Abstract
Background
Total knee arthroplasty (TKA) instrumentation and implant designs have been evolving, with one of the current innovations being patient-specific implants (PSIs).
Purpose
To evaluate whether there is a significant difference in surgical time, intraoperative blood loss, postoperative range of motion, and length of stay between PSI and conventional TKA.
Study Design
Cohort study; Level of evidence, 3.
Methods
A consecutive series of 621 TKA patients, 307 with PSIs and 314 with conventional implants, was reviewed. Differences in estimated blood loss, length of stay, range of motion, and surgical time/tourniquet time between the 2 cohorts were analyzed.
Results
Linear regression analysis demonstrated that PSI decreased estimated blood loss by 44.72 mL (P < .01), decreased length of stay by 0.39 days (P < .01), decreased postoperative range of motion by 3.90° (P < .01), and had a negligible difference on surgical and tourniquet time.
Conclusion
The use of PSI is associated with decreased estimated blood loss, decreased length of stay, decreased range of motion, and no discernible difference in surgical or tourniquet time, all of which are unlikely to be clinically significant.
Keywords: total knee arthroplasty, patient-specific implants, estimated blood loss, length of stay, range of motion, surgical time, tourniquet time
Approximately 700,000 knee replacement procedures are performed annually in the United States. The demand for primary total knee arthroplasties (TKAs) is projected to grow by 673% to 3.48 million procedures by 2030.13 Between 1991 and 2010, annual primary TKA volume increased 161.5% from 93,230 to 243,802 procedures. Per capita, utilization increased 99.2%, from 31.2 procedures per 10,000 Medicare enrollees in 1991 to 62.1 procedures per 10,000 Medicare enrollees in 2010.8 In light of these projections and trends, there have been attempts to improve the efficiency of these procedures, improve implant technologies, and decrease associated costs.
Total knee arthroplasty has been evolving since John Insall and colleagues12 implanted the first total condylar prosthesis in 1974. Now, 40 years later, we are investigating the role of patient-specific implants (PSIs) in TKA. PSIs use magnetic resonance imaging (MRI) or computed tomography (CT) scans of the knee to create custom alignment guides for the femur and tibia. The data obtained from the scans allow for the dimensions of the knee to be measured precisely to better select component size. The surgeon can then map the procedure in 3-dimensional software prior to surgery to make adjustments for alignment of components, thickness of resections, translation of components, reference points for components, and sizing and implant options. After the surgeon creates the preoperative plan, custom-made jigs are manufactured that reflect the surgeon’s preoperatively planned bone resections.
This study investigated the use of ConforMIS implants (ConforMIS Inc). ConforMIS offers a novel system that differs from that of other PSI manufacturers. In addition to creating custom cutting templates, ConforMIS manufactures a patient-specific total knee implant. Individualized femoral and tibial implants, as well as polyethylene tibial inserts, are constructed using the 3-dimensional model of the patient’s knee and are delivered along with the PSI.
Literature on both PSI and customized implants is growing, and there is currently no gold standard indication for their use when compared with conventional instrumentation and implants. There has been no consensus on the benefits and risks of PSI in TKA with regard to surgical time,1–5,7,9,10,13–17,22–24 blood loss,2,4,5,7,15,16,22,23 changes in range of motion (ROM), or length of hospital stay.4,14,16 Our study goal was to evaluate whether there is a significant difference in surgical time, intraoperative blood loss, postoperative ROM, and length of stay between the patient-specific and conventional TKA instrumentation and implants.
Methods
Study Design
The study was approved by our institutional review board. A consecutive series of cases were selected from our electronic medical records between January 2008 and June 2013. The Current Procedural Terminology (CPT) code used was 27447. The patients’ clinical notes and operative reports were then reviewed. All surgeries were performed by 2 senior surgeons with experience utilizing both implants compared in this study. In addition, all patients underwent surgery at the same 2 institutions.
Patient-Specific Implant Versus Conventional Group
The patients’ charts were reviewed and separated into 4 groups: (1) surgeon A patients who received a PSI, (2) surgeon B patients who received a PSI, (3) surgeon A patients who received a conventional implant, and (4) surgeon B patients who received a conventional implant. The procedures with conventional implants were performed between January 2008 and December 2010. The procedures involving the PSIs were performed between January 2011 and June 2013. There were no significant changes in protocol for the TKA procedure during this time period. We chose to select patients this way to collect a consecutive cohort of patients from each group and to minimize selection bias between the 2 implants.
Surgical Procedure and Protocol
All cases utilized a medial parapatellar incision and a multimodal pain management regimen. All patients were mobilized as soon as possible with no restrictions. Conventional implants (January 2008–December 2010) included the following models: Genesis 2 (Smith & Nephew), Sigma (DePuy), and PFC Sigma (DePuy). The PSI groups (January 2011–June 2013) received the ConforMIS iTotal G2 implant.
Measures of Outcomes
Our study was set to analyze specific outcomes measured from the patients’ operative notes and charts: surgical time, intraoperative blood loss, change in postoperative ROM, and length of stay. ROM, reported as a range (eg, 0°-120°), was measured both preoperatively and 1 year postoperatively using a goniometer for both the PSI and conventional implant cohorts. We then analyzed ROM postoperatively compared with preoperatively based on a scale of total change in ROM degrees. For example, 0° to 120° was converted to 120° of motion. We then subtracted the preoperative from the postoperative values to calculate ROM as an absolute variable that we could compare and analyze. A positive ROM value indicated an increase in ROM postoperatively, while a negative value indicated a decrease of ROM postoperatively compared with preoperatively. Both surgeons A and B reported ROM values. Length of stay was measured by the number of nights spent in the hospital after the TKA procedure and was gathered from the patient’s discharge summary. Both surgeons A and B reported length of stay. Intraoperative blood loss or estimated blood loss was measured by the surgeon based on amount of blood accumulated in the suction canister and surgical sponges and gathered from the operative notes. Surgeon A reported blood loss, however surgeon B did not report blood loss for his cohort of patients. Surgical time was reported in minutes as the time from skin incision to wound closure. Tourniquet time was measured as the length of time in minutes that the surgeon used the tourniquet during the surgery. Surgeon A reported surgical time while surgeon B did not report surgical time, so we opted to evaluate his timing with the use of tourniquet time. Surgeon B used a standard protocol in which the tourniquet was inflated just before skin incision and released after implant cementation. We realize that surgical time and tourniquet time are not comparable measures of operative time, so we did not analyze these groups against each other.
Statistical Analysis
The analysis of the data was performed using Rstudio (v 0.98.507; Rstudio Inc). We performed linear regression analyses to analyze intraoperative blood loss, ROM, surgical and tourniquet time, and length of stay. The factors we used in the linear regression were the surgeon performing the procedure, the type of implant used in the procedure, age, sex, body mass index, and left or right side of implant. This allowed us to control for these variables when analyzing the data assessing the differences between the types of implants. We then analyzed the significance based on each factor of the linear regression, with a significance of P < .01.
Results
Patient Demographics
There were a total of 621 patients included in the retrospective analysis, with 307 patients in the patient-specific instrumentation group and 314 included in the conventional group (Table 1). The mean age was 63.19 years (range, 20-89 years). The mean body mass index (BMI) was 31.48 kg/m2 (range, 17.8-57.6 kg/m2). There were 232 males and 389 females. There were 307 left-sided implants, 310 right-sided implants, and 4 bilateral implants. Surgeon A performed 407 procedures, and surgeon B performed 214 procedures.
TABLE 1.
Patient Demographicsa
| PSI | Conventional Implant | Total | P Value | |
|---|---|---|---|---|
| Mean age, y | 61.39 | 64.95 | 63.19 | 1.01E-05 |
| Sex, n | .2587 | |||
| Male | 122 | 110 | 232 | |
| Female | 185 | 204 | 389 | |
| Mean BMI, kg/m2 | 30.85 | 32.11 | 31.48 | .01929 |
| Side of implant, n | .1357 | |||
| Left | 141 | 166 | 307 | |
| Right | 162 | 148 | 310 | |
| Bilateral | 4 | 0 | 4 | |
| Patients per surgeon, n | ||||
| A | 208 | 199 | 407 | |
| B | 99 | 115 | 214 |
aBMI, body mass index; PSI, patient-specific implant.
Patient-Specific Implant Versus Conventional Group
There was no statistical difference between the groups with respect to BMI, sex, or laterality (P = .02, .26, and .14, respectively). There was a statistically significant difference between groups with respect to age (P < .01). For PSIs, surgeon A performed 208 procedures and surgeon B performed 99 procedures. For conventional implants, surgeon A performed 199 procedures and surgeon B performed 115 procedures (Table 1).
Estimated Blood Loss
Estimated blood loss was recorded for 341 of the 621 original patients. Surgeon A had a total of 341 patients with 142 PSI and 199 conventional implants. Surgeon B did not record estimated blood loss, so his patients were excluded from this analysis. Overall, the mean estimated blood loss was 290.6 mL (range, 50-500 mL). The mean estimated blood loss for PSIs was 261.97 mL (range, 50-500 mL); for the conventional implant, it was 311.31 mL (range, 100-500 mL) (Figure 1). According to this linear model, there was a statistically significant difference between implant types with regard to estimated blood loss during the TKA procedure, with a decrease in blood loss of 44.72 mL for PSI compared with the conventional procedure (P < .01).
Figure 1.
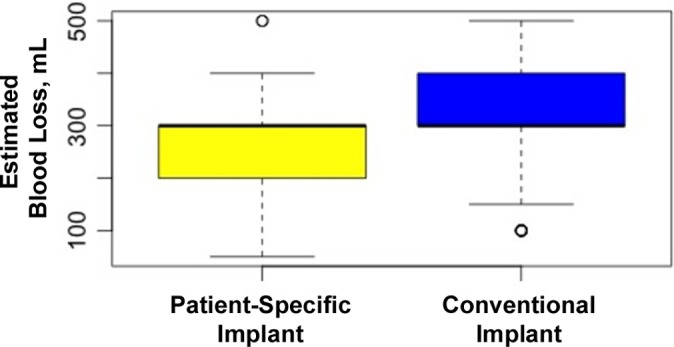
Mean estimated blood loss for each implant group. Error bars indicate SD; circles indicate outliers.
Surgical Time and Tourniquet Time
Surgeon A and surgeon B did not have available comparable measures for length of surgery. Surgeon A used surgical time, while surgeon B used tourniquet time. Surgeon A recorded surgical time for 302 of the 407 original patients. Surgeon A had a total of 302 patients with 148 PSIs and 154 conventional implants. For surgeon A, mean surgical time was 99.53 minutes (range, 57-192 minutes); mean surgical time for PSIs was 99.92 minutes (range, 57-185 minutes), and mean surgical time for conventional implants was 99.16 minutes (range, 72-192 minutes) (Figure 2). Surgeon B recorded tourniquet time for 207 of the 219 original patients. Surgeon B had a total of 207 patients, with 96 PSIs and 111 conventional implants. For surgeon B, mean tourniquet time overall was 72.43 minutes (range, 11-117 minutes); mean tourniquet time for PSIs was 72.47 minutes (range, 50-111 minutes), and mean tourniquet time for conventional implants was 72.41 minutes (range, 11-117 minutes) (Figure 3). No statistical significance was found with regard to the type of implant used during the procedure and surgical time or tourniquet time (P < .88 and < .16, respectively).
Figure 2.
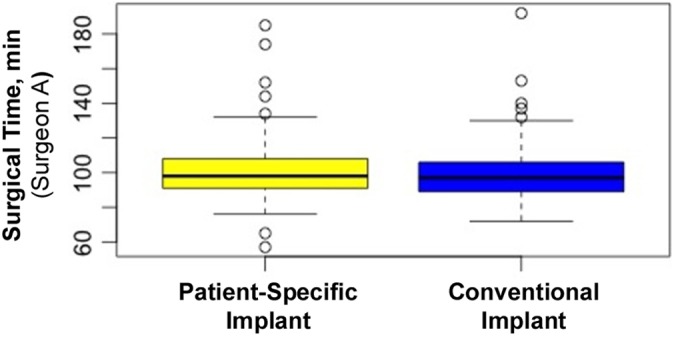
Mean surgical time for surgeon A. Error bars indicate SD; circles indicate outliers.
Figure 3.
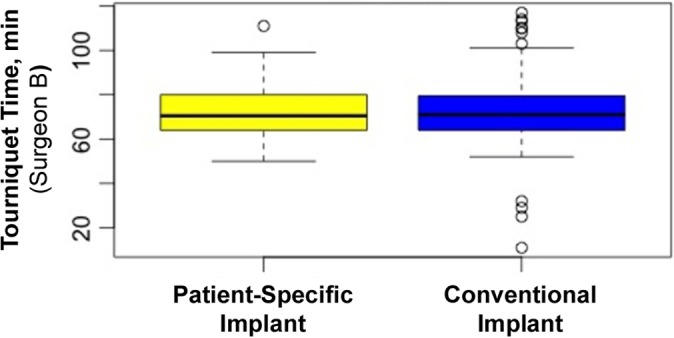
Mean tourniquet time for surgeon B. Error bars indicate SD; circles indicate outliers.
Length of Stay
Length of hospital stay was recorded for 520 of the 621 original patients. Surgeon A had a total of 385 patients with 186 PSIs and 199 conventional implants; surgeon B had a total of 135 patients with 20 PSIs and 115 conventional implants. The PSI group had an average length of stay of 2.44 days (range, 1-12 days), while the conventional group had an average length of stay of 3.18 days (range, 1-10 days) (Figure 4). According to this linear model, there was a statistically significant decrease in hospital stay between implant types, with a 0.39-day decrease after PSIs compared with conventional implants (P < .01).
Figure 4.
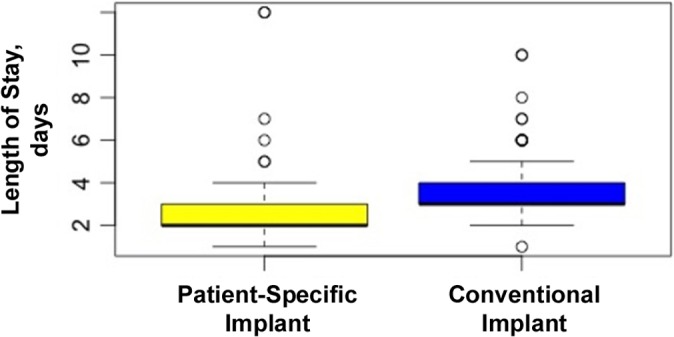
Mean length of stay for each implant group. Error bars indicate SD; circles indicate outliers.
Range of Motion
ROM was recorded for 466 of the 621 original patients. Surgeon A had a total of 275 patients, and surgeon B had a total of 191 patients. We found a statistically significant difference between implant groups when analyzing the difference in ROM measured postoperatively versus preoperatively. The overall ROM difference was pre- to postoperative decrease of 0.36° (range, –83° to 90°). For PSI, the ROM difference was a decrease of 3.44° (range, –83° to 55°), and for conventional TKA it was an increase of 1.54° (range, –80° to 90°). According to this linear model, there was a statistically significant decrease in pre- and postoperative ROM of 3.90° for PSIs compared with conventional implants (P < .01) (Figure 5), thus showing an increase postoperative ROM for the conventional TKA cohort compared to the PSI cohort.
Figure 5.
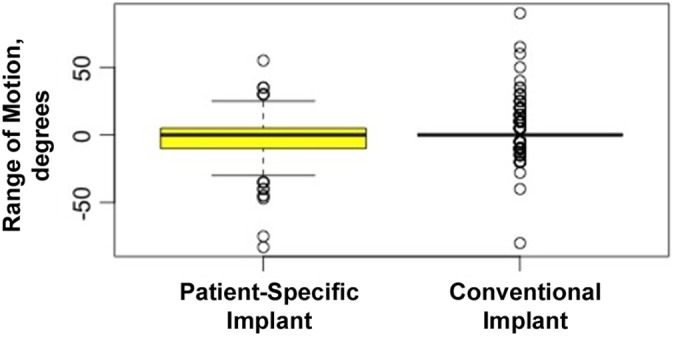
Mean range of motion for each implant group. Error bars indicate SD; circles indicate outliers.
Discussion
This is the second-largest cohort study to date to investigate perioperative and functional outcomes comparing the use of patient-specific implants to conventional implants in TKA. Ng et al16 reviewed 569 TKAs performed with patient-specific positioning guides and 155 with conventional instrumentation. They focused on coronal alignment and did not investigate surgical time, blood loss, length of stay, or changes in postoperative ROM. DeHaan et al9 performed a retrospective study reviewing 356 TKAs, with 306 being PSIs and 50 being conventional implants. They found that the PSI group averaged 20.4 minutes less surgical time (P < .01), with no significant changes in blood loss or length of hospital stay. Similarly, Noble et al15 and Stronach et al23 reported no difference in blood loss between conventional TKA and PSI procedures.
In contrast, our analysis did reveal statistically significant differences in several of these parameters. Our results build on data from Boonen et al4 that demonstrated a 100-mL (P < .01) decrease in blood loss and a 5-minute (P < .01) reduction in operative time for PSIs compared with off-the-shelf implants. Our data, derived from a larger cohort (621 individuals vs 180 individuals analyzed by Boonen et al4), demonstrated statistically significant differences in estimated blood loss, length of stay, and postoperative ROM. Notably, we reported a decrease in estimated blood loss of 44.72 mL (P < .01) for PSI. Boonen et al4 reported no difference in length of stay between the 2 groups. However, our data showed a decrease in length of stay of 0.39 days (P < .01) for patients receiving PSIs compared with conventional implants.
Hoeffel11 demonstrated that PSIs have been used successfully in obese patients and stated that significant preoperative planning leads to a reduced dependence on intraoperative bony landmark palpation, identification, and sizing accuracy. Previous studies have reported decreased intraoperative time with increased preoperative planning, decreased tourniquet time, and decreased length of stay.14 Others have reported that PSIs neither shorten surgical time nor improve alignment but did decrease the amount of trays used during surgery.6,10,19 Bali et al2 reported that in bilateral knee replacement wherein patient-specific cutting guides were used on 1 side and either computer-navigated or conventional guides were used on the other, there was a decreased mean skin-to-skin time and mean blood loss when using patient-specific guides. Sigman and Proverb21 discussed that PSIs improved outcomes in patients identified as outliers in anatomic structure. Collins7 reported on the perceived advantages and disadvantages of PSIs in his practice. The perceived advantages were decreased operative time, decreased blood loss, improved alignment, potential long-term cost benefits, and increased referrals and personal stimulus. The perceived disadvantages were increased cost of preoperative MRI/CT imaging, increased radiation exposure if CT imaging was used, increased cost of resection guides, and a tendency for the surgeon to rely too heavily on the engineer’s preoperative assessments rather than on the surgeon’s intraoperative assessments. The use of PSIs may give residents and fellows less experience in determining proper bone cuts during training.7
To determine the cost effectiveness of PSI, one must take into account the cost of the instrument manufacturing, the aforementioned operating room time, MRI/CT costs, and the potential for added revenue with reduction of operative time. Barrack et al3 noted that the US$322 savings with reduction in operative time and instrumentation set up with PSI was masked by the additional cost of MRI (or CT) along with cost of the PSI itself, which led to an overall loss of US$1178 per procedure compared with conventional implants.
Although prior studies have compared postoperative ROM between conventional TKA and PSI groups, we are not aware of any other studies analyzing differences in range of motion between conventional and customized implant groups. Yaffe et al25 demonstrated a 1.9° (P = .86) greater change in ROM at 6 months postoperative for manual instrumentation groups versus PSI groups. Our data indicate that PSIs are associated with a decrease of 3.9° (P < .01) in ROM after surgery compared with conventional implants. Ideally, there would have been a positive change in ROM after surgery; however, the PSI group lost an average of 3.44° in ROM postsurgery. We analyzed ROM data from various time points in the postoperative period, and these time points were after the early rehabilitation period and the same among each surgeon’s cohorts. We believe this outcome is most likely without any clinical significance and more likely due to nonunified ROM measurement protocol, both pre- and postsurgery.
Our study had a few limitations. First of all, the retrospective nature of our study limited our ability to uniform the measurement criteria of the different evaluated variables, thus leading to possible bias. Our study used data for the 2 cohorts from different time periods. The conventional implants were performed between January 2008 and December 2010, while the PSIs were performed between January 2011 and June 2013. This could allow for possible bias due to factors such as improved pain management and improved protocols. Our study focused on interpreting the differences in ROM as a measure of difference between the PSI and conventional implants. Because of the retrospective nature of the study, there was no standardized approach for documenting both pre- and postsurgical ROM measurements. By not following a specific protocol for collecting ROM values, there was possibly a variability in measurements based on how ROM was measured and by whom. For future studies, it would be more meaningful to address these issues in a prospective study to lessen the amount of variation. In the future, it would be important to address factors such as quality of life and patient-reported outcome measures. Ideally, a follow-up study should address these measurements in a prospective, randomized controlled fashion.
Our data demonstrated statistically significant differences in estimated blood loss and length of stay; however, it seems that these differences may not represent any clinically significant differences. This is best explained by the negligible change in estimated blood loss (44.72 mL) and length of stay (0.39 days) between cohorts. Nonetheless, whether these intraoperative and postoperative benefits of individualized implants are truly associated with long-term favorable outcomes for patients remains to be evaluated. That postoperative increase in ROM was less in the patient-specific group compared with the conventional group suggests that further analysis of other parameters such as postoperative knee score, pain score, and alignment will be useful in determining any long-term advantage of customized instruments and implants. Of note, conventional instrumentation may be associated with increased operative times and intraoperative blood loss due to its reliance on manual intramedullary alignment guides.18,20 Furthermore, patient-specific implants provide greater bone coverage, thus eliminating exposed bone, and may contribute to decreased postoperative blood loss compared with conventional implants.
Conclusion
Our retrospective cohort study focused on measuring the differences between PSI and conventional implants in TKA procedures over a 6-year period. Our results demonstrated that PSI resulted in a statistically significant difference in decreasing estimated blood loss and length of stay, although we interpret these values as unlikely to have a significant clinical difference between the 2 cohorts. With regard to ROM, we believe that the difference observed is also unlikely to have clinical significance, and future studies need to address quality of life and patient-reported functional outcome measurements between the 2 cohorts.
Footnotes
The authors declared that they have no potential conflicts of interest in the authorship and publication of this contribution.
References
- 1. Abane L, Anract P, Boisgard S, Descamps S, Courpied JP, Hamadouche M. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: a multicentre randomised controlled trial. Bone Joint J. 2015;97-B:56–63. [DOI] [PubMed] [Google Scholar]
- 2. Bali K, Walker P, Bruce W. Custom-fit total knee arthroplasty: our initial experience in 32 knees. J Arthroplasty. 2012;27:1149–1154. [DOI] [PubMed] [Google Scholar]
- 3. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 suppl A):95–99. [DOI] [PubMed] [Google Scholar]
- 4. Boonen B, Schotanus MG, Kerens B, van der Weegen W, van Drumpt RA, Kort NP. Intra-operative results and radiological outcome of conventional and patient-specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21:2206–2212. [DOI] [PubMed] [Google Scholar]
- 5. Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J. 2013;95B:354–359. [DOI] [PubMed] [Google Scholar]
- 6. Chen JY, Yeo SJ, Yew AK, et al. The radiological outcomes of patient-specific instrumentation versus conventional total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:630–635. [DOI] [PubMed] [Google Scholar]
- 7. Collins M. The impact patient specific instrumentation has on my practice in the last 5 years. Am J Orthop. 2014;XLIII:3S. [PubMed] [Google Scholar]
- 8. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29:2065–2069. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton WG, Parks NL, Saxena A. Patient-specific instrumentation does not shorten surgical time: a prospective, randomised trial. J Arthroplasty. 2013;28(suppl):96–100. [DOI] [PubMed] [Google Scholar]
- 11. Hoeffel D. Patient specific instrumentation for the obese patient. Am J Orthop. 2014;XLIII:3S. [PubMed] [Google Scholar]
- 12. Insall JN. Historic development, classification, and characteristics of knee prostheses In: Scott WN, ed. Insall & Scott Surgery of the Knee. 5th ed New York, NY: Churchill Livingstone; 2012:952–987. [Google Scholar]
- 13. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 14. Lachiewicz P, Henderson R. Patient-specific instruments for total knee arthroplasty. J Am Acad Orthop Surg. 2013;21:513–518. [DOI] [PubMed] [Google Scholar]
- 15. Noble JW, Jr, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27:153–155. [DOI] [PubMed] [Google Scholar]
- 16. Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV. Improved accuracy of alignment with patient-specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res. 2012;470:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pietsch M, Djahani O, Zweiger C, et al. Custom-fit minimally invasive total knee arthroplasty: effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc. 2013;21:2234–2240. [DOI] [PubMed] [Google Scholar]
- 19. Roh YW, Kim TW, Lee S, Seong SC, Lee MC. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of 1 system. Clin Orthop Relat Res. 2013;471:3988–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schnurr C, Csécsei G, Eysel P, König D. The effect of computer navigation on blood loss and transfusion rate in TKA. Orthopedics. 2010;33:474. [DOI] [PubMed] [Google Scholar]
- 21. Sigman S, Proverb K. Patient specific instrumentation: total knee arthroplasty in sports medicine. Am J Orthop. 2014;XLIII:3S. [PubMed] [Google Scholar]
- 22. Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2009;33:1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stronach BM, Pelt CE, Erickson J, Peters CL. Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res. 2013;471:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watters TS, Mather RC, 3rd, Browne JA, Berend KR, Lombardi AV, Jr, Bolognesi MP. Analysis of procedure-related costs and proposed benefits of using patient-specific approach in total knee arthroplasty. J Surg Orthop Adv. 2011;20:112–116. [PubMed] [Google Scholar]
- 25. Yaffe M, Luo M, Goyal N, et al. Clinical, functional, and radiographic outcomes following total knee arthroplasty with patient-specific instrumentation, computer-assisted surgery, and manual instrumentation: a short-term follow-up study. Int J Comput Assist Radiol Surg. 2014;9:837–844. [DOI] [PubMed] [Google Scholar]


