Abstract
Background
Autologous platelet-rich plasma (PRP) has been widely used for the treatment of sports injuries. It has been associated with improved healing and regeneration of soft tissues in elite athletes. Athletes are commonly receiving nonsteroidal anti-inflammatory drugs (NSAIDs). As yet, the effect of these drugs on platelet function in PRP formulations has not been taken into consideration.
Hypothesis
The function of platelets in PRP produced under the influence of NSAIDs is inhibited and may lessen a possible healing effect on the site of injury.
Study Design
Controlled laboratory study.
Methods
PRP was collected from patients receiving NSAIDs after elective orthopaedic surgery, and platelet function was evaluated using light transmission aggregometry (LTA). Results were compared with those obtained from healthy volunteers without a history of NSAID intake during the previous 2 weeks. Two different systems for blood collection and PRP production (Arthrex ACP double-syringe system and standard 4.5-mL sodium citrate blood collection tubes) were used and compared regarding the quality of PRP that was produced.
Results
For both groups, the baseline platelet counts of whole blood and the platelet counts of PRP formulations were found to be in the normal range. Both collection systems for PRP produced comparable results without significant differences between the groups. Platelet function testing with LTA revealed significantly impaired platelet aggregation in both PRP preparations, obtained from patients taking NSAIDs, irrespective of the type of NSAID (P < .001). All subjects from the control group showed normal platelet aggregation patterns when tested with LTA.
Conclusion
Autologous PRP produced from subjects after NSAID medication shows significantly impaired platelet function and may result in lower quality regarding the content of bioactive compounds.
Clinical Relevance
If required, the administration of NSAIDs should be performed after blood collection for preparation of autologous PRP; otherwise, the therapeutic effect may be limited.
Keywords: platelet-rich plasma, nonsteroidal anti-inflammatory drugs, platelet function
Platelet-rich plasma (PRP) describes an autologous blood product containing a high concentration of platelets within a small volume of plasma. Nowadays, PRP is widely used for the treatment of sports injuries (eg, injuries of hamstring muscles or augmentation in surgical reconstructive procedures) to achieve accelerated healing and regeneration, especially in elite athletes who require rapid return to competition.9,33 The World Anti-Doping Agency (WADA) has removed PRP from its list due to insufficient evidence that PRP provides performance enhancement.4,31,32
Animal studies and preliminary human studies show that the main therapeutic effects of PRP rely on the delivery of a high concentration of bioactive compounds such as cytokines and growth factors to the site of injury.12,16,29 However, robust clinical studies to support the beneficial use of PRP are still rare. Some prospective randomized controlled studies have shown positive effects with the use of PRP (eg, in augmenting rotator cuff repairs, Achilles tendon repairs, and anterior cruciate ligament reconstruction), but some studies do not show a clear benefit of PRP.#
There is intensive ongoing debate regarding the ideal volume of PRP to administer, the frequency of application, the exact site of administration of PRP, and which technique/preparation system of manufacture to use.3,25,26 While these questions have not been fully answered and remain open for discussion, another important issue has not yet been addressed: the fact that nonsteroidal anti-inflammatory drugs (NSAIDs) may influence platelet function such as adequate platelet aggregation. This effect on platelet function is crucial to the release of stored bioactive compounds and growth factors from granules into PRP preparations. As a consequence, the therapeutic effects of autologous PRP may be significantly lowered.
In this single-center pilot study, the in vivo effect of NSAIDs on platelet function in autologous PRP was investigated using 2 different systems for preparation of PRP.
Methods
Study Subjects, Medication, Sample Collection, and Preparation of PRPs
This study was approved by the local ethics committee. A total of 21 study participants were investigated in the pilot study: 11 patients treated with NSAIDs after orthopaedic injuries followed by surgical intervention served as the study group (5 males, 6 females; mean age, 45.1 ± 19.5 years), and 10 healthy volunteers without a history of NSAID intake within the previous 2 weeks served as the control group (5 males, 5 females; mean age, 36.1 ± 12.3 years). NSAID use consisted of either diclofenac 75 mg or dexibuprofen 400 mg taken twice daily (at 8 am and 8 pm). The time of NSAID intake prior to PRP preparation was a mean (±SD) 3.2 ± 2.1 days. All surgical interventions were performed by the same surgeon. Subjects were included if they gave written informed consent and they had normal platelet counts within the reference range of 150 to 400 × 109/L. Subjects were excluded for any of the following reasons: a history of medication classified as platelet function inhibitors (including acetylsalicylic acid, clopidogrel, prasugrel, or ticagrelor) or a history of hepatic disease. Detailed baseline characteristics are presented in Table 1.
TABLE 1.
Baseline Characteristics of the Study Groupa
| Underlying Condition | n | Surgical Intervention | Type of NSAID | Duration of NSAID Before PRP Preparation, d |
|---|---|---|---|---|
| ACL rupture | 1 | ACL reconstruction | Dexibuprofen 400 mg twice daily | 3 |
| Gonarthrosis | 1 | Total knee endoprosthesis | Diclofenac 75 mg twice daily | 4 |
| Subacromial impingement | 3 | Arthroscopic subacromial decompression | Diclofenac 75 mg twice daily | 2 |
| Bony Bankart lesion of the shoulder | 1 | Open revision and screw fixation of the fragment | Dexibuprofen 400 mg twice daily | 3 |
| Rotator cuff tear | 3 | Arthroscopic rotator cuff repair | Dexibuprofen 400 mg twice daily | 2 |
| Postfemoral locking nail | 1 | Hardware removal | Diclofenac 75 mg twice daily | 5 |
| Chronic patellar dislocation | 1 | Tibial tubercle transfer | Diclofenac 75 mg twice daily | 2 |
aACL, anterior cruciate ligament; NSAID, nonsteroidal anti-inflammatory drug; PRP, platelet-rich plasma.
For the preparation of PRP, whole blood was obtained from each study participant by venipuncture of the cubital vein using a 21-gauge needle. Using this venipuncture, blood was consecutively collected using 2 different collection systems: the Arthrex ACP double-syringe system using 13.5 mL of whole blood mixed with prefilled 1.5 mL of 3.8% concentrated sodium citrate (Arthrex Inc) and standard 3 × 4.5–mL sodium citrate tubes (3.8% wt/vol) with a blood-to-citrate ratio of 9:1 (Greiner Bio One). All blood samples were transferred within 1 hour to the platelet research laboratory and processed immediately. Samples collected with the Arthrex ACP system were handled in accordance with the manufacturer’s instructions, using the designated Hettich Rotofix 32 swing out rotor centrifuge (Hettich Lab Technology) for centrifugation at 350g for 5 minutes. After centrifugation, the plasma supernatant containing the PRP was aspirated with the attached second syringe. Samples collected with the Greiner Bio One standard tubes were also centrifuged at 115g for 15 minutes, producing clear PRP. Two milliliters of PRP was collected in sterile Falcon tubes. The remaining plasma in the tubes was centrifuged again at 2880g for 15 minutes to produce platelet-poor plasma (PPP), which was used for baseline value calibration with light transmission aggregometry (LTA), as described in detail below. Both PRP preparations were measured for total platelet count on a hematological analyzer (XE-5000; Sysmex) and were subsequently used for platelet function testing with LTA using the gold standard method.
Platelet Function Testing Using LTA
Both PRP preparations using the Arthrex ACP system and the standard blood collection tubes were further processed using standard laboratory protocols. Samples containing 450 µL of PRP were stimulated with standard inductors of platelet aggregation in the following fashion: 5 µL of arachidonic acid (AA; final concentration, 50 mmol/L), 5 µL of adenosine diphospate (ADP; final concentration, 1 mmol/L), 1 µL of collagen (COL; final concentration, 1 mg/mL), and 20 µL of thrombin receptor–activated peptide–6 (TRAP-6; final concentration, 1 mmol/L) to provide an internal run control reflecting basis activity of platelets. LTA was performed on a lumi-aggregometer (Chronolog 700; Chronolog Corp), and results were analyzed using the Aggrolink 8.1.2.2 software package (Chronolog). Values of aggregation were expressed as the maximum percentage change in light transmission from baseline, depicted as areas under the curve (AUCs). Parallel testing the PPP of the corresponding PRP samples for 10 minutes served as the calibrated baseline value.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software (version 22.0; IBM Corp). Student t tests for paired observations were conducted, and box plots were generated. P < .05 was considered statistically significant. The final sample size of 11 participants in the NSAID study group and the 10 healthy volunteers in the control group yields 100% power to detect a 5% variation in the laboratory parameters measured for platelet aggregation, assuming an alpha error of 0.05.
Results
A total of 21 study participants were investigated in this pilot study, including 11 subjects treated with NSAIDs after orthopaedic surgery (study group; see Table 1) and 10 healthy volunteers without a history of NSAID intake within the previous 2 weeks (control group). Baseline platelet counts in whole blood were found to be within normal reference ranges in both groups, resulting in a mean platelet count of 160 ± 34 × 109/L in the study group versus a mean platelet count of 190 ± 8 × 109/L in the control group. In PRP formulations, mean platelet counts were also found to be similar and comparable between PRP formulations prepared with the 2 different methods; in addition, there was no significant difference between mean platelet counts of PRP formulations between the 2 groups (Table 2).
TABLE 2.
Results of Platelet Function Testing (Mean ± SD) Using LTA and Standard Inductors of Platelet Aggregationa
| LTA Parameter | NSAID Group (n = 11) | P Valuec | Control Group (n = 10) | ||||
|---|---|---|---|---|---|---|---|
| P Valueb | ACP | SCT | ACP | SCT | P Valueb | ||
| Platelet PRP (109/L) | .853 | 335 ± 139 | 325 ± 118 | .179 | 388 ± 139 | 392 ± 176 | .877 |
| % Collagen | .999 | 76 ± 6 | 76 ± 15 | .127 | 80 ± 11 | 83 ± 8 | .518 |
| AUC | .850 | 629 ± 48 | 621 ± 128 | .118 | 658 ± 98 | 680 ± 71 | .574 |
| % ADP | .856 | 70 ± 7 | 69 ± 15 | .561 | 71 ± 18 | 73 ± 18 | .765 |
| AUC | .886 | 614 ± 53 | 607 ± 140 | .627 | 614 ± 143 | 644 ± 151 | .658 |
| % AA | .711 | 2.7 ± 3.7 | 2.2 ± 3.0 | <.001 | 78 ± 11 | 80 ± 7 | .694 |
| AUC | .679 | 25 ± 19 | 21 ± 31 | <.001 | 563 ± 61 | 612 ± 74 | .126 |
| % TRAP-6 | .283 | 83 ± 9 | 87 ± 7 | .499 | 83 ± 11 | 84 ± 8 | .766 |
| AUC | .249 | 750 ± 96 | 793 ± 75 | .590 | 734 ± 95 | 779 ± 80 | .260 |
aResults are reported as mean ± SD. Boldfacing indicates statistically significant difference in platelet function. AA, arachidonic acid; ACP, Arthrex ACP collection system for PRP preparation; ADP, adenosine diphosphate; AUC, area under the curve; LTA, light transmission aggregometry; PRP, platelet-rich plasma; SCT, standard collection tubes for PRP preparation; TRAP-6, thrombin receptor–activated peptide–6.
bStatistically significant difference between types of PRP preparation systems (P < .05).
cStatistically significant difference between study group and control group (P < .05).
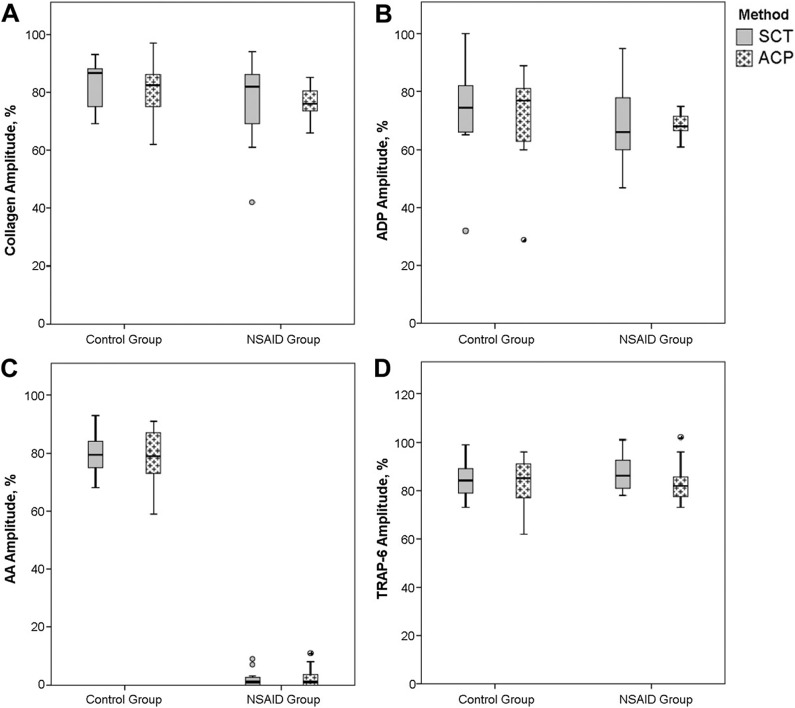
Results of platelet function testing with LTA were not influenced by the method of blood collection for production of PRP. In both PRP formulations produced differently with platelet aggregation response patterns after stimulation of PRP with thrombin receptor–activated peptide–6 (TRAP-6), the “internal control” reflected adequate basis activity of platelets, and no significant differences could be observed throughout all measurements performed (Table 2, Figures 1 and 2).
Figure 1.
Distribution of platelet aggregation patterns under stimulation of platelet-rich plasma (PRP) samples with standard inductors: (A) collagen, (B) adenosine diphosphate (ADP), (C) arachidonic acid (AA), and (D) thrombin receptor–activated peptide–6 (TRAP-6). Box plots indicate amplitude ranges (in % platelet aggregation) obtained by light transmission aggregometry in patients receiving nonsteroidal anti-inflammatory drugs (NSAIDs) and in control group subjects. PRP samples were prepared with 2 different methods (standard collection tubes for PRP preparation [SCT] and the Arthrex ACP collection system for PRP preparation [ACP]) and tested in parallel. Circles indicate outliers.
Figure 2.
Representative platelet aggregation patterns obtained by light transmission aggregometry in (A) a patient who received nonsteroidal anti-inflammatory drugs (NSAIDs) and (B) a control group subject. Platelet-rich plasma samples were stimulated with standard inductors: collagen, adenosine diphosphate (ADP), arachidonic acid (AA), and thrombin receptor–activated peptide–6 (TRAP-6).
When LTA results obtained from the NSAID study group were compared with those obtained from the control group, we observed a massive inhibition of platelet aggregation under stimulation with arachidonic acid in PRP preparations from study subjects taking NSAIDs (P < .001). Stimulation with collagen, ADP, and TRAP-6 showed no significant differences when compared with control subjects (Table 2, Figures 1 and 2). All subjects from the control group showed adequate platelet aggregation patterns after stimulation with all 4 standard inductors for platelet aggregation (Table 2, Figures 1 and 2). No difference was observed regarding the type of NSAID administered or the duration of NSAID intake.
Discussion
In sports and orthopaedic medicine, there is extensive discussion about the benefits of administering autologous PRP to the site of injury.29 The healing effect of the administration of PRP relies on the fact that bioactive compounds like growth factors and cytokines are being released (by activation and aggregation) directly to the site of injury.2,4,12,16,17,29 Debate continues about the following: timing, site, content, and volume of delivery to achieve accelerated healing and return to sports.1,3,6,10,25,26
The quality of the platelets contained in autologous PRP (mainly reflected by the potential of activation and aggregation of platelets) has not yet been investigated. In particular, NSAIDs are described to have negative effects on platelets such as decrease in storage of alpha granules and inhibition of activation and aggregation of platelets.19,35 Commonly, an increased risk of bleeding associated with trauma and operations has been described with antiplatelet drugs classified as NSAIDs (eg, aspirin).15,22
The production and application of autologous PRP depends on the release of bioactive compounds induced by activation and aggregation, and this needs to be considered to achieve optimal function. Since athletes are frequently taking NSAIDs, the negative influence of these drugs on platelet activation and aggregation is important, because an inferior quality of autologous PRP is produced after NSAID therapy.
In this pilot study, the systemic effect of NSAID intake on platelet function (activation and aggregation) was measured in autologous PRP samples obtained from subjects taking NSAIDs. Autologous PRP samples should contain at least a 2- to 3-fold concentration of platelets in a small amount of sample matrix. This is achieved by multiple centrifugation steps during the process of preparation, and the quality often depends on the level of standardization of the collection system(s).10,25,31 To ensure high quality of sample material, autologous PRP preparations were produced by 2 standardized, commercially available blood collection systems in this study. There were no significant differences in platelet counts of both PRP preparations produced with these 2 different systems. This allowed for reliable comparison of results obtained from consecutively performed platelet function/platelet aggregation testing.
Platelet function testing was performed using LTA. There are numerous test devices or test systems available to evaluate platelet function, but most of these only refer to whole blood testing of patients in perioperative settings.15,22 In contrast, LTA is developed exclusively for the use of PRP as a sample matrix for testing platelet function. It is considered to be the gold standard method, stimulating platelets with standard inductors of platelet activation and aggregation.5 The consistent significant inhibition of platelet function/platelet aggregation found after stimulation with arachidonic acid in the NSAID study group was irrespective of type and duration of NSAID intake and of the blood collection system used for PRP preparation. These effects could not be observed in the healthy control group without NSAID intake. Arachidonic acid is used in routine platelet aggregation studies for the differential diagnosis of aspirin-like release defects and storage pool disease (alpha granules) of platelets; it is also used to evaluate the inhibitory effect of aspirin or other NSAIDs on platelet function.13,15 The pathophysiological effect is based on the fact that in vitro addition of platelets with arachidonic acid results in a burst of oxygen consumption, which highly induces and activates platelet aggregation via the phospholipase A2-cyclo-oxygenase–thromboxane A2 pathway. Therefore, ingestion of aspirin or aspirin-like compounds such as NSAIDs inhibits cyclo-oxygenase–mediated oxygen consumption, thus precluding all subsequent events leading to platelet activation and aggregation.18 However, in the presence of NSAIDs in our study group, these reactions are absent. Also, bioactive compounds stored in the alpha granules such as growth factors, transforming growth factor–β, and platelet factor 4 cannot be adequately released if this pathway is blocked by NSAIDs, and platelet function is severely impaired. These conditions support our hypothesis that autologous PRP produced after NSAID intake is of lesser quality and therefore may negatively affect the healing effect. Consequently, if NSAID intake is necessary after sports injury and therapeutic, autologous PRP is considered, NSAID use should be commenced only after blood collection for the preparation of autologous PRP to avoid an adverse effect on platelet function and the quality of PRP.
This study has some limitations, such as the small sample size, as a pilot study. However, the numbers of participants derived from sample size calculation are sufficient to achieve statistical significance with a 95% confidence interval for the parameters investigated. The results should be considered as preliminary. Additionally, our control group consisted of only healthy individuals not undergoing orthopaedic surgery and therefore cannot be fully matched. We chose healthy volunteers as controls for ethical reasons, as patients after orthopaedic surgery cannot be precluded from receiving NSAIDs. Third, our study group consisted of orthopaedic patients undergoing elective surgery and did not include athletes with sports injuries. As NSAIDs should have the same therapeutic in vivo effects irrespective of the reason for administration, this should not weaken our findings. Last, we did not quantitate growth factors or cytokines in our PRPs. Therefore, we can only hypothesize or extrapolate that the pathophysiological mechanism extensively described resulted in a PRP with impaired effect on the site of administration. To study such direct effects, a larger prospective study including sports athletes with and without NSAID intake treated with PRP for sports injury must be performed. A strength of our study group is that participants who were chosen from a homogenous collective were all operated on and followed up with by the same surgeon. This ensured optimal adherence with the NSAID regimen.
Conclusion
Autologous PRP produced from subjects after NSAID medication use shows significantly impaired platelet function. If required, the administration of NSAIDs should be performed after blood collection for the preparation of autologous PRP; otherwise, the therapeutic effect may be reduced.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
REFERENCES
- 1. Andia I, Latorre PM, Gomez MC, Burgos-Alonso N, Abate M, Maffulli N. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99–115. [DOI] [PubMed] [Google Scholar]
- 2. Andia I, Sanchez M, Maffulli N. Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther. 2010;10:1415–1426. [DOI] [PubMed] [Google Scholar]
- 3. Anitua E, Sánchez M, Padilla S. More on platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;371:1264. [DOI] [PubMed] [Google Scholar]
- 4. Banfi G, Corsi MM, Volpi P. Could platelet rich plasma have effects on systemic circulating growth factors and cytokine release in orthopaedic applications? Br J Sports Med. 2006;40:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. [DOI] [PubMed] [Google Scholar]
- 6. Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. 2014;42:1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. [DOI] [PubMed] [Google Scholar]
- 8. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28:1718–1727. [DOI] [PubMed] [Google Scholar]
- 9. Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med. 2014;42:2067–2074. [DOI] [PubMed] [Google Scholar]
- 10. Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang C-Q, Pinto NR, Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 11. Everts PA, Devilee RJJ, Brown Mahoney C, et al. Exogenous application of platelet-leukocyte gel during open subacromial decompression contributes to improved patient outcome. A prospective randomized double-blind study. Eur Surg Res. 2008;40:203–210. [DOI] [PubMed] [Google Scholar]
- 12. Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents. 2012;26(suppl 1):35S–42S. [PubMed] [Google Scholar]
- 13. Ingerman CM, Smith JB, Shapiro S, Sedar A, Silver MJ. Hereditary abnormality of platelet aggregation attributable to nucleotide storage pool deficiency. Blood. 1978;52:332–344. [PubMed] [Google Scholar]
- 14. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39:2082–2090. [DOI] [PubMed] [Google Scholar]
- 15. Mahla E, Raggam R, Toller W. Platelet function testing to time surgery in patients on dual antiplatelet therapy? Hämostaseologie. 2014;34:40–45. [DOI] [PubMed] [Google Scholar]
- 16. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. [DOI] [PubMed] [Google Scholar]
- 17. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. [DOI] [PubMed] [Google Scholar]
- 18. Moncada S, Vane JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979;300:1142–1147. [DOI] [PubMed] [Google Scholar]
- 19. Munsterhjelm E, Niemi TT, Ylikorkala O, Silvanto M, Rosenberg PH. Characterization of inhibition of platelet function by paracetamol and its interaction with diclofenac in vitro. Acta Anaesthesiol Scand. 2005;49:840–846. [DOI] [PubMed] [Google Scholar]
- 20. Nin JR, Gasque GM, Azcárate AV, Beola JDA, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25:1206–1213. [DOI] [PubMed] [Google Scholar]
- 21. Orrego M, Larrain C, Rosales J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;24:1373–1380. [DOI] [PubMed] [Google Scholar]
- 22. Prüller F, Drexler C, Archan S, Macher S, Raggam RB, Mahla E. Low platelet reactivity is recovered by transfusion of stored platelets: a healthy volunteer in vivo study. J Thromb Haemost. 2011;9:1670–1673. [DOI] [PubMed] [Google Scholar]
- 23. Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26:50–57. [DOI] [PubMed] [Google Scholar]
- 24. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–528. [DOI] [PubMed] [Google Scholar]
- 25. Reurink G, Goudswaard GJ, Moen MH, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546–2547. [DOI] [PubMed] [Google Scholar]
- 26. Reurink G, Verhaar JA, Tol JL. More on platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;371:1264–1265. [DOI] [PubMed] [Google Scholar]
- 27. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–251. [DOI] [PubMed] [Google Scholar]
- 29. Sánchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39:345–354. [DOI] [PubMed] [Google Scholar]
- 30. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39:38–47. [DOI] [PubMed] [Google Scholar]
- 31. Schippinger G, Fankhauser F, Oettl K, Spirk S, Hofmann P. Does single intramuscular application of autologous conditioned plasma influence systemic circulating growth factors? J Sports Sci Med. 2012;11:551–556. [PMC free article] [PubMed] [Google Scholar]
- 32. Schippinger G, Oettl K, Fankhauser F, Spirk S, Domej W, Hofmann P. Influence of intramuscular application of autologous conditioned plasma on systemic circulating IGF-1. J Sports Sci Med. 2011;10:439–444. [PMC free article] [PubMed] [Google Scholar]
- 33. Schippinger G, Studencnik G, Fankhauser F. PRP indications in sports medicine—a review of the literature. Sports Orthop Traumatol. 2015;31:45–53. [Google Scholar]
- 34. Vavken P, Sadoghi P, Murray MM. The effect of platelet concentrates on graft maturation and graft-bone interface healing in anterior cruciate ligament reconstruction in human patients: a systematic review of controlled trials. Arthroscopy. 2011;27:1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiss HJ, Lages B. Platelet malondialdehyde production and aggregation responses induced by arachidonate, prostaglandin-G2, collagen, and epinephrine in 12 patients with storage pool deficiency. Blood. 1981;58:27–33. [PubMed] [Google Scholar]