2009). Despite these differences, it was still generally assumed that stem cell regulatory mechanisms and repair mechanisms were similar in craniofacial and long bone MSCs. The repair of a critical-sized calvarial bone defect is routinely used as a standard assay for evaluating the regeneration potential of various types of MSCs.
The craniofacial periosteum has been proposed to be the source of progenitor cells responsible for injury repair of the adult skull, as it is in long bones (Lin et al. 2014). In long bones, an acute inflammation response is initiated in the periosteum 1 or 2 d after injury. Progenitor cells within the periosteum are then activated into proliferation, causing the periosteum to thicken. A bone callus is formed via endochondral ossification that heals the injury site. Pericytes surrounding the vasculature were proposed to be the progenitor cells within the periosteum (Pape et al. 2010). However, some studies have suggested that the craniofacial periosteum has distinct biological features compared to the long bone periosteum (Lin et al. 2014). When mandibular periosteum is introduced into a tibial bony defect, intramembranous ossification occurs instead of endochondral ossification. If tibial periosteum cells are transplanted into a mandibular defect, endochondral ossification occurs (Leucht et al. 2008).
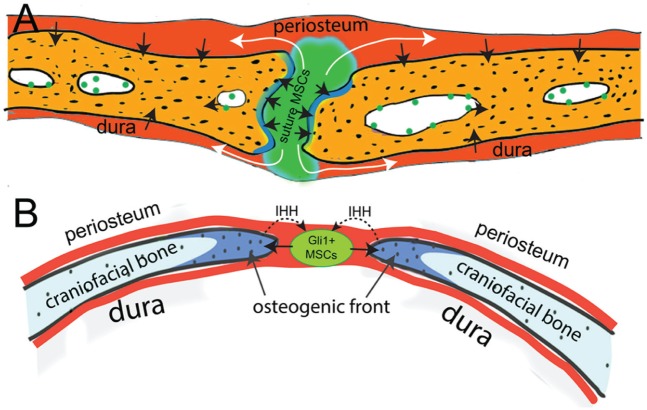
In addition, a recent study revealed that Gli1+ cells are MSCs residing in all craniofacial sutures (Fig. 3; Zhao et al. 2015). These Gli1+ cells support the turnover and injury repair of adult craniofacial bones. During postnatal turnover, suture Gli1+ cells can give rise to the periosteum and dura (Fig. 2). They are quiescent stem cells and can be activated upon injury. The specific ablation of Gli1+ cells leads to the closure of all craniofacial sutures and severe osteoporosis, indicating that they are an indispensable stem cell population. These Gli1+ cells are not related to the vasculature, and their distribution is gradually restricted to the suture mesenchyme during postnatal development. Although Gli1+ cells do not express typical MSC markers, such as CD44, CD73, or CD146, in vivo, they can undergo trilineage differentiation and highly express all the typical MSC markers after culture in vitro, indicating that they are MSCs. Gli1+ cells in the suture mesenchyme are not related to the vasculature and are regulated by IHH secreted from the osteogenic front, which contains committed osteogenic cells (Fig. 3). Blocking the Hedgehog signaling pathway leads to severe osteoporosis, but the sutures remain patent, suggesting that Hedgehog signaling mainly regulates the differentiation, but not maintenance, of these MSCs.
Figure 3.
The suture mesenchyme provides a niche for adult craniofacial bone mesenchymal stem cells (MSCs). (A) Gli1+ MSCs within the suture mesenchyme contribute to the periosteum, osteogenic front, and dura. (B) The osteogenic front secretes IHH to regulate the differentiation of Gli1+ MSCs in the suture mesenchyme.
The above study also identified Gli1+ cells within the craniofacial bone marrow space. However, their number and function are much less significant than suture Gli1+ MSCs based on cellular quantification and transplantation experiments (Zhao et al. 2015). Craniofacial bones contain much less bone marrow space than that of long bones in the adult. Therefore, suture MSCs might be the most important, if not the only, stem cell population for craniofacial bones. The craniofacial periosteum is derived from the suture and therefore is not the most primitive source of stem cells.
These findings provide a new perspective for understanding craniosynostosis. The closure of sutures after stem cell ablation suggests that craniosynostosis might be caused by the premature loss of the stem cell population within the suture mesenchyme. After Gli1-LacZ mice were crossed with Twist1+/– mice, which are a classical model for studying craniosynostosis (Behr et al. 2011), Gli1+ cell number within the sutures was significantly reduced. In addition, although synostosis initiates at around 3 wk after birth in Twist1+/– mutants, the number of Gli1+ cells was already significantly reduced prior to that, consistent with a causative link between the Gli1+ cell reduction and craniosynostosis (Zhao et al. 2015).
Conclusions
Teeth and bones are 2 organs that share many similar developmental and stem cell regulatory mechanisms. Craniofacial bones have typically also been considered similar to long bones with regard to their repair and stem cell regulation mechanisms. Dental and craniofacial stem cell studies have benefited greatly from earlier MSC and long bone studies. Nevertheless, recent craniofacial studies indicate that these organs are in fact quite different from each other. Craniofacial bones are distinct from long bones, not only in their developmental origins, but also in their stem cell sources and repair mechanisms. This information will have a significant impact on the craniofacial surgery clinic.
In addition, recent MSC studies of mouse incisors and craniofacial bones have challenged the traditional definition of MSCs, which is based on in vitro cellular properties and might not be appropriate for identifying MSCs in vivo. As incisor and suture studies have both shown, the majority of Gli1+ MSCs in these organs do not express typical MSC markers, such as CD146 and Sca1. They are unipotential odontogenic or osteogenic stem cells in vivo, even though they can be multipotential in vitro. They are not always perivascular, in contrast to previous proposals that perivascular cells were the in vivo counterparts of MSCs (Feng et al. 2010; Kfoury and Scadden 2015).
The stem cell research field is transitioning from in vitro study to a greater emphasis on in vivo study. Although only a few in vivo models have been established to study stem cells in dental and craniofacial tissues, these studies have already provided valuable information that could not be obtained through in vitro approaches. The lessons learned from these studies will help us to design better strategies for the future use of stem cells for tissue engineering purposes.
Author Contributions
H. Zhao, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Chai, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Samuel Bridget and Julie Mayo for critical reading of the manuscript.
Footnotes
H. Zhao acknowledges training grant support from the National Institute of Dental and Craniofacial Research, National Institutes of Health (R90 DE022528). This study was supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health (R37 DE012711, R01 DE022503, and U01 DE024421) to Y. Chai.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. 2006. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 38(6):758–768. [DOI] [PubMed] [Google Scholar]
- Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, Gazit Z. 2006. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 24(7):1728–1737. [DOI] [PubMed] [Google Scholar]
- Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. 1999. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 5(3):267–277. [DOI] [PubMed] [Google Scholar]
- Behr B, Longaker MT, Quarto N. 2011. Craniosynostosis of coronal suture in twist1 mice occurs through endochondral ossification recapitulating the physiological closure of posterior frontal suture. Front Physiol. 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. 2010. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 26:581–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. 1991. Mesenchymal stem cells. J Orthop Res. 9(5):641–650. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Schiro R, Orazi A, Soligo D, Colombo MP. 1993. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 81(7):1726–1738. [PubMed] [Google Scholar]
- Chung IH, Yamaza T, Zhao H, Choung PH, Shi S, Chai Y. 2009. Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells. 27(4):866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. 2008. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 26(9):2287–2299. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. 2011. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 108(16):6503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Mantesso A, Sharpe PT. 2010. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther. 10(10):1441–1451. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Gerasimov UV. 1987. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 20(3):263–272. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Latzinik NW, Grosheva AG, Gorskaya UF. 1982. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol. 10(2):217–227. [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. 1968. Heterotopic of bone marrow: analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 6(2):230–247. [PubMed] [Google Scholar]
- Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. 2004. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 22(4):617–624. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. 2002. Stem cell properties of human dental pulp stem cells. J Dent Res. 81(8):531–535. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova M, Lo DD, Montoro D, Hyun JS, Chung MT, Wan DC, Longaker MT. 2012. Models of cranial suture biology. J Craniofac Surg. 23(7 Suppl 1):1954–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. 2005. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 7(5):393–395. [DOI] [PubMed] [Google Scholar]
- Kadakia S, Helman SN, Badhey AK, Saman M, Ducic Y. 2014. Treacher Collins syndrome: the genetics of a craniofacial disease. Int J Pediatr Otorhinolaryngol. 78(6):893–898. [DOI] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, et al. 2014. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 513(7519):551–554. [DOI] [PubMed] [Google Scholar]
- Kfoury Y, Scadden DT. 2015. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 16(3):239–253. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. 2015. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 16(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Dupin E. 2012. The neural crest in vertebrate evolution. Curr Opin Genet Dev. 22(4):381–389. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. 2008. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 135(17):2845–2854. [DOI] [PubMed] [Google Scholar]
- Levi B, Wan DC, Wong VW, Nelson E, Hyun J, Longaker MT. 2012. Cranial suture biology: from pathways to patient care. J Craniofac Surg. 23(1):13–19. [DOI] [PubMed] [Google Scholar]
- Lin Z, Fateh A, Salem DM, Intini G. 2014. Periosteum: biology and applications in craniofacial bone regeneration. J Dent Res. 93(2):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi Y, Morikawa S, Harada S, Niibe K, Suzuki S, Renault-Mihara F, Houlihan DD, Akazawa C, Okano H, Matsuzaki Y. 2013. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Reports. 1(2):152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon HJ, Bee J, von der Mark K, Owen ME. 1987. Development of osteogenic tissue in diffusion chambers from early precursor cells in bone marrow of adult rats. Cell Tissue Res. 250(1):157–165. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, et al. 2005. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 20(3):399–409. [DOI] [PubMed] [Google Scholar]
- McCulloch EA, Till JE. 1960. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 13:115–125. [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. 2003. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 53:1–114. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Scadden DT, Sanchez-Aguilera A. 2015. Bone marrow stem cells: current and emerging concepts. Ann N Y Acad Sci. 1335:32–44. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. 2005. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 24(2):155–165. [DOI] [PubMed] [Google Scholar]
- Ono N, Ono W, Nagasawa T, Kronenberg HM. 2014. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 16(12):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman LA. 2000. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 219(4):472–485. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. 1988. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 136:42–60. [DOI] [PubMed] [Google Scholar]
- Pape HC, Marcucio R, Humphrey C, Colnot C, Knobe M, Harvey EJ. 2010. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 24(9):522–525. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. 2012. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 10(3):259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe J, Cipriani C, Pilotto R, De Lucia F, Castro C, Lenge L, Russo S, Guarnieri V, Scillitani A, Carnevale V, et al. 2011. Sporadic and hereditary primary hyperparathyroidism. J Endocrinol Invest. 34(7 Suppl):40–44. [PubMed] [Google Scholar]
- Ricalde P, Magliocca KR, Lee JS. 2012. Craniofacial fibrous dysplasia. Oral Maxillofac Surg Clin North Am. 24(3):427–441. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. 2007. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 131(2):324–336. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. 2004. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149–155. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. 2003. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 18(4):696–704. [DOI] [PubMed] [Google Scholar]
- Slater BJ, Lenton KA, Kwan MD, Gupta DM, Wan DC, Longaker MT. 2008. Cranial sutures: a brief review. Plast Reconstr Surg. 121(4):170e–178e. [DOI] [PubMed] [Google Scholar]
- Smith CE, Warshawsky H. 1975. Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. Anat Rec. 183(4):523–561. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, et al. 2006. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. 2008. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 34(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. 1992. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 71(6):973–985. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, Ninomiya C, doAmaral C, Peters H, Habal M, et al. 2001. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 28(2):125–126. [DOI] [PubMed] [Google Scholar]
- Vollkommer T, Gosau M, Felthaus O, Reichert TE, Morsczeck C, Gotz W. 2015. Genome-wide gene expression profiles of dental follicle stem cells. Acta Odontol Scand. 73(2):93–100. [DOI] [PubMed] [Google Scholar]
- Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, et al. 2015. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 160(1–2):269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T, Saito M, Kiyono T, Iseki S, Kosaka K, Nishida E, Tsubakimoto T, Harada H, Eto K, Noguchi T, et al. 2007. Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res. 327(2):301–311. [DOI] [PubMed] [Google Scholar]
- Zhao H, Bringas P, Jr, Chai Y. 2006. An in vitro model for characterizing the post-migratory cranial neural crest cells of the first branchial arch. Dev Dyn. 235(5):1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Ho TV, Grimes W, Urata M, Chai Y. 2015. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 17(4):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. 2014. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 14(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. 2014. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 15(2):154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]