Abstract
Background
Complete rupture of the distal biceps tendon from its osseous attachment is most often treated with operative intervention. Knowledge of the overall tendon morphology as well as the orientation of the collagenous fibers throughout the musculotendinous junction are key to intraoperative decision making and surgical technique in both the acute and chronic setting. Unfortunately, there is little information available in the literature.
Purpose
To comprehensively describe the morphology of the distal biceps tendon.
Study Design
Descriptive laboratory study.
Methods
The distal biceps terminal musculature, musculotendinous junction, and tendon were digitized in 10 cadaveric specimens and data reconstructed using 3-dimensional modeling.
Results
The average length, width, and thickness of the external distal biceps tendon were found to be 63.0, 6.0, and 3.0 mm, respectively. A unique expansion of the tendon fibers within the distal muscle was characterized, creating a thick collagenous network along the central component between the long and short heads.
Conclusion
This study documents the morphologic parameters of the native distal biceps tendon. Reconstruction may be necessary, especially in chronic distal biceps tendon ruptures, if the remaining tendon morphology is significantly compromised compared with the native distal biceps tendon. Knowledge of normal anatomical distal biceps tendon parameters may also guide the selection of a substitute graft with similar morphological characteristics.
Clinical Relevance
A thorough description of distal biceps tendon morphology is important to guide intraoperative decision making between primary repair and reconstruction and to better select the most appropriate graft. The detailed description of the tendinous expansion into the muscle may provide insight into better graft-weaving and suture-grasping techniques to maximize proximal graft incorporation.
Keywords: distal biceps tendon, graft, morphology, 3D computer modeling
Distal biceps tendon (DBT) ruptures are most commonly seen in the dominant elbow of men in their fourth decade of life, with an increased incidence in smokers.25 The mechanism of injury usually involves a sudden eccentric load to a forcefully flexed elbow with the DBT often avulsing from its insertion on the radial tuberosity.8,10,11,18 Although DBT ruptures represent only 3% of injuries to the biceps muscle complex, they have been associated with pain in the antecubital fossa, deformity, and cramping.4 Nonoperative treatment is reported to result in a loss to forearm supination and flexion in terms of strength (40% and 30%, respectively) and endurance (79% and 30%, respectively).2,21 Operative repair of the ruptured DBT has been shown to improve pain and function, thus becoming the standard of care in healthy active individuals.20,21,24
Acute repairs are safer, technically easier, and fare better than chronic repairs.15 Although there are varied opinions as to the time frame that defines a “chronic” injury, the descriptive characteristics appear consistent across studies: loss of tunnel passage for the tendon with associated retraction and scarring of the tendon to the underlying brachialis.6 With further time delay and retraction, particularly when the bicipital aponeurosis ruptures concomitantly, the DBT undergoes atrophy and degeneration. However, when the bicipital aponeurosis remains intact, a primary repair in the chronic setting may be possible since retraction of the DBT is often mitigated and “sufficient” tendon length, caliber, and tissue quality may persist. If the remaining tendon is insufficient, a graft reconstruction of the DBT that mimics the normal length, caliber, and tissue quality should be considered.
A variety of grafts (autografts, allografts, and synthetics) have been reported in the literature to reconstruct the DBT. Reported graft sources include autologous fascia lata,3 synthetic ligament augmentation with fascia lata,14 bicipital aponeurosis,12 flexor carpi radialis,7,19 semitendinosus,13,29 and tendoachilles allograft.6,16,22,26,30 The decision to proceed with a graft and the choice of graft should be guided by the understanding of normal DBT morphology. In addition, knowledge of the orientation of the tendon fibers near the musculotendinous junction (MTJ) can help guide graft-weaving orientation and suture placement for improved proximal fixation. These data are currently lacking in the literature.
The purpose of the present study was to determine the key morphology of the DBT (length, width, and thickness) and describe the tendon expansion running within the deep muscle layers using a unique anatomical 3-dimensional (3D) modeling technique. This knowledge will assist with intraoperative decision making in both chronic and acute settings: when to use a graft, which graft best matches the native tendon characteristics of the DBT, as well as which suture orientation and tendon weaving techniques may help to augment the reconstruction.
Methods
Ethics approval was obtained from the University of Toronto Health Sciences Research Ethics Board, Toronto, Ontario, Canada. A total of 10 formalin-embalmed human cadaveric upper limbs (mean age, 78.3 ± 16.2 years; 6 women, 1 men) were used in this study. Sex and age were only available for 7 of the 10 specimens. There were 6 left upper limbs and 4 right (hand dominance of the donors was unknown). Exclusion criteria included prior surgical intervention and any evidence of musculoskeletal pathology.
Dissection
The skin and subcutaneous tissue were removed from the upper limb of each specimen. Next, the full length of the biceps brachii muscle complex was exposed by removing the superficial fascia. Distally, the brachioradialis and extensor carpi radialis longus and brevis were released from the lateral epicondyle and removed along with the bicipital aponeurosis. The brachialis, except for the most distal portion overlying the antecubital fossa, was left in situ to support the biceps brachii during the digitization process. To allow full forearm supination and elbow extension, the joint capsule was released as necessary. At the end of the dissection process, the DBT was visualized along its full length to its insertion on the radial tuberosity.
Each specimen was secured, in full extension and supination, to a wooden base with metal plates and screws (Figure 1). Three additional screws were placed in the base as reference markers at the level of the medial epicondyle, proximal third of the radius, and at the distal third of the humerus. The center of each screw was digitized in the same sequence for later reconstruction of the data points. This task was performed prior to digitizing each new layer of muscle or tendon to ensure there was no change in specimen positioning. In addition, the wooden base was secured to the table with the use of 2 metallic C-clamps to prevent micromotion.
Figure 1.
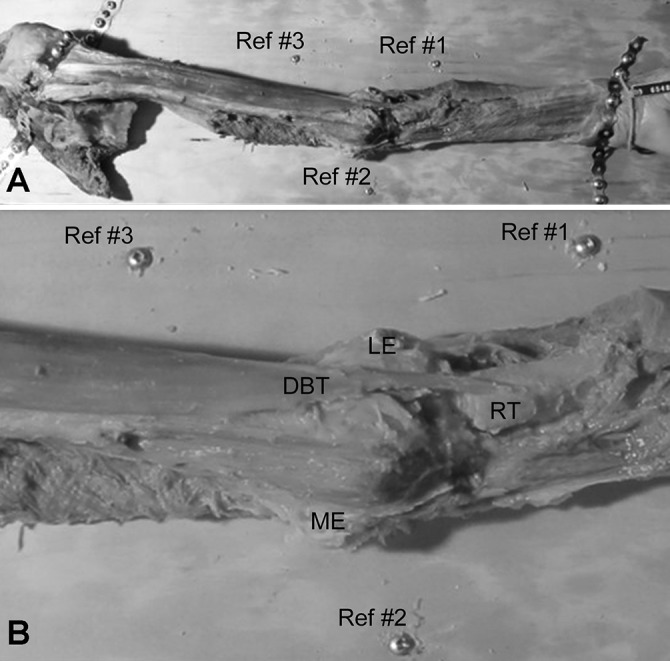
(A) Photograph illustrating the specimen setup for a left elbow. The limb was dissected and secured to a wooden board using metal plates and screws (scapula to the left, hand to the right). The elbow was positioned in full extension with the forearm maximally supinated. Three reference screws (Ref #1-#3) were positioned about the elbow. (B) An enlarged photograph from above the distal biceps brachii muscle and tendon. DBT, distal biceps tendon; LE, lateral epicondyle; ME, medial epicondyle; RT, radial tuberosity.
Data Collection
Ten specimens were digitized from the distal third of the muscle belly to the tendon insertion onto the radial tuberosity (see the Video Supplement). Data points were collected and recorded using the Microscribe 3DX Digitizer (0.3-mm accuracy; Immersion Corp). Individual muscle fiber bundles were identified, followed, and digitized from proximal to distal with data points collected every 2 to 4 mm. This process was repeated along the entire width of the muscle layer to gain an accurate representation of the anatomy. Each layer was then dissected and removed, repeating the same data collection on the following layer, continuing from superficial to deep. As the superficial muscle layers were removed, proximal extensions of the tendon were seen (deep aponeurosis); these layers were digitized in a similar fashion as the muscle fibers but distinctly labeled under a separate heading as internal tendon, defined as the tendinous tissue extending proximally from the MTJ deep to the muscle fiber bundles. Any portion of the internal tendon that was thin and lacked true substance was not digitized. The external tendon was defined as the visible tendon seen on gross anatomic inspection, from the MTJ extending distally to its insertion on the radial tuberosity. Both the internal and external tendon lengths and widths (mediolateral dimension) were recorded (Figure 2), as well as the thickness (anteroposterior dimension) of the external tendon.
Figure 2.

The internal and external tendons on a left elbow specimen midway through the digitization process. The radial tuberosity is on the right and the muscle belly (with evidence of layers dissected off) on the far left. ET, external tendon; IT, internal tendon.
Data collection was then incorporated using a 3D modeling technique to obtain a volumetric reconstruction of the distal muscle belly, MTJ, and the DBT as it attaches onto the radial tuberosity. This was done using the Autodesk Maya software platform (Autodesk Inc) with custom software plug-ins developed in our laboratory. Once all the muscle had been dissected off the internal tendon framework, the external tendon was pinned in situ to the underlying brachialis muscle and cut transversely beginning at the MTJ and proceeding distally in 5-mm intervals. The circumference of the tendon was then digitized after each cut with data points collected in <2-mm intervals to capture the variability in shape and orientation along its course and to capture a true representation of the overall width and thickness at each transection. Measurements for the width of the internal tendon were taken at the widest portion of the tendon in each specimen. Thickness measurements were not obtained for the internal tendon as the tendon was extremely thin. Because the tendon is quite flat at both the MTJ and insertion, the average width and thickness of the external tendon was averaged from the values collected from the middle third of the tendon for each specimen. All data were analyzed, reconstructed, and then modeled using Autodesk Maya 3D animation software.
Results
Total length of the tendon (both internal and external) was 126.7 ± 10.7 mm. Average internal tendon length was 63.8 ± 11.6 mm, while the average external tendon length was 63.0 ± 9.4 mm. The average width of the internal tendon was quite broad at 12.0 ± 4.2 mm, as it expanded across both the short and long muscle heads. The width and thickness averaged within the middle third of the external tendon and then averaged across specimens is also shown in Table 1. These values were 6.0 ± 1.0 and 3.0 ± 0.5 mm, respectively. The internal and external tendon parameters are summarized in Table 1.
TABLE 1.
Dimensions of the Internal and External Distal Biceps Tendon Using a 3-Dimensional Anatomic Digitizera
| Measurement, mm | |
|---|---|
| Internal tendon | |
| Length | 63.8 ± 11.6 |
| Width | 12.0 ± 4.2 |
| External tendon | |
| Length | 63.0 ± 9.4 |
| Width | 6.0 ± 1.0 |
| Thickness | 3.0 ± 0.5 |
aValues are reported as mean ± SD. Measurements of the width of the internal tendon were taken at the widest portion of the tendon in each specimen. Measurements of the width and thickness of the external tendon were taken from the middle third.
There was no consistent relationship between the internal and external tendon lengths, but across all specimens, the internal tendon length extended proximally more than 4.5 cm from the MTJ. The width of the internal tendon tended to be much wider than that of the external tendon.
The orientation of the external tendon was quite predictable across all specimens. A flattened and broader tendon appearance was seen at the MTJ and at the insertion, with the central third being more consistent and ovoid in shape. The tendon took a predictable 90° external rotation (supination) at the level of the anticubital fossa, causing the medial fibers of the short head to become anterior on its attachment to the radial tuberosity (Figure 3). Selected 3D representative data from a left-sided specimen dissection is shown in Figure 4. As the superficial muscle fibers are removed, the full length of the internal tendon could be appreciated.
Figure 3.
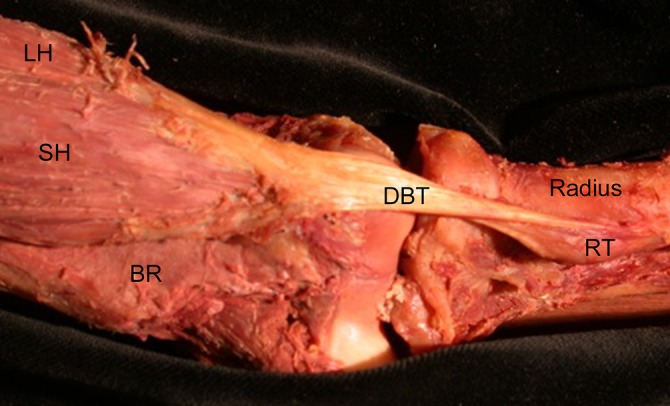
A representative left elbow dissection showing both the short and long muscle bellies and the 90° external rotation of the distal biceps tendon as it crosses the joint and inserts onto the radial tuberosity. The most medial border of the short head becomes the most distal insertion point on the radial tuberosity. BR, brachialis; DBT, distal biceps tendon; LH, long head; RT, radial tuberosity; SH, short head.
Figure 4.
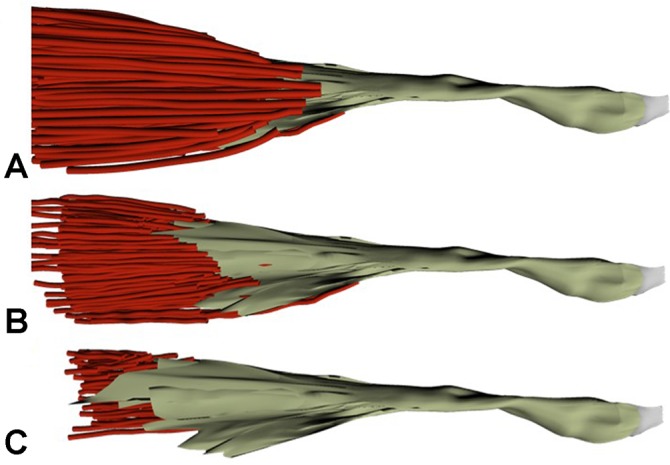
A 3-dimensional representation of the distal muscle tendon unit of a single left elbow specimen chosen at 3 distinct times during the digitization process. (A) Prior to any dissection of the muscle layers, (B) after about half of the muscle fibers have been dissected off the tendon, and (C) after the majority of the muscle fibers have been dissected off the tendon, revealing the full length of the internal tendon structure.
The width and thickness of the middle third of the external tendon was measured in <5-mm transected intervals and averaged across the specimen (Table 1). After each transection, the shape of the tendon was assessed, both grossly as well as the digitized representation. The tendon had an ovoid cross-sectional shape. There were distinct bifid tendons surrounded by a common sleeve in 4 of the 10 specimens.
A common complex orientation of the internal tendon was seen across all specimens. There was a thick collagenous band extending proximally from the MTJ, centrally located with the thickest portion vertically oriented in the sagittal plane (Figure 5). This band was seen more proximally as the fascial division between the long and short head; however, extending distally, there was more interdigitation across the muscle fibers of the short and long heads. The band was quite substantial, measuring several millimeters in thickness.
Figure 5.
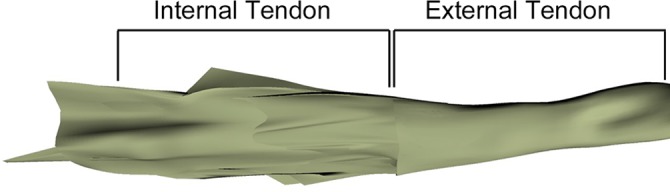
A 3-dimensional representation of the peaks and valleys of the internal tendon in a left elbow specimen. The large vertical projection can be well appreciated in this specimen, and suture grasping within this area would likely yield increased strength at time zero.
Discussion
Although there is much literature on the anatomy of the insertion of the DBT onto the radial tuberosity, there are very few studies addressing the morphology of the distal tendon itself. This knowledge is key to intraoperative decision making: deciding when a graft is needed to supplement or replace the native tendon, to help guide the decision process into which graft source is most appropriate, and to better inform the method of proximal graft incorporation to the distal biceps muscle.
Presently, we have found only 2 studies in the peer-reviewed literature that have obtained some objective measurements of the DBT morphology. Cucca et al5 investigated the relationship and anatomical variations between the 2 muscle bellies, the length of the DBT, and the width measured at the level of the MTJ in 20 cadaveric specimens. Length was measured from MTJ to insertion using standard calipers (57 ± 12 mm), and width measured only at the MTJ (15 ± 3 mm). Although objective measurements are provided, the use of calipers is a crude measurement tool, and tendon width was only recorded at the MTJ where width is greatest and is not necessarily representative of the average width of the tendon, which would be beneficial for graft selection. Our measurements of external tendon lengths (63.0 ± 9.4 mm) fall within the above range. However, our measurement of the width of the external tendon was taken from the middle third, a more narrow ovoid section, and therefore not comparable, although our measurement of the width of the internal tendon is similar to that of Cucca et al.5 Kulshreshtha et al17 examined the width and thickness of the DBT at the antecubital fossa in 74 cadaveric arms but did not measure tendon length. Although measurements were taken using Vernier calipers, we feel this location of measurement is more appropriate than the MTJ for determining characteristics for graft selection. They did, however, only measure from 1 location on each specimen, whereas we feel we gain a more accurate representation by taking the average of multiple points from the middle third of the external tendon. Nonetheless, the values of width and thickness from their study (5.2 ± 1.0 and 2.6 ± 0.5 mm, respectively)17 fall within the range of our current study (6.0 ± 1.0 and 3.0 ± 0.5 mm, respectively).
Cucca et al5 also examined the relationship between the 2 muscle bellies as they merged into the tendon. Of their specimens, 30% (6/20) had complete separation of the muscle bellies leading up to the tendon. Using our 3D anatomic modeling, we were able to identify the relationship of the 2 heads within the tendon and identified 4 of the 10 to show complete separation as distinct tendons surrounded by a thin common sheath.
Lastly, there is little information in the literature to help guide surgical and suturing techniques to incorporate the graft proximally into the remaining distal biceps muscle and tendon stump. Although there is a clear visual distinction between muscle and tendon on gross inspection, there is a complex expansion of the internal tendon proximally, hiding beneath the more superficial muscle layers as depicted in our 3D modeling. A better understanding of the orientation of these fibers as well as the unique characteristics of the tendon itself may provide new insight into techniques to improve the strength of the repair by better orienting the graft and/or sutures. To our knowledge, this has not been reported in the literature.
Repair techniques described for chronic reconstructions are technically challenging. Docking the distal end first means fixing the proximal part of the graft to the inadequate tendon stump and distal muscle with the arm bent at 45°.26 This is technically difficult and minimizes the access to the deep portions of the internal tendon. Similarly, when the graft is secured and positioned in a blanket-type fashion with Krackow stitching along the periphery, incorporation of the graft into the thick aponeurotic expansion that exists under the superficial muscle fibers, which could allow for greater graft security, is being missed. In addition, the bifid relationship of the distal tendon can be best understood with layer dissection. As this is not possible clinically in a surgical repair setting, it would be prudent to assume a bifid relationship exists in each patient and to select a suture grasping technique that is adequate to secure both layers. This conclusion is supported by the work of Fogg et al,9 where their anatomical study suggests that nonsutured tendinous bands may slip in the direction of muscle contraction, contributing to a less robust repair and poor clinical function postoperatively. By suturing this internal tendon aponeurosis, it will not only ensure even distribution of forces between both muscle heads, but provide better control when replicating the anatomic docking by placing the medial aspect of the tendon (short head) more distal into the radial tuberosity and maximizing supination, although this has not been confirmed with biomechanical or outcome studies.1 It is important to acknowledge that even though it is ultimately our goal to restore native anatomy, anatomic reconstruction does not necessarily lead to a superior outcome.
Our technique of data collection allows the capturing of the entire muscle and tendon volume as well as a detailed look at the cadaveric architecture, and in this case, a detailed description of the muscle fiber orientation and collagenous extensions. This process enables a greater resolution and accuracy to a submillimeter level (0.3 mm) to that offered with standard calipers. The disadvantage, however, of this technique is that it is an extremely time-intensive technique for serial dissection and digitization.
Limitations
This study is a unique 3D representation of the important morphological characteristics of the DBT. There are, however, some limitations to this study. First, the cadavers were embalmed in formalin. Although still quite flexible, this is not as natural as fresh specimens, which were not feasible at our institution. Second, a smaller sample size was selected secondary to the demands of the digitization process. Although similar in number to previous digitization studies, there are fewer specimens than some of the other anatomic studies investigating the distal biceps anatomy. Regardless, we feel we have an excellent representation of the anatomy secondary to the precision of these measurements and this technique. Lastly, the demographics of the cadavers did not represent the population typically burdened by this injury, with the majority being female and with an average age of 78.3 years. Given the sex and age of specimens, results of the present study likely represent the low range of width and thickness measurements but are likely a fair representation of the length of the tendon.
Conclusion
Surgeons can use the dimensions of the native distal biceps tendon when deciding whether reconstruction is necessary, as well as for graft selection of similar morphology. If there is significant loss of length or caliber compared to “normal,” the decision to augment and/or reconstruct with a graft should be considered. Based on our anatomical 3D study, we suggest graft consideration to substitute for a normal tendon of approximately 63.0 mm in length. In cases of reconstruction, a graft should be chosen that has similar characteristics as the native tendon (width, 6.0 mm; thickness, 3.0 mm) and account for the additional length necessary for graft incorporation (63.0 mm for the external tendon, 50.0 mm for pulvertaft weave, and 5.0 mm for docking distally into an osseous trough), for a total length between 115 to 120 mm. A wider graft means a larger hole for docking, and hence, increased risk of fracture. Trimming a graft to meet the desired width and thickness means disrupting the collagen fibers and increasing the risk of adhesions. Tubularizing a graft to reproduce the shape of the native tendon weakens its structural and mechanical properties.28 For all these reasons, finding a graft most similar to the native tendon will yield more predictable results.
We currently use autogenous semitendinosus from the contralateral leg but have successfully used flexor carpi radialis from the ipsilateral arm. Although variation in measurements exist, the semitendinosus tendon is at least on average 235 ± 20 mm in length27 with a minimum cross-sectional area of 11.4 ± 2.06 mm2,23 providing a width and thickness of approximately 3.4 mm each.23,27,28,31 This provides sufficient length to substitute for the distal biceps tendon as well as similar ovoid morphology. We use a pulvertaft weave with 2 passes from medial to lateral, 1 proximally and 1 distally, to take advantage of the orientation of the thick internal tendon and maximize strength of the repair at time zero, and 1 intervening pass from anterior to posterior. The pulvertaft weave is started just high enough to get 3 passes, each 90° to one another, to achieve a solid connection to the stump that hopefully does not lengthen with physiologic load or rehabilitation. We are not attempting to replicate the entire internal tendon length. The graft is cycled under tension to remove any residual slack and confirm secure fixation. It is then truncated at the “normal” anatomical length of the external tendon (with about 5.0 mm additional length for intraosseous docking), and reconstruction proceeds similar to an acute distal biceps tendon 2-incision repair technique to the radial tuberosity.
Acknowledgment
The authors thank Lu Zheng and Stanley Hung for their assistance with data collection.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: A.E. is a consultant for Arthrex.
A Video Supplement for this article is available at http://ojsm.sagepub.com/supplemental.
References
- 1. Athwal GS, Steinmann SP, Rispoli DM. The distal biceps tendon: footprint and relevant clinical anatomy. J Hand Surg Am. 2007;32:1225–1229. [DOI] [PubMed] [Google Scholar]
- 2. Baker B, Bierwagen D. Rupture of the distal tendon of the biceps brachii: operative versus non-operative treatment. J Bone Joint Surg Am. 1985;67:414–417. [PubMed] [Google Scholar]
- 3. Bayat A, Neumann L, Wallace WA. Late repair of simultaneous bilateral distal biceps brachii tendon avulsion with fascia lata graft. Br J Sports Med. 1999;33:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon rupture; a historical perspective and current concepts. Am J Orthop. 2001;30:193–200. [PubMed] [Google Scholar]
- 5. Cucca Y, McLay S, Okamoto T, Ecker J, McMenamin P. The biceps brachii muscle and its distal insertion: observations of surgical and evolutionary relevance. Surg Radiol Anat. 2010;32:371–375. [DOI] [PubMed] [Google Scholar]
- 6. Darlis NA, Sotereanos DG. Distal biceps tendon reconstruction in chronic ruptures. J Shoulder Elbow Surg. 2006;15:614–619. [DOI] [PubMed] [Google Scholar]
- 7. Dirim B, Brouha SS, Pretterklieber ML, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. AJR Am J Roentgenol. 2008;191:W248–W255. [DOI] [PubMed] [Google Scholar]
- 8. Dobbie R. Avulsion of the lower biceps brachii tendon; analysis of fifty-one previously unreported cases. Am J Surg. 1941;51:662–683. [Google Scholar]
- 9. Fogg QA, Hess BR, Rodgers KG, Ashwood N. Distal biceps brachii tendon anatomy revisited from a surgical perspective. Clin Anat. 2009;22:346–351. [DOI] [PubMed] [Google Scholar]
- 10. Friedmann E. Rupture of the distal biceps brachii tendon: report on 13 cases. JAMA. 1963;184:60–63. [DOI] [PubMed] [Google Scholar]
- 11. Gilcreest EL. Rupture of muscle and tendons, particularly subcutaneous rupture of the biceps flexor cubiti. JAMA. 1925;84:1819–1822. [Google Scholar]
- 12. Hamer MJ, Caputo AE. Operative treatment of chronic distal biceps tendon ruptures. Sports Med Arthrosc. 2008;16:143–147. [DOI] [PubMed] [Google Scholar]
- 13. Hang DW, Bach BR, Jr, Bojchuk J. Repair of chronic distal biceps brachii tendon rupture using free autogenous semitendinous tendon. Clin Orthop. 1996;323:188–191. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan FT, Rokito A, Birdzell MG, Zuckerman JD. Reconstruction of chronic distal biceps tendon rupture with use of fascia lata combined with a ligament augmentation device: a report of 3 cases. J Shoulder Elbow Surg. 2002;11:633–636. [DOI] [PubMed] [Google Scholar]
- 15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of repair of the distal biceps tendon with the modified two-incision technique. J Bone Joint Surg Am. 2000;82-A:1575–1581. [DOI] [PubMed] [Google Scholar]
- 16. Kelly EW, Sanchez-Sotello J, Morrey B, O’Driscoll SW. Repair of chronic distal biceps tendon ruptures: indications and use of tendon grafts. Oper Tech Sports Med. 2003;11:55–59. [Google Scholar]
- 17. Kulshreshtha R, Singh R, Sinha J, Hall S. Anatomy of the distal biceps brachii tendon and its clinical relevance. Clin Orthop Relat Res. 2006;456:117–120. [DOI] [PubMed] [Google Scholar]
- 18. Lee H. Traumatic avulsion of tendon of insertion of biceps brachii. Am J Surg. 1951;82:290–292. [DOI] [PubMed] [Google Scholar]
- 19. Levy HJ, Mashoff AA, Morgan D. Repair of chronic ruptures of the distal biceps tendon using flexor carpi radialis tendon graft. Am J Sports Med. 2000;28:538–540. [DOI] [PubMed] [Google Scholar]
- 20. Morrey BF. Biceps tendon injury. Instr Course Lect. 1999;48:405–410. [PubMed] [Google Scholar]
- 21. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67:418–421. [PubMed] [Google Scholar]
- 22. Patterson RW, Sharma J, Lawton JN, Evans PJ. Tendoachilles allograft: a modification of the endobutton technique utilizing an ACL reconstruction system. J Hand Surg Am. 2009;34:545–552. [DOI] [PubMed] [Google Scholar]
- 23. Pichler W, Tesch NP, Schwantzer G, et al. Differences in length and cross-section of semitendinosus and gracilis tendons and their effect on anterior cruciate ligament reconstruction. J Bone Joint Surg Br. 2008;90:516–519. [DOI] [PubMed] [Google Scholar]
- 24. Rantanen J, Orava S. Rupture of the distal biceps tendon. A report of 19 patients treated with anatomic reinsertion, and a meta-analysis of 147 cases found in the literature. Am J Sports Med. 1999;27:128–132. [DOI] [PubMed] [Google Scholar]
- 25. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275–283. [PubMed] [Google Scholar]
- 26. Sanchez-Sotelo J, Morrey BF, Adams RA, O’Driscoll SW. Reconstruction of chronic ruptures of the distal biceps tendon with use of an Achilles tendon allograft. J Bone Joint Surg Am. 2002;84:999–1005. [DOI] [PubMed] [Google Scholar]
- 27. Tohyama H, Beynnon BD, Johnson RJ, Nichols CE, Renstrom PA. Morphology of the semitendinosus and gracilis tendons with application to anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1993;1:143–147. [DOI] [PubMed] [Google Scholar]
- 28. Wang R, Arciero RA, Obopilwe E, Mazzocca A. A comparison of structural and mechanical properties of tubularized and native semitendinosus graft. Am J Sports Med. 2010;38:1246–1249. [DOI] [PubMed] [Google Scholar]
- 29. Wiley WB, Noble JS, Dulaney TD, Bell RH, Noble DD. Late reconstruction of chronic distal biceps tendon ruptures with a semitendinosus autograft technique. J Shoulder Elbow Surg. 2006;15:440–444. [DOI] [PubMed] [Google Scholar]
- 30. Wright T. Late distal biceps repair. Tech Hand Upper Extrem Surg. 2004;8:167–172. [DOI] [PubMed] [Google Scholar]
- 31. Yasumoto M, Deie M, Sunagawa T, Adachi N, Kobayashi K, Ochi M. Predictive value of preoperative 3-dimensional computer tomography measurement of semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:259–264. [DOI] [PubMed] [Google Scholar]


