Abstract
Background:
Patients with anterior cruciate ligament (ACL)–injured knees are at an increased risk of posttraumatic osteoarthritis (OA). OA changes secondary to ACL injuries have many variations, and when and where early cartilage degenerative change begins has not yet been established.
Purpose:
To characterize the location of cartilage degeneration after ACL injury associated with time since injury using T1rho (T1ρ) mapping.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
In this study, 49 knees with ACL injuries and 14 normal knees from uninjured volunteers were imaged with a 3.0-T magnetic resonance scanner. Three regions of interest (ROIs) were defined in the cartilage at the weightbearing area of the femoral condyles (anterior, middle, and posterior zones). Two ROIs were defined in the tibial plateau (anterior and posterior zones). The T1ρ values within the ROIs were measured. Patients were allocated into 3 groups based on time since injury: <12 weeks (group A; 28 patients), 12 weeks to 2 years (group B; 14 patients), and >2 years to 5 years (group C; 7 patients).
Results:
Mean T1ρ values were significantly greater in the anterior and middle ROIs of the medial femoral condyle in group C compared with those in other groups (P < .05). Patients with medial meniscus injury, for whom the time since injury was ≥12 weeks, exhibited significantly greater T1ρ values in the middle areas of the medial femoral condyle versus normal knees and ACL-injured knees without medial meniscus injury.
Conclusion:
The risk of cartilage degeneration in the area of the femoral condyle that contacts the tibia during small degrees of flexion increased when the time since injury was longer than 2 years. In addition, medial meniscus injury was associated with cartilage degeneration at the medial femoral condyle in the chronic phase.
Clinical Relevance:
Cartilage degeneration occurs more than 2 years after ACL injury and increases with medial meniscus injury. Early intervention may be desirable for meniscus injury.
Keywords: anterior cruciate ligament (ACL), meniscus, cartilage, osteoarthritis, magnetic resonance imaging (MRI)
Patients with anterior cruciate ligament (ACL)–injured knees are at an increased risk of posttraumatic osteoarthritis (OA).9,10 Over 40% of patients with ACL deficiency develop OA changes visible on radiographs 10 to 20 years after injury, primarily in the medial compartment.12,14,18 Progressive deterioration of the cartilage has been attributed to abnormal laxity and abnormal loading patterns associated with ACL injuries.2,12,13 OA changes relevant to ACL injuries have many variations, and when and where early cartilage degenerative change begins has not yet been established.
In recent years, T1rho (T1ρ) and T2 mapping have attracted attention as relatively new magnetic resonance imaging (MRI) methods that can be used to evaluate the quality of cartilage matrix. T1ρ values are known to correlate with cartilage proteoglycan content. Some studies have suggested that T1ρ is more sensitive than T2 for detecting early cartilage degeneration.7,17,20 T1ρ mapping methodology was used in some reports that investigated early cartilage degeneration of knees with ligament and meniscus injuries.1,11,15,22 However, in the previous studies reporting the T1ρ values of the cartilage in knees with ACL injury, the period of time from ACL injury until the MRI evaluation varied considerably. We hypothesized that cartilage degeneration becomes obvious as the time that elapses after injury increases; however, research questions exist about when and where the early degenerative changes occur.
The purpose of this study was to investigate cartilage degeneration using T1ρ mapping at various regions of interest (ROIs) in the cartilage of ACL-injured knees by comparing patient groups partitioned on the basis of time since injury. The relationship between changes in the T1ρ values and meniscal tears was also studied.
Methods
Subjects
A total of 63 subjects were recruited for this cross-sectional study and were assigned to either the ACL-injured group of patients or to the control group of subjects who did not have knee injuries and had no clinical symptoms of OA. Eligible patients with ACL injuries had Kellgren-Lawrence grading scales between 0 and 1 and were without other ligament injuries or surgeries. Patients evaluated at <12 weeks from knee injury were considered to be in the acute phase (group A), and patients evaluated ≥12 weeks after injury were defined as being in the chronic phase. The rationale for the phase division at 12 weeks was that it corresponded with the approximate time when acute injury–related symptoms tended to abate and when patients returned to their daily lives and/or sports activities. Also, considering the duration after injury varied widely among those in the chronic phase, a further division of this group into 2 subgroups was made as follows: ≥12 weeks to ≤2 years (group B) and >2 years to 5 years (group C) since injury. Group B consisted of patients who had instability symptoms, such as giving way, during their sports activities or daily lives after initial nonsurgical treatment. Group C consisted of patients who had been left untreated for a long time and had repeated injuries or frequent instability symptoms in daily life. Patients in group C had not been referred to a specialist until they visited us, resulting in the 2-year gap before treatment.
The absence of OA and any injuries to the cartilage, ligaments, or menisci in the control group was confirmed by MRI. The presence of meniscus injury in ACL-injured patients was determined surgically. The relationship between meniscus status and cartilage degeneration was also assessed based on the time since injury; it was similarly classified as belonging to the acute or chronic phase for consistency and to avoid further complicating classifications. A stress radiography examination was also performed for the knee in 30° of flexion with a 15-kg anterior stress applied by a Telos arthrometer to evaluate the degree of laxity. Ethical approval was obtained from the internal review board at our institution, and all subjects gave their informed consent before they were included in the study. Prior to ACL reconstruction surgery, all patients were evaluated using the MRI protocol that follows.
Magnetic Resonance Imaging Protocol
The MRI was performed on a 3-T system (Achieva 3.0T, Quasar Dual; Philips Healthcare) using an 8-channel phased-array knee coil. Sagittal and coronal fat-suppressed, turbo spin echo, T2-weighted images were obtained using the following parameters: repetition time/echo time (TR/TE), 4675 /71 ms; field of view (FOV), 140 × 140 mm; matrix, 400 × 400; slice thickness, 3 mm; slice gap, 0 mm; flip angle, 90°; bandwidth, 31.54 Hz/pixel; number of slices, 26; and total scan time, 3 min 33 s. Fat-suppressed, turbo spin echo, T2-weighted images were used to define the cartilage area and the clinical diagnosis.
Two-dimensional (2D) sagittal T1ρ mapping was calculated from T1ρ-prepared images using the fast-field echo technique. The imaging parameters were as follows: TR/TE, 4.7/2.4 ms; FOV, 140 × 140 mm; matrix, 320 × 320; slice thickness, 3 mm; slice gap, 0 mm; flip angle, 35°; bandwidth, 31.54 Hz/pixel; spin-lock pulses, 1/20/40/60/80 ms; spin-lock pulse frequency, 500 Hz; number of slices, 26; total scan time, 16 min 15 s. A low flip angle was used, but it did not affect T1ρ contrast because a 6000-ms shot interval was used between each slice acquired, which filled the k-space using low-high ordering. T1ρ mapping was produced with Philips Research Integrated Development Environment (PRIDE) software written in Interactive Data Language (IDL 6.3; ITT Inc) and was used in the quantitative assessment.
Imaging Assessment of the T1ρ Mapping
The T1ρ values were calculated using MIPAV (medical image processing, analysis, and visualization) software (Biomedical Imaging Research Services Section, Center for Information Technology, National Institutes of Health).
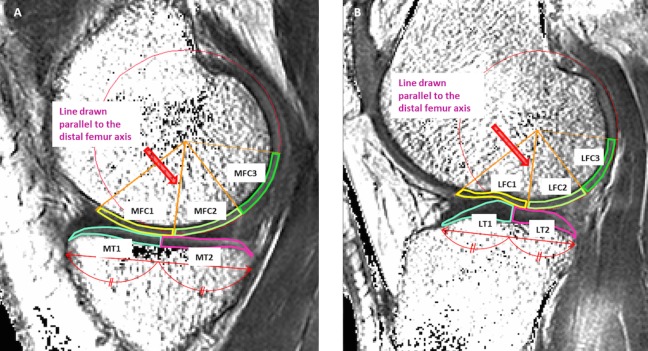
Four compartments of the knee were evaluated as follows: medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibia (MT), and lateral tibia (LT). Femoral ROIs in the cartilage were partitioned into 3 areas. From the center of the circle marking the approximate circumference of each posterior femoral condyle, a line was drawn parallel to the distal femur axis. Areas on either side of the line drawn parallel to the femoral axis were defined as follows: anterior area 45° from the line (MFC1, LFC1), middle area 45° posterior from the line (MFC2, LFC2), and the posterior area 45° to 90° posterior from the line (MFC3, LFC3). ROIs on the tibial cartilage were divided into anterior (MT1, LT1) and posterior areas (MT2, LT2) (Figure 1). A total of 3 MR sagittal images, consisting of a center slice of the medial or lateral compartment and both adjacent slices, were analyzed within each ROI. Segmentation was corrected manually to avoid synovial fluid or other surrounding tissue causing artifacts. All measurements were performed by 1 observer and were repeated in a blinded manner during the course of 2 sessions at least 1 month apart. Another observer independently made measurements of 5 randomly selected knees.
Figure 1.
Each region of interest (ROI) was defined as follows: ROIs on the femoral articular cartilage were divided into an anterior area 45° from the line (MFC1, LFC1), a middle area 45° posterior from the line (MFC2, LFC2), and a posterior area 45° to 90° posterior from the line (MFC3, LFC3). ROIs on the tibial articular cartilage were divided into an anterior (MT1, LT1) and posterior area (MT2, LT2). (A) Medial compartment. (B) Lateral compartment. LFC, lateral femoral condyle; LT, lateral tibia; MFC, medial femoral condyle; MT, medial tibia.
Statistical Analysis
All data are expressed as mean ± SD unless otherwise stated. Analysis of variance (ANOVA) and a post hoc comparison using the Tukey honestly significant difference (HSD) test were used to assess differences between group T1ρ values for each ROI on the femoral and tibial articular cartilage. Statistical tests were performed with JMP software version 9.0 (SAS Institute). P < .05 was considered significant for the analysis. A 95% CI was also reported. The intra- and interobserver reliabilities were evaluated using the intraclass correlation coefficient (ICC) by R software version 2.15.2 (R development core team).
Results
This cross-sectional study included 49 patients with ACL knee injuries (30 males and 19 females; mean age, 25.2 ± 8.6 years; age range, 15-45 years) and 14 patients in the control group without any knee injuries and no clinical symptoms of OA (14 males; mean age, 37.1 ± 6.0 years; age range, 28-46 years). Of these study participants, 28 ACL-injured patients, evaluated <12 weeks from knee injury, were assigned to the acute phase cohort (group A), and 21 patients, evaluated ≥12 weeks after injury, were allocated to the chronic phase group of patients. The chronic phase was further divided into 2 groups: group B (≥12 weeks to ≤2 years; 14 patients) and group C (>2 years to 5 years; 7 patients). Patient activity level was assessed with the Tegner activity score. The Lysholm knee score was used as a general knee evaluation. There was no significant difference in either the Tegner activity score or the Lysholm knee score among the patient groups. To estimate knee laxity in each group, we evaluated anterior displacement difference between injured and intact sides with anterior drawer stress radiography. There was no significant difference in laxity between each group. Subject baseline characteristics are shown in Table 1.
TABLE 1.
Baseline Patient Characteristicsa
| n | Sex, n (%) | Age, y, Mean ± SD (Range) | Time From Injury to MRI, d, Mean ± SD (Range) | Tegner Activity Score, Median (Range) | Lysholm Score at MRI, Mean ± SD | ADD at MRI, mm, Mean ± SD | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Before Injury | At MRI | ||||||
| ACL status | |||||||||
| Control | 14 | 14 (100.0) | 0 (0.0) | 37.1 ± 6.0 (28-46) | — | — | 5 (3-7) | 100 ± 0.0 | — |
| Acute (<12 wk) | |||||||||
| Group A | 28 | 16 (57.1) | 12 (42.9) | 27.1 ± 10.0 (16-45) | 44.1 ± 16.0 (0-76) | 5 (3-9) | 2 (2-2) | 63.8 ± 14.7 | 5.8 ± 3.3 |
| Chronic (≥12 wk) | |||||||||
| Group B (≤2 y) | 14 | 10 (71.4) | 4 (28.6) | 22.0 ± 5.4 (15-36) | 256.3 ± 171.2 (97-551) | 7.5 (6-9) | 6 (2-7) | 72.1 ± 10.0 | 5.9 ± 3.1 |
| Group C (>2 y) | 7 | 4 (57.1) | 3 (42.9) | 24.4 ± 6.7 (16-36) | 1328.7 ± 397.2 (744-1738) | 7 (6-8) | 6 (4-8) | 61.0 ± 12.0 | 6.5 ± 4.7 |
| Meniscusb | |||||||||
| Acute (<12 wk) | |||||||||
| MM(+) | 9 | 4 (44.4) | 5 (55.6) | 21.3 ± 6.6 (16-34) | 47.6 ± 15.7 (25-76) | 7 (6-9) | 2 (2-2) | 64.2 ± 14.2 | 5.6 ± 3.3 |
| MM(–) | 19 | 12 (63.2) | 7 (36.8) | 29.8 ± 10.3 (16-45) | 42.4 ± 16.3 (0-67) | 7 (3-7) | 2 (2-2) | 63.6 ± 15.4 | 5.9 ± 3.4 |
| LM(+) | 12 | 6 (50.0) | 6 (50.0) | 25.5 ± 10.8 (16-45) | 44.3 ± 20.0 (0-76) | 7 (6-7) | 2 (2-2) | 67.7 ± 11.1 | 6.5 ± 3.9 |
| LM(–) | 16 | 10 (62.5) | 6 (37.5) | 28.3 ± 9.5 (16-45) | 43.9 ± 12.9 (25-67) | 7 (3-9) | 2 (2-2) | 60.9 ± 16.7 | 5.3 ± 2.8 |
| Chronic (≥12 wk) | |||||||||
| MM(+) | 10 | 5 (50.0) | 5 (50.0) | 22.1 ± 6.5 (15-36) | 751.9 ± 487.1 (123-1561) | 7 (6-9) | 6 (4-8) | 65.8 ± 15.1 | 6.3 ± 4.6 |
| MM(–) | 11 | 9 (81.8) | 2 (18.2) | 23.5 ± 5.3 (16-36) | 488.2 ± 647.7 (97-1816) | 8 (6-9) | 4 (2-7) | 70.8 ± 7.3 | 5.9 ± 2.6 |
| LM(+) | 6 | 4 (66.7) | 2 (33.3) | 20.3 ± 3.4 (15-24) | 301.8 ± 172.4 (123-518) | 8 (7-9) | 6 (4-7) | 69.2 ± 14.1 | 5.3 ± 3.1 |
| LM(–) | 15 | 10 (66.7) | 5 (33.3) | 23.8 ± 6.4 (16-36) | 738.5 ± 639.7 (97-1816) | 7 (6-9) | 6 (2-8) | 68.1 ± 11.1 | 6.4 ± 3.8 |
aDashes indicate no data available. ACL, anterior cruciate ligament; ADD, anterior displacement difference; LM, lateral meniscus; MM, medial meniscus; MRI, magnetic resonance imaging.
b(+), with meniscal tears; (–), without meniscal tears.
The T1ρ values in each area of the cartilage on the femoral condyles and tibial plateau are summarized in Table 2. Comparisons among the 4 groups (control group and groups A, B, and C) showed T1ρ values were significantly higher in the MFC1 region of group C than in the MFC1 region in the other groups (95% CI: controls, 1.58-11.76 [P < .01]; group A, 0.62-9.91 [P = .02]; group B, 0.35-10.52 [P = .03]) (Figure 2A) and significantly higher in the MFC2 region of group C than the same region in the other groups (95% CI: control, 3.15-12.02 [P < .001]; group A, 0.58-8.68 [P = .02]; group B, 1.21-10.09 [P < .01]) (Figure 2B). There were no significant differences in other ROIs among the groups.
TABLE 2.
Magnetic Resonance Imaging T1ρ Mapping of Knee Jointsa
| Cartilage ROI T1ρ Values, msb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Medial Femoral Condyle | Medial Tibial | Lateral Femoral Condyle | Lateral Tibial | |||||||
| MFC1 | MFC2 | MFC3 | MT1 | MT2 | LFC1 | LFC2 | LFC3 | LT1 | LT2 | |
| ACL status | ||||||||||
| Control | 43.9 ± 3.3 | 44.8 ± 2.2 | 48.1 ± 2.8 | 43.4 ± 2.8 | 43.8 ± 2.2 | 42.1 ± 3.0 | 46.1 ± 2.4 | 46.9 ± 3.9 | 38.4 ± 3.2 | 41.6 ± 3.6 |
| Acute (<12 wk) | ||||||||||
| Group A | 45.3 ± 3.6 | 47.8 ± 3.3 | 49.7 ± 2.6 | 43.0 ± 2.7 | 44.3 ± 3.3 | 44.2 ± 3.1 | 49.0 ± 4.9 | 49.8 ± 3.8 | 38.7 ± 2.5 | 43.1 ± 2.8 |
| Chronic (≥12 wk) | ||||||||||
| Group B (≤2 y) | 45.2 ± 3.3 | 46.7 ± 2.6 | 47.5 ± 3.3 | 44.1 ± 5.2 | 43.4 ± 2.5 | 44.0 ± 3.2 | 47.0 ± 2.8 | 47.9 ± 5.0 | 38.2 ± 3.1 | 41.8 ± 3.0 |
| Group C (>2 y) | 50.6 ± 8.0d | 52.4 ± 7.4e | 49.8 ± 3.3 | 42.3 ± 2.0 | 44.2 ± 2.1 | 46.4 ± 6.6 | 49.1 ± 6.1 | 48.6 ± 4.5 | 37.0 ± 0.8 | 39.9 ± 2.1 |
| Meniscusc | ||||||||||
| Acute (<12 wk) | ||||||||||
| MM(+) | 44.9 ± 4.1 | 48.9 ± 3.4 | 49.6 ± 2.0 | 42.5 ± 1.7 | 44.7 ± 2.4 | — | — | — | — | — |
| MM(–) | 45.5 ± 3.4 | 47.2 ± 3.2 | 49.7 ± 2.9 | 43.3 ± 3.1 | 44.1 ± 3.7 | — | — | — | — | — |
| LM(+) | — | — | — | — | — | 44.3 ± 3.3 | 50.2 ± 6.4 | 49.4 ± 4.2 | 38.5 ± 2.8 | 44.0 ± 2.6 |
| LM(–) | — | — | — | — | — | 44.1 ± 3.0 | 48.1 ± 5.0 | 50.0 ± 3.7 | 38.9 ± 2.4 | 42.5 ± 2.9 |
| Chronic (≥12 wk) | ||||||||||
| MM(+) | 49.2 ± 7.4f | 51.5 ± 6.5g | 49.3 ± 3.2 | 42.6 ± 2.9 | 43.9 ± 2.4 | — | — | — | — | — |
| MM(–) | 45.0 ± 2.8 | 46.0 ± 1.7 | 47.4 ± 3.4 | 44.2 ± 5.6 | 43.5 ± 2.3 | — | — | — | — | — |
| LM(+) | — | — | — | — | — | 44.1 ± 4.1 | 46.9 ± 1.5 | 49.6 ± 5.8 | 37.4 ± 3.6 | 41.3 ± 2.9 |
| LM(–) | — | — | — | — | — | 45.0 ± 4.9 | 48.0 ± 4.8 | 47.6 ± 4.3 | 38.0 ± 2.2 | 41.1 ± 3.0 |
aData are reported as mean ± SD. Dashes indicate no data were available. LM, lateral meniscus; MM, medial meniscus; MRI, magnetic resonance imaging; ROI, region of interest.
bSee Figure 1 for definitions of ROIs.
c(+), with meniscal tears; (–), without meniscal tears.
dSignificant difference: P < .01 versus control, P = .02 versus group A, P = .03 versus group B.
eSignificant difference: P < .001 versus control, P = .02 versus group A, P < .01 versus group B.
fSignificant difference: P = .03 versus control.
gSignificant difference: P < .001 versus control, P = .03 versus acute MM(–), P < .01 versus chronic MM(–).
Figure 2.
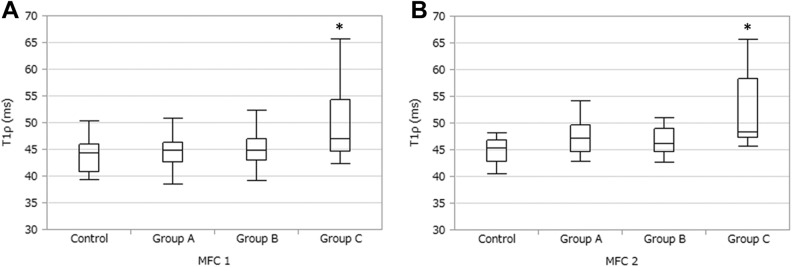
Comparisons among the 4 groups. (A) The MFC1 region. *Significant difference (P < .01) versus controls, P = .02 versus group A, and P = .03 versus group B. (B) The MFC2 region. *Significant difference (P < .001) versus controls, P = .02 versus group A, and P < .01 versus group B. See Figure 1 for definition of regions of interest.
With regard to the presence or absence of meniscus injury, T1ρ values were significantly higher in the MFC1 region of chronic phase (≥12 weeks) ACL-injured patients with medial meniscus injury versus the control group (95% CI, 0.29-10.26 [P = .03]) (Figure 3A), significantly higher in the MFC2 region of chronic phase ACL-injured patients with medial meniscus injury than the same region in other groups (95% CI: control, 2.45-10.85 [P < .001]; acute phase without medial meniscus injury, 0.29-8.22 [P = .03]; chronic phase without medial meniscus injury, 0.97-9.84 [P < .01]) (Figure 3B). There was no significant difference in T1ρ values of the lateral compartment with or without lateral meniscus injury.
Figure 3.
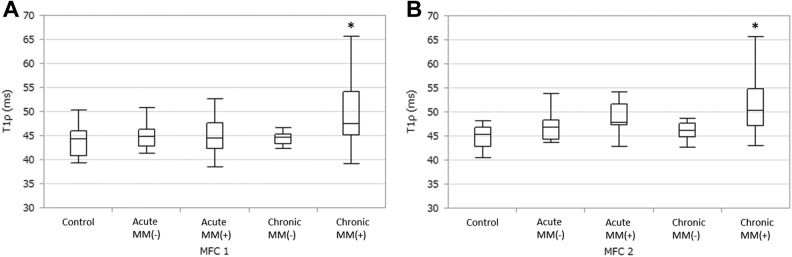
Comparisons with regard to the presence (+) or absence (–) of medial meniscus (MM) tears. (A) The MFC1 region. *Significant difference (P = .03) versus controls. (B) The MFC2 region. *Significant difference (P < .001) versus controls, P = .03 versus acute MM(–), and P < .01 versus chronic MM(–). See Figure 1 for definition of regions of interest.
Intraobserver reliability of the measurements was 0.95. Interobserver reliability of the measurements was 0.84.
Discussion
This study demonstrated that T1ρ values were significantly greater in the anterior and middle zone of the weightbearing area of the MFC (MFC1, MFC2) in the patients assessed more than 2 years postinjury (group C) compared with those in other groups (uninjured controls, patients in the acute phase, and those in the chronic phase who injured their knee ≤2 years before MRI assessment). Furthermore, group C had large standard deviations—especially in the MFC1 and MFC2 regions. The patients with higher T1ρ value were associated with medial meniscus injury.
Patients with a medial meniscus injury whose ACL tears were chronic exhibited significantly greater T1ρ values in the middle area of the medial femoral condyle compared with normal knees and ACL injured knees without medial meniscus injury.
In this study, T1ρ MRI techniques were employed to characterize early cartilage degeneration of ACL-injured knees before surgical reconstruction. Considering T1ρ is sensitive to articular cartilage proteoglycan content, and a loss of proteoglycans from cartilage matrices is one of the pathological changes observed with the onset of OA, increased T1ρ values can identify early cartilage degeneration that cannot be diagnosed by other conventional radiographic imaging modalities. OA changes after ACL injury can vary, and it is important to elucidate the cause. We focused on the time since ACL injury. An extensive perusal of the literature revealed that no studies have investigated early macromolecular changes in cartilage in ACL-injured knees in terms of the duration since injury.
There are some limitations in our study. First, because this is a cross-sectional study, it cannot be concluded that the degenerative change observed in this study was attributed to the time since injury. Furthermore, the effect of meniscal injury on cartilage damage in the chronic phase is unclear. It is virtually impossible to judge, without a longitudinal study, whether the presence of meniscus injury may be the cause or the result of cartilage degeneration. Hawkins et al4 reported that secondary meniscal tears occurred in 16% of the ACL injuries treated nonoperatively during the follow-up period for an average of 4 years. Second, grouping bias could affect the results. We partitioned the patients into 3 groups based on time since injury: <12 weeks (group A), 12 weeks to 2 years (group B), and >2 years to 5 years (group C). We considered that patients in group A represent those who had not returned to sports activities, group B represent those who had once returned to the sports activities after the initial nonsurgical treatment but come back to our office for surgery due to the residual instability symptoms, and group C represent those who had been left untreated for a long time due to lack of chance to visit a specialist until they were referred to us. However, the times for 12 weeks or 2 years were not based on scientific evidence. In addition, group C had a small sample size of only 7 subjects. It is not common for ACL deficiency to be left untreated for a long time because of an elevated awareness of ACL injury and an increased use of advanced medical imaging by a general physician.6 A low statistical power due to this small sample size might have influenced the lack of significance in other ROIs such as the lateral compartment. Third, there are various types of medial meniscus injuries, and each type may affect cartilage in a different way. Although it may have been better to perform the analyses based on the types of meniscal tears separately, too many meniscal tear categories precluded this approach. Last, data from uninjured contralateral knees in patients with ACL injuries were not available. Therefore, data from healthy subjects with uninjured knees were used for the control group. The mean age of subjects in the control group was higher than for the ACL-injured group of patients. However, the fact that the cartilage quality in the ACL-injured patients was worse than that of the older patients in the control group suggests that the cartilage damage in the ACL-injured patients represents a considerable significance.
Results for the acute phase patients (group A, injured for <12 weeks) showed that there was no significant difference in each ROI compared with the control. Bolbos et al3 performed a quantitative assessment of the knee cartilage in 15 healthy controls and 16 ACL-injured patients within 3 to 12 weeks of injury using a T1ρ mapping technique. They found that T1ρ values were significantly higher at the posterior lateral tibial plateau of injured patients compared with uninjured controls. Similar results were reported from the same institute for an evaluation of cartilage damage in 12 acute ACL-injured knees using the T1ρ mapping technique. This difference may be caused by the way in which the ROIs were partitioned. For the purposes of our research, the lateral tibial plateau was divided into 2 ROIs, whereas the institute partitioned this area into 3 ROIs. The reason why cartilage degenerative change was less in the acute phase may be that the patients had not yet experienced the repetitive instability of the knee during this short period of time. Because ACL injury-related damage only occurred once, the location of cartilage damage may be limited to the posterior lateral tibial plateau where bone bruising more commonly appears.
Results for patients in the late chronic phase showed the high T1ρ values were observed in MFC1 and MFC2 of group C (>2 to 5 years since injury) compared with other groups, and LFC1 of group C compared with uninjured controls. There was no significant difference among other groups at each ROI. These areas are where contact occurs with the tibia during extension together with a small degree of flexion at the knee. The cartilage damage at these areas in the late chronic phase may be attributed to the repetitive instability of the knee during sports activities or activities of daily living. We also speculate that degenerative changes anterior to the weightbearing area of the MFC may be because those areas come into constant contact with the tibial plateau during anterior instability of the tibia.
Since our results compared patients with and without meniscus injury, high T1ρ values were observed in the MFC1 and MFC2 regions in the chronic phase (≥12 weeks), with accompanying medial meniscus injury. There was no significant difference between other groups with or without lateral meniscus injury. The reason why the MFC was more susceptible to the meniscus injury compared with the LFC might be associated with the role of the medial meniscus as a secondary stabilizer to limit the anteroposterior translation of the tibia, especially in cases of ACL insufficiency.16 Previous studies also assessed the cartilage damage of ACL injury in the acute phase using T1ρ mapping MRI comparing the knees with or without meniscal injury. Li et al8 compared the knees of 12 ACL-injured patients with 10 uninjured controls using T1ρ mapping at baseline (mean time since injury, 38.5 days). Their results showed that no differences of T1ρ values were found between the uninjured control and injured knees, with or without medial meniscus injury. Su et al19 also reported no significant effect of meniscal injury on T1ρ values in acute ACL-injured knees at baseline or at 2 years postoperatively. In the present study, patients with a medial meniscus injury had significantly greater T1ρ values in the weightbearing area of the medial compartment of the knee compared with uninjured controls, although there was no significant difference in T1ρ values between the patients without medial meniscus injury and uninjured controls. Theologis et al21 also reported similar results of significantly greater T1ρ values at the medial compartments in ACL-reconstructed knees with meniscal injury compared with the contralateral knees 12 to 16 months after ACL reconstruction but no differences for the knees without meniscal injury. These findings coincide with our observations. Hirose et al5 assessed the cartilage in 23 ACL-injured patients using a T1ρ mapping technique, and compared the baseline (mean time since injury, 21.0 weeks) with 1-year results after ACL reconstructions. No difference was evident between the patients with a meniscal injury versus without a meniscal injury at both baseline and 1 year after ACL reconstruction. Our results showed there were significant differences in the results between patients in the chronic phase with a medial meniscus injury and uninjured controls; however, Hirose et al5 did not compare outcomes with uninjured controls. Furthermore, none of those studies have evaluated patients with chronic ACL insufficiency and meniscus injuries. Our results were influenced by the presence or absence of meniscal injury, which was dependent on the time since ACL injury. Ideally, patients should be evaluated with consideration given to the time since injury. Variations in the time since injury may lead to increased variability of results.
In our study, the risk of cartilage degeneration was shown to increase when the time since ACL injury exceeded 2 years. Cases involving medial meniscus injury are more concerning because T1ρ values vary widely under these circumstances and could include higher values indicative of progressive cartilage degeneration.
Conclusion
We evaluated cartilage degeneration in ACL-injured knees using T1ρ mapping within the context of time since injury. There was greater cartilage degeneration in ACL-injured patients in the group assessed 2 years after injury; this was evident in the middle region of the femoral condyle that contacted the tibia while the degree of flexion was small. Moreover, medial meniscus injury was associated with cartilage degeneration at the medial femoral condyle during the chronic phase.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was partly supported by the Grant of Japan Sports Medicine Foundation 2013. Ken Okazaki is a paid speaker for Zimmer, Johnson & Johnson, and Daiichi Sankyo Co Ltd.
References
- 1. Akella S, Regatte R, Gougoutas A, et al. Proteoglycan-induced changes in T-1 rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. [DOI] [PubMed] [Google Scholar]
- 2. Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH. The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young-adults. Clin Orthop Relat Res. 1990;(259):192–199. [PubMed] [Google Scholar]
- 3. Bolbos RI, Link TM, Ma CB, Majumdar S, Li X. T1ρ relaxation time of the meniscus and its relationship with T1ρ of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage. 2009;17:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawkins R, Misamore G, Merritt T. Follow-up of the acute nonoperated isolated anterior cruciate ligament tear. Am J Sports Med. 1986;14:205–210. [DOI] [PubMed] [Google Scholar]
- 5. Hirose J, Nishioka H, Okamoto N, et al. Articular cartilage lesions increase early cartilage degeneration in knees treated by anterior cruciate ligament reconstruction: T1ρ mapping evaluation and 1-year follow-up. Am J Sports Med. 2013;41:2353–2361. [DOI] [PubMed] [Google Scholar]
- 6. LaBella CR, Hennrikus W, Hewett TE; Council on Sports Medicine and Fitness, and Section on Orthopaedics. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. 2014;133:e1437–e1450. [DOI] [PubMed] [Google Scholar]
- 7. Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T-1p mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1ρ and T2—initial experience with 1-year follow-up. Radiology. 2011;258:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. [DOI] [PubMed] [Google Scholar]
- 10. Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. [DOI] [PubMed] [Google Scholar]
- 11. Matsubara H, Okazaki K, Takayama Y, et al. Detection of early cartilage deterioration associated with meniscal tear using T1ρ mapping magnetic resonance imaging. BMC Musculoskelet Disord. 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mcdaniel W, Dameron T. Untreated ruptures of the anterior cruciate ligament—a follow-up-study. J Bone Joint Surg Am. 1980;62:696–705. [PubMed] [Google Scholar]
- 13. Neyret P, Donell ST, Dejour H. Results of partial meniscectomy related to the state of the anterior cruciate ligament. Review at 20 to 35 years. J Bone Joint Surg Br. 1993;75:36–40. [DOI] [PubMed] [Google Scholar]
- 14. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65:154–162. [DOI] [PubMed] [Google Scholar]
- 15. Okazaki K, Takayama Y, Osaki K, et al. Subclinical cartilage degeneration in young athletes with posterior cruciate ligament injuries detected with T1ρ magnetic resonance imaging mapping [published online December 7, 2014]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3469-4. [DOI] [PubMed] [Google Scholar]
- 16. Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med. 2001;29:226–231. [DOI] [PubMed] [Google Scholar]
- 17. Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1ρ relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1ρ with T2. J Magn Reson Imaging. 2006;23:547–553. [DOI] [PubMed] [Google Scholar]
- 18. Sherman MF, Warren RF, Marshall JL, Savatsky GJ. A clinical and radiographical analysis of 127 anterior cruciate insufficient knees. Clin Orthop Relat Res. 1988;(227):229–237. [PubMed] [Google Scholar]
- 19. Su F, Hilton JF, Nardo L, et al. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takayama Y, Hatakenaka M, Tsushima H, et al. T1ρ is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: radiological-pathological correlation after total knee arthroplasty. Eur J Radiol. 2013;82:E192–E198. [DOI] [PubMed] [Google Scholar]
- 21. Theologis AA, Haughom B, Liang F, et al. Comparison of T1ρ relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsushima H, Okazaki K, Takayama Y, et al. Evaluation of cartilage degradation in arthritis using T1ρ magnetic resonance imaging mapping. Rheumatol Int. 2012;32:2867–2875. [DOI] [PubMed] [Google Scholar]