The effect of systemic administration of conditioned media (CM) and extracellular vesicles (EVs) from both human and mouse bone marrow-derived mesenchymal stromal cells (MSCs) in a murine model of severe experimental asthma was studied. Systemic administration of CM and the isolated EVs significantly ameliorated the challenge-induced increases in airway hyperreactivity, lung inflammation, and antigen-specific CD4 T-cell phenotype. EVs isolated from human MSCs generally were more potent than those from mouse MSCs.
Keywords: Mesenchymal stromal cells, Extracellular vesicles, Conditioned media, EDCI, Asthma, Mouse
Abstract
An increasing number of studies demonstrate that administration of either conditioned media (CM) or extracellular vesicles (EVs) released by mesenchymal stromal cells (MSCs) derived from bone marrow and other sources are as effective as the MSCs themselves in mitigating inflammation and injury. The goal of the current study was to determine whether xenogeneic administration of CM or EVs from human bone marrow-derived MSCs would be effective in a model of mixed Th2/Th17, neutrophilic-mediated allergic airway inflammation, reflective of severe refractory asthma, induced by repeated mucosal exposure to Aspergillus hyphal extract (AHE) in immunocompetent C57Bl/6 mice. Systemic administration of both CM and EVs isolated from human and murine MSCs, but not human lung fibroblasts, at the onset of antigen challenge in previously sensitized mice significantly ameliorated the AHE-provoked increases in airway hyperreactivity (AHR), lung inflammation, and the antigen-specific CD4 T-cell Th2 and Th17 phenotype. Notably, both CM and EVs from human MSCs (hMSCs) were generally more potent than those from mouse MSCs (mMSCs) in most of the outcome measures. The weak cross-linking agent 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride was found to inhibit release of both soluble mediators and EVs, fully negating effects of systemically administered hMSCs but only partly inhibited the ameliorating effects of mMSCs. These results demonstrate potent xenogeneic effects of CM and EVs from hMSCs in an immunocompetent mouse model of allergic airway inflammation and they also show differences in mechanisms of action of hMSCs versus mMSCs to mitigate AHR and lung inflammation in this model.
Significance
There is a growing experience demonstrating benefit of mesenchymal stromal cell (MSC)-based cell therapies in preclinical models of asthma. In the current study, conditioned media (CM) and, in particular, the extracellular vesicle fraction obtained from the CM were as potent as the MSCs themselves in mitigating Th2/Th17-mediated allergic airway inflammation in a mouse model of severe refractory clinical asthma. Moreover, human MSC CM and extracellular vesicles were effective in this immunocompetent mouse model. These data add to a growing scientific basis for initiating clinical trials of MSCs or extracellular vesicles derived from MSCs in severe refractory asthma and provide further insight into the mechanisms by which the MSCs may ameliorate the asthma.
Introduction
The mechanisms by which mesenchymal stromal cells (MSCs), isolated from bone marrow, adipose tissue, umbilical cord blood, and other sources, exert immunomodulatory actions in in vitro mixed lymphocyte and other assays remain incompletely understood. Even less well understood are the mechanisms by which systemic administration of syngeneic, allogeneic, or xenogeneic MSCs result in anti-inflammatory actions in vivo. Postulated mechanisms include release of soluble anti-inflammatory, antibacterial, and other peptides, as well as mitochondrial transfer through connexin-43-mediated direct cell-cell contact [reviewed in 1–5]. Data from a variety of different preclinical lung disease models, including acute lung injury, hyperoxia, and acute Th2-mediated eosinophilic allergic airway inflammation, demonstrate that systemic administration of conditioned media (CM) alone, obtained from cultured MSCs, can convey the same protective actions as administration of the MSCs themselves [6–13]. Data also suggest that the extracellular vesicle (EV) fraction, also variably denoted as exosomes, microvesicles, or microparticles, released by the MSCs and present in conditioned media may convey the protective effects [14–18]. However, the specific responsible mediators, such as soluble proteins, EVs, or other components of the CM, have not yet been identified and are likely to be different for each lung injury model [19]. In particular, EVs contain a number of components, including miRNAs that may mitigate their actions. Initial information is emerging regarding the roles of specific miRNAs and other EV components in mediating the protective effects of MSC administration in preclinical lung disease models, but there is much as yet unknown [15, 17].
We and others have demonstrated that administration of syn-, allo-, or xenogeneic MSCs can mitigate both Th2-mediated eosinophilic and more severe Th2/Th17 neutrophilic-mediated allergic airway inflammation in mice [6, 20–30]. The latter is a model of severe refractory clinical asthma and provides a potential basis for clinical use of MSCs in severe asthma [30–32]. We have demonstrated that xenogeneic administration of human bone marrow-derived MSCs (hMSCs) is equally effective, if not more so, as administration of murine bone marrow-derived MSCs (mMSCs) in mitigating airway hyperresponsiveness and lung inflammation in a model of mixed Th2/Th17 allergic airway inflammation provoked by repeated airway mucosal exposure to Aspergillus fumigatus hyphal extract (AHE) [33].
Thus, in the current study, we hypothesized that CM or EVs isolated from CM obtained from either hMSCs or mMSCs would also able to mitigate airway hyperresponsiveness and lung inflammation in this model. Moreover, we aimed to compare the efficacy between CM and EVs obtained from hMSCs versus mMSCs. Finally, we aimed to block the release of soluble mediators and EVs from MSCs and assess whether this would differentially affect the ameliorating effects of hMSCs versus mMSCs.
Materials and Methods
Mice
C57Bl/6 mice (male, 8–12 weeks old, n = 72; The Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were housed in microisolator cages and used in accordance with the University of Vermont (UVM) Institutional Animal Care and Use Committee under all applicable Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Cells and Cell Culture
Murine bone marrow-derived mesenchymal stromal cells from C57Bl/6 mice were obtained from the Texas A&M Stem Cell core facility [34]. Human mesenchymal stem cells (hMSCs) derived from bone marrow of normal human volunteers were obtained from the National Heart, Lung, and Blood Institute’s Production Assistance for Cellular Therapies (D.H.M.). These cells have been extensively characterized for cell surface marker expression and differentiation capacity [35, 36]. mMSCs were expanded in culture using Iscove’s Modified Dulbecco’s Medium (IMDM) (HyClone/GE Healthcare, Rockford, IL, http://www.gelifesciences.com), 10% fetal bovine serum (FBS) (HyClone/GE Healthcare), 10% horse serum (HyClone/GE Healthcare), 1% penicillin/streptomycin (Pen/Strep) (Invitrogen, Life Technologies, Grand Isle, NY), and 2 mM l-glutamine (Invitrogen). hMSCs were cultured in Minimal Essential Medium (MEM) with Earle’s balanced salts, 20% FBS, 1% Pen/Strep, and 2 mM l-glutamine. Normal adult human lung fibroblasts (HLF) (catalog no. CCL-199; American Type Culture Collection, Manassas, VA, http://www.atcc.org) were expanded in culture with Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com/), 10% FBS, 1% Pen/Strep, and 2 mM l-glutamine. hMSCs, mMSCs, and HLFs were maintained in culture at confluence no greater than 70% and used at passage 6 or lower. Cells were passaged approximately every 3 days during these studies.
For use in experiments, the cells were harvested using 2.5% trypsin/ethylenediaminetetraacetic acid (Invitrogen). Cell density and viability were determined using trypan blue staining and counted using a hemacytometer. Cell pellets were then resuspended in 1× phosphate-buffered saline (PBS) to a final concentration of 1 × 106 cells per 200 µl immediately prior to injection. Cell viability, density, and final concentration (1 × 106 viable cells per 200 µl of PBS) was determined by trypan blue exclusion and by counting, using a hemacytometer as described for cultured MSC preparations [26, 30].
Preparation of Conditioned Media
Mouse and human MSCs were grown between 70% and 80% confluence. The medium was discarded and cells were rinsed three times with PBS. Cells were then cultured with serum-free medium for 24 hours. The conditioned media were collected and filtered through a 0.2-μm filter to remove cellular debris. Adherent cells were trypsinized, stained with trypan blue, and counted. The media from 1 × 106 cells yielded 15 ml of primary CM that were further concentrated approximately 25-fold (i.e., 200 μl CM) using ultrafiltration units with a 3-kDa molecular weight cutoff (Amicon Ultra-PL 3; EMD Millipore Corporation, Billerica, MA, http://www.emdmillipore.com). CM was similarly collected from HLF as a control [6, 7, 12].
Isolation and Characterization of Extracellular Vesicles
EVs were obtained from the supernatant of MSCs and HLFs as previously described by Zhu et al. [17]. Briefly, MSCs or HLFs were cultured until 100% confluent and then serum starved for 48 hours in fresh MEM supplemented with 0.5% bovine albumin fraction (MP Biomedicals, LLC, Santa Ana, CA, http://www.mpbio.com). To isolate EVs, conditioned media from MSCs or HLFs were collected and centrifuged at 3,000g for 20 minutes to remove cellular debris, then ultracentrifuged at 100,000g (Beckman Coulter Optima L-100XP ultracentrifuge, rotor RW 40Ti; Beckman Coulter, Brea, CA, http://www.beckmancoulter.com) for 1 hour at 4°C to sediment the EVs [17]. EVs were then washed in PBS and submitted to a second ultracentrifugation at 100,000g for 1 hour. MSC or HLF EVs were resuspended in PBS according to the final cell count of MSCs or HLFs after 48 hours of serum starvation (at 10 μl per 1 × 106 cells) and stored at −80°C until further use.
The total protein content of the EV fraction was quantified by Bradford assay. The EV particle-size distribution was determined by diffraction analysis using a NS300 particle-size tracker and Nanosight NTA 3.0 software using light scatter mode (Malvern Instruments Ltd., Technologies, Malvern, U.K., http://www.malvern.com) [37, 38]. Samples were diluted as needed in PBS to achieve an approximate concentration of 107 to 109 particles per ml during the Nanosight NTA analysis. Three (PBS) or five (EVs media, CM, EV pellet) replicates were analyzed for each sample and results were averaged; mean and SD are reported for each sample tested. Aliquots of representative EV pellets were fixed in 1.5% uranyl acetate and 2% phosphotungstic acid and visualized by transmission electron microscopy (JEOL 1400 TEM [JEOL USA, Inc., Peabody, MA, http://www.jeolusa.com] operating at 60 kV).
Induction of Allergic Airway Inflammation
AHE aliquots at a concentration of 1.466 mg/ml in 1× PBS, generously provided by the Whittaker laboratory at UVM and previously used by us, were thawed and vortexed immediately prior to use, diluted to a final concentration of 5 µg of AHE in 40 µl of sterile 1× PBS [30–32]. Mice were anesthetized by isoflurane inhalation and received an oropharyngeal administration of PBS (naïve [N]) or AHE solution (A) on days 0 and 7 to initiate the immune response (sensitization), then challenged for 3 days on days 14–16 with oropharyngeal inoculations using the same AHE preparation (supplemental online Fig. 1) [30].
Systemic Administration of Cells, Conditioned Media, or Extracellular Vesicles
On day 14, immediately after the AHE inoculation, mice received a systemic (tail vein) injection of 1 × 106 cells in 200 µl of 1× PBS (C) or 1× PBS control (P). As previously described, animals received mMSCs, hMSCs, HLFs, their respective CM, or EVs. Some mice received cells treated with the cross-linker 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDCI) prior to injection, to prevent release of soluble mediators as previously described [26, 30]. The amount of CM administered to each mouse (200 μl) reflects that obtained from 106 MSCs after concentration. In accordance with Zhu et al. [17], we used the amount of EVs released by 3 × 106 cells to maximize any potential effects. Mice were euthanized on day 19 and inflammation and lung function were measured as described in Assessment of Airway Inflammation (supplemental online Fig. 1).
Respiratory Mechanics
Pulmonary function was analyzed using the forced oscillation technique (flexiVent; SCIREQ Scientific Respiratory Equipment, Tempe, AZ, http://www.scireq.com), as previously described [26, 30, 33, 39]. The peak responses for airway resistance (RN), overall tissue resistance (G), and elasticity within the lung (H) were determined in response to the following inhalation sequence of methacholine in nebulized saline: 3.125 mg/ml, 12.5 mg/ml, and 25 mg/ml.
Assessment of Airway Inflammation
Following evaluation of lung mechanics, mice were euthanized by lethal intraperitoneal injection of sodium pentobarbital. Bronchoalveolar lavage fluid (BALF) was collected by administering 1 ml of sterile 1× PBS to the airways through a tracheal cannula and rinsing the lungs 3 times prior to recovery. BALF was centrifuged at 5,000 rpm for 5 minutes at 4°C, and the supernatant was collected in separate tubes and stored at −80°C. The Bioplex Cytokine Assay System (Bio-Rad, Hercules, CA, http://www.bio-rad.com) was used to examine undiluted BALF samples for soluble inflammatory cytokines, using a mouse 23-plex panel. Concentrations were determined using the Bio-Plex Manager Software (Bio-Rad). The cell pellet was resuspended and an aliquot was used to determine total cell count by the ADVIA Hematology Analyzer (Siemens Diagnostics, Johnson City, TN, http://usa.healthcare.siemens.com). Cytospins were made using 3 × 104 cells centrifuged onto precleaned, pretreated glass slides (Corning Inc., Corning, NY) at 800 rpm for 8 minutes, dried overnight, and stained using DiffQuick (Hema 3 Stain Set; Fisher Scientific, Pittsburgh, PA, https://www.fishersci.com). Different cell populations were determined by blinded manual count of 200 cells performed by 3 individuals. Following BALF collection, the trachea and heart/lung block were removed, and the right lobes of the lung were removed and snap frozen in liquid nitrogen. The left lobe was then gravity fixed (20 cm H2O) for 1 hour with 4% paraformaldehyde, and 5-µm paraffin sections subsequently were stained with hematoxylin and eosin. Airway inflammation (10 airways per animal, at least 6 animals for each group), evaluated by 3 individuals in blinded fashion, was based on the presence and intensity of peribronchial cell infiltrates compared with positive and negative controls, using an established semiquantitative scoring system with a 0–3 range, as previously described [26, 30].
Mediastinal Lymph Node Mixed-Lymphocyte Assessments
Mediastinal lymph nodes (MLNs) were isolated by dissection from each mouse and placed in T-cell medium (Roswell Park Memorial Institute medium; 5% FBS; 1× Pen/Strep; 2 mM l-glutamine; 2,500 mg/ml glucose; 1 mg/ml folate in 2 g/l sodium bicarbonate; 1 mM sodium pyruvate; and 50 µM of β-mercaptoethanol). To ensure we would have enough cells for the assay, MLN cells from mice of the same experimental group (at least 6 animals for each group) were pooled and pressed through a 40-µm mesh filter into a single cell suspension. Cells were then washed twice in 1× PBS and resuspended for counting. One million cells per time point (24, 48, and 72 hours) were plated in duplicate for each group in a 24-well dish in 500 µl of T-cell medium. In half the wells, cells were stimulated with 1 µg of AHE in the medium for 24 or 48 hours; the other wells were left unstimulated for the same time points. Total contents of each well were collected at the indicated time points and were centrifuged for 5 minutes at 5,000 rpm to pellet cells and debris. Supernatants were moved to a new tube and frozen at −20°C. Content of representative Th1, Th2, and Th17 soluble mediators (interleukin [IL]-4, IL-5, and IL-17; and interferon [IFN]-γ) were assessed by enzyme-linked immunosorbent assay (Biolegend, San Diego, CA, http://www.biolegend.com).
Statistical Analyses
All data were graphed and analyzed using the GraphPad Prism version 6.0 statistical software package (GraphPad Software, La Jolla, CA, http://www.graphpad.com). The normality of the data (Kolmogorov-Smirnov test with Lilliefors’ correction) and the homogeneity of variances (Levene median test) were tested. Parametric data are expressed as mean ± SD. Differences between the groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test. Nonparametric data were analyzed using ANOVA on ranks, followed by Dunn’s post hoc test. Statistical significance was established at p ≤ .05.
Results
Characterization of Extracellular Vesicles
Using NanoSight particle-size tracking analyses, the size range of the EVs released from each cell type (hMSC, mMSC, HLF) was determined (Fig. 1A) [18, 37, 38]. The majority of particles isolated from the ultracentrifugation-concentrated EV pellets were in a size range of approximately 50–150 nm, consistent with the designation of exosomes according to recent consensus guidelines from the International Society for Extracellular Vesicles [38]. However, smaller amounts of larger particles (approximately 200–500 nm) were also observed, consistent with the designation of microvesicles [38]. A similar pattern of EV particle sizes was observed in the unconcentrated conditioned media; however, the amounts were significantly lower. No detectable levels of EVs were observed in either the medium alone or in the PBS vehicle. Transmission electron microscopy (TEM) of the EVs obtained from the concentrated pellet demonstrated a range of particle sizes consistent with those measured by particle-size tracking analyses. Representative images are shown in Figure 1B.
Figure 1.
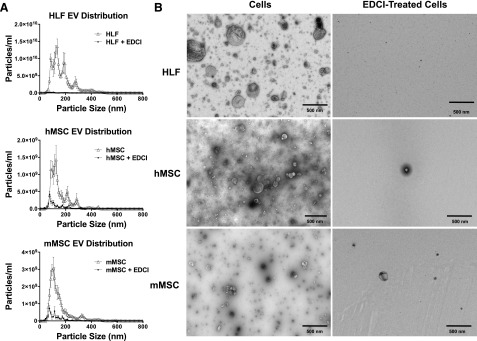
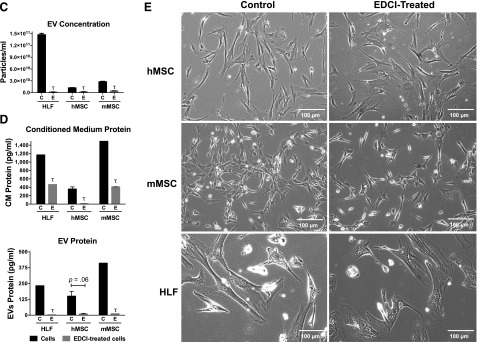
EDCI inhibits release of soluble proteins and of extracellular vesicles from cultured hMSCs, mMSCs, and HLFs. (A): Particle size analyses. EVs in the ultracentrifugation pellet collected after 48 hours of incubation from control cells and EDCI-treated HLFs, hMSCs, and mMSCs were analyzed using a NS300 machine and Nanosight NTA 3.0 software using light scatter mode. n = 3-5 for each group. Samples were diluted as needed in PBS to achieve an approximate concentration of 107 to 109 particles per milliliter. Results are presented as mean ± SD of triplicate measurements for each sample. (B): Representative transmission electron micrographs of EVs collected from ultracentrifuged pellets of conditioned media collected from control cells and EDCI-treated cells. Scale bars = 500 nm. (C): Extracellular vesicle concentration quantified on the ultracentrifugation pellet, collected after 48-hour incubation, from control cells and EDCI-treated cells, using the NS300 machine and Nanosight NTA 3.0 software using light scatter mode (n = 3–5 for each group). Data are presented as mean ± SD of triplicate measurements for each sample. Significance compared with control cell is indicated by τ. (D): Total protein content of raw conditioned media and of the ultracentrifuge pellets obtained from control cells and EDCI-treated cells after 48-hour incubation (n = 2 for each group). Data are presented as mean ± SD of triplicate measurements for each sample. Significance compared with control cells is indicated by τ. (E): Representative photomicrographs (contrast phase) of control and EDCI-treated cells after 48-hour incubation. Original magnification ×20. Scale bars = 100 μm. Abbreviations: EDCI, 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride; EV, extracellular vesicle; HLF, human lung fibroblast; hMSC, human mesenchymal stromal cell; mMSC, mouse mesenchymal stromal cell.
The weak cross-linker EDCI is known to inhibit cell release of soluble proteins such as cytokines [26, 30]. To initially assess EDCI effects on EV production by cells, 3 × 106 cells of each type were incubated for 1 hour at room temperature with EDCI and the conditioned media were collected after 48 hours. EVs were collected from 3 × 106 cells of each type to reflect the amount of EVs used in the in vivo model. Notably, as assessed by both particle-size tracking analyses and by TEM, treatment of each cell type with EDCI significantly and substantially reduced the number of EVs in both the unconcentrated conditioned media and in the EV fraction following ultracentrifugation (Fig. 1A–1C).
To further assess whether EV release was also inhibited following EDCI treatment, 1 × 106 cells of each type were incubated in vitro at room temperature with EDCI for 1 hour. Then the cells were washed and the conditioned media collected after 48 hours to quantify the overall protein content (Fig. 1D). In addition to a significant decrease in EV particles concentration and in the overall conditioned media protein content in EDCI-treated cells, a significant decrease was observed in the total protein content of the EV fraction following ultracentrifugation (Fig. 1D). No obvious cell toxicity was observed following the EDCI exposure, consistent with what we have previously observed (Fig. 1E) [26, 30]. These results demonstrate that EDCI treatment inhibits release of EVs, as well as of soluble proteins.
Airway Hyperresponsiveness
The experimental design is depicted in supplemental online Figure 1. Sensitization and challenge with AHE resulted in a significant increase in large-airway resistance, tissue resistance, and lung elasticity compared with naïve mice (Fig. 2). As we have previously shown, administration of either mMSCs or hMSCs significantly decreased each measure of methacholine-mediated AHR, whereas the administration of the HLF, a control cell population, had no effect (Fig. 2) [33].
Figure 2.
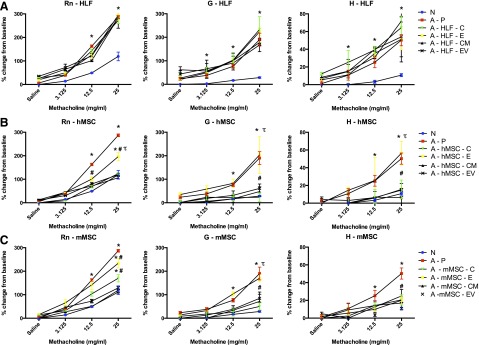
Systemic administration of human or mouse MSCs or their respective conditioned media or extracellular vesicles significantly ameliorates the airway hyperresponsiveness induced by Aspergillus hyphal extract. (A): Analysis of Rn, G, and H according to methacholine dose of N and A treated with P, human lung fibroblast (C), E, CM, or EV (n = 6 for all combinations except the following: 17 N and 15 A-P). (B): Analysis of Rn, G, and H of N and A treated with the vehicle P, hMSCs (C), E, CM, or EV (n = 6 for all combinations except the following: 17 N, 15 A-P, and 10 A-hMSC-C). (C): Analysis of Rn, G, and H of naive and AHE-exposed mice treated with the vehicle P, mMSCs (C), E, CM, or EV (n = 6 for all treatment combinations except the following: 17 N and 15 A-P). Data are presented as peak response normalized to the baseline, and then expressed as percent increase over the baseline ± SD. Statistical significance set at p ≤ .05. ∗, significantly different from N; #, significantly different from A-P; τ, significantly different from each of the three cell types. Abbreviations: A, Aspergillus hyphal extract-exposed mice; C, cells; CM, conditioned media; E, EDCI-treated cells; EV, extracellular vesicle; G, overall tissue resistance; H, lung elasticity; HLF, human lung fibroblast; N, naïve mice; P, phosphate-buffered saline; Rn, airway resistance.
Notably, EDCI-treated hMSCs were ineffective in reducing G and H and only partly reduced the AHE-stimulated increase in RN following systemic in vivo administration (Fig. 2). EDCI-treated mMSCs were as effective as untreated mMSCs in reducing H but had only partial effects in reducing AHE-stimulated increases in RN and G. This indicates that the secretome from hMSCs and mMSCs, including potentially both soluble mediators and EVs, contributes, albeit differentially, to reduce airway hyperresponsiveness.
To evaluate their relative contribution, CM or EVs obtained from hMSCs or mMSCs were administered, in parallel experiments, on day 14 at the onset of antigen challenge. Since EVs released by 106 MSCs only partially abrogate inflammation in other models of lung injury, as demonstrated by Zhu and colleagues, we used the amount of EVs secreted by 3 × 106 cells in each experimental animal to maximize potential beneficial effects [17]. Notably, CM or EVs derived from hMSCs or mMSCs, but not from HLFs, were each as effective as their respective cell of origin in decreasing AHR (Fig. 2).
Lung Inflammation
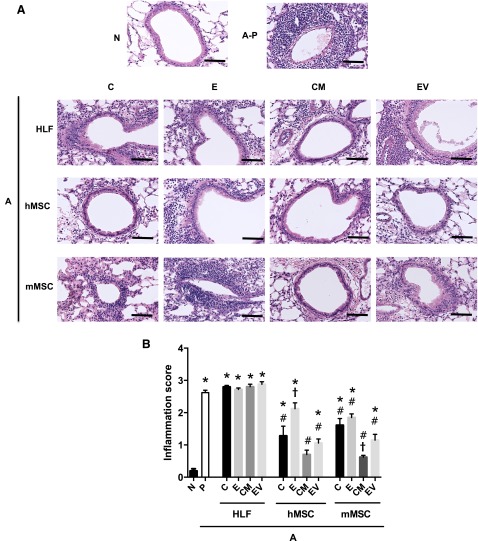
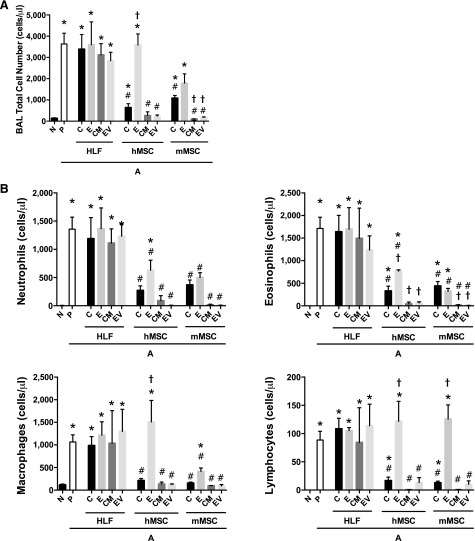
AHE sensitization and challenge resulted in a significant increase in histologic and BALF inflammatory cell content compared with naïve mice (Figs. 3, 4). Systemic administration of either hMSCs, mMSCs, and their respective CM or EVs, significantly decreased both histologic inflammation (Fig. 3A, 3B) and BALF total and differential cell counts (Fig. 4). Conditioned media were more effective than cells (significantly for mMSCs and nearing significance for hMSCs), whereas EVs alone were generally comparable to their respective cell of origin in reducing histologic inflammation (Fig. 3). Administration of either CM or EVs from either hMSCs or mMSCs was equally effective, if not more so, in decreasing AHE-stimulated increases in BALF total cells, neutrophils, eosinophils, macrophages, and lymphocytes (Fig. 4). In particular, CM and EVs were more potent than their respective cells of origin in reducing numbers of neutrophils and eosinophils.
Figure 3.
Systemic administration of human or mouse MSCs or their respective conditioned media or extracellular vesicles significantly reduces histologic lung inflammation provoked by Aspergillus hyphal-extract sensitization and challenge. (A): Representative photomicrographs of H&E-stained lung section. (B): Inflammation score (range: 0–3 ± SEM) of airways in N and A mice treated with HLF, hMSCs, and mMSCs (C), CM, or EVs (n = 6 for all treatment combinations except the following: 17 N, 15 A-P, and 10 A-hMSC-C). Data are presented as mean ± SD. Statistical significance set at p ≤ .05. ∗, significantly different from N; #, significantly different from A-P; τ, significantly different from each of the three cell types. Original magnification ×10; scale bars = 100 μm. Abbreviations: A, Aspergillus hyphal extract-exposed mice; C, cells; CM, conditioned media; E, EDCI-treated cells; EV, extracellular vesicle; HLF, human lung fibroblast; hMSC, human mesenchymal stromal cell; mMSC, mouse mesenchymal stromal cell; N, naïve mice; P, phosphate-buffered saline.
Figure 4.
Systemic administration of human or mouse MSCs or their respective conditioned media or extracellular vesicles significantly reduces increases in BALF inflammatory cells provoked by Aspergillus hyphal-extract sensitization and challenge. (A): Total cell number within the BALF in N and A mice treated with HLF, hMSCs, and mMSCs (C), CM, or EVs. (B): Differential cell population within the BALF normalized to total cell numbers of neutrophils, eosinophils, macrophages and lymphocytes (n = 6 for all treatment combinations except the following: 17 N, 15 A-P, and 0 A-hMSC-C). Data are presented as mean ± SD. Statistical significance set at p ≤ .05. ∗, significantly different from N; #, significantly different from A-P; τ, significantly different from each of the three cell types. Abbreviations: A, Aspergillus hyphal extract-exposed mice; BALF, bronchoalveolar lavage fluid; C, cells; CM, conditioned media; E, EDCI-treated cells; EV, extracellular vesicle; HLF, human lung fibroblast; hMSC, human mesenchymal stromal cell; mMSC, mouse mesenchymal stromal cell; N, naïve mice; P, phosphate-buffered saline.
EDCI-treated hMSCs were not as effective in reducing histologic lung inflammation, whereas EDCI-treated mMSCs were as effective as mMSCs in attenuating inflammation around the airways (Fig. 3). This suggests that mMSCs might be acting through a cell-to-cell interaction in addition to paracrine effects. Similarly, EDCI treatment significantly abrogated the protective effect of hMSCs but not of mMSCs on BALF neutrophils and eosinophils (Fig. 4). EDCI treatment reduced the effect of mMSCs on total cell and macrophage numbers but had no effect on BALF lymphocytes (Fig. 4). Administration of HLFs, EDCI-treated HLFs, or HLF-conditioned media or EVs had no effects on the AHE-provoked histologic or BALF inflammation.
Modulation of Th1, Th2, and Th17 Pathways
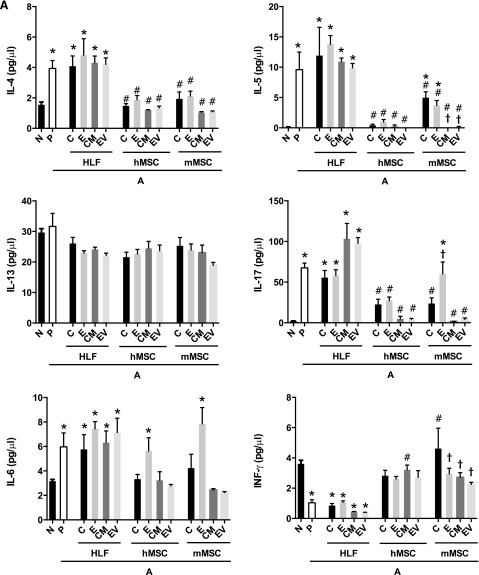
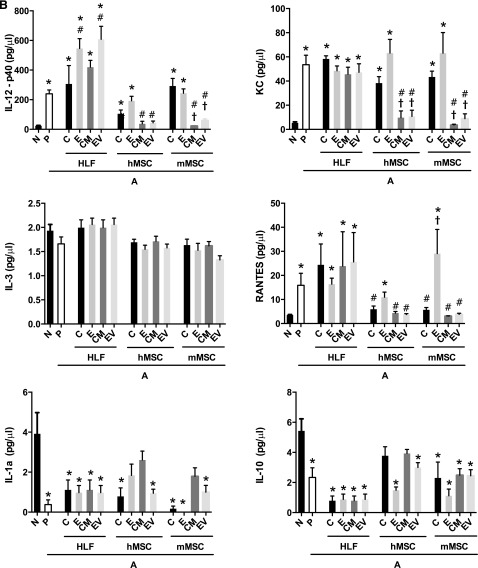
Systemic administration of hMSCs, mMSCs, HLFs, or their respective CM or EVs had mixed effects on levels of BALF cytokines (Fig. 5). hMSCs and mMSCs, as well as their respective CM or EVs, had similar effects in decreasing the AHE-provoked increases in BALF levels of IL-4, IL-5, IL-6, IL-17, and RANTES (Fig. 5A, 5B). In contrast, each of these reversed the AHE-provoked decrease in the level of IFN-γ. Notably, CM and EVs from hMSCs were more effective than hMSCs in reducing AHE-induced alterations in BALF levels of IL-12 and the chemokine keratinocyte chemoattractant (KC). CM and EVs from mMSCs had similar effects in decreasing AHE-induced augmentation in levels of IL-4, IL-17, IL-6, and RANTES. CM and EVs obtained from mMSCs were more effective than the mMSCs in reducing BALF levels of IL-5, IL-12, and KC. EDCI-hMSC administration did not have the same effects (i.e., downregulation) on levels of IL-6 in the BALF, while EDCI-mMSC did not have the same effects (i.e., downregulation) on levels of IL-17, IL-6, KC, and RANTES in the BALF when compared with untreated cells. IL-3 and IL-13 levels were not affected by any cell type or their respective CM or EVs. IL-10 levels in the BALF were increased by administration of either hMSCs or mMSCs or of their respective CM or EVs. HLFs, EDCI-treated HLFs, HLF-CM, and EVs generally yielded no effects on the levels of any cytokines over those produced by AHE exposure alone except for increases in IL-12p40 levels after administration of either EDCI-HLF or HLF EVs (Fig. 5A, 5B).
Figure 5.
Systemic administration of human or mouse mesenchymal stromal cells or their respective conditioned media or extracellular vesicles significantly reduces the increased BALF content of proinflammatory soluble cytokines and chemokines provoked by Aspergillus hyphal-extract sensitization and challenge. (A): Soluble BALF cytokines associated with Th2 (IL-4, IL-5, IL-13), Th17 (IL-6, IL-17a), and Th1 inflammation (IFN-γ). (B): Soluble BALF further Th17 inflammation-associated cytokines (IL-12, KC), alternate inflammatory cytokines (IL-3, RANTES), and cytokines previously identified as secreted by MSCs in immunomodulation (IL-1A, IL-10) (n = 6 for all treatment combinations except the following: 17 N, 15 A-P, 10 A-hMSC-C). Data are presented as mean ± SD. Statistical significance set at p ≤ .05. ∗, significantly different from N; #, significantly different from A-P; τ significantly different from each of the three cell types. Abbreviations: A, Aspergillus hyphal extract-exposed mice; BALF, bronchoalveolar lavage fluid; CM, conditioned media; E, EDCI-treated cells; EV, extracellular vesicle; HLF, human lung fibroblast; hMSC, human mesenchymal stromal cell; IL, interleukin; INF, interferon; KC, keratinocyte chemoattractant; mMSC, mouse mesenchymal stromal cell; N, naïve mice; P, phosphate-buffered saline.
Antigen-Specific Release of Th2 and Th17 Mediators
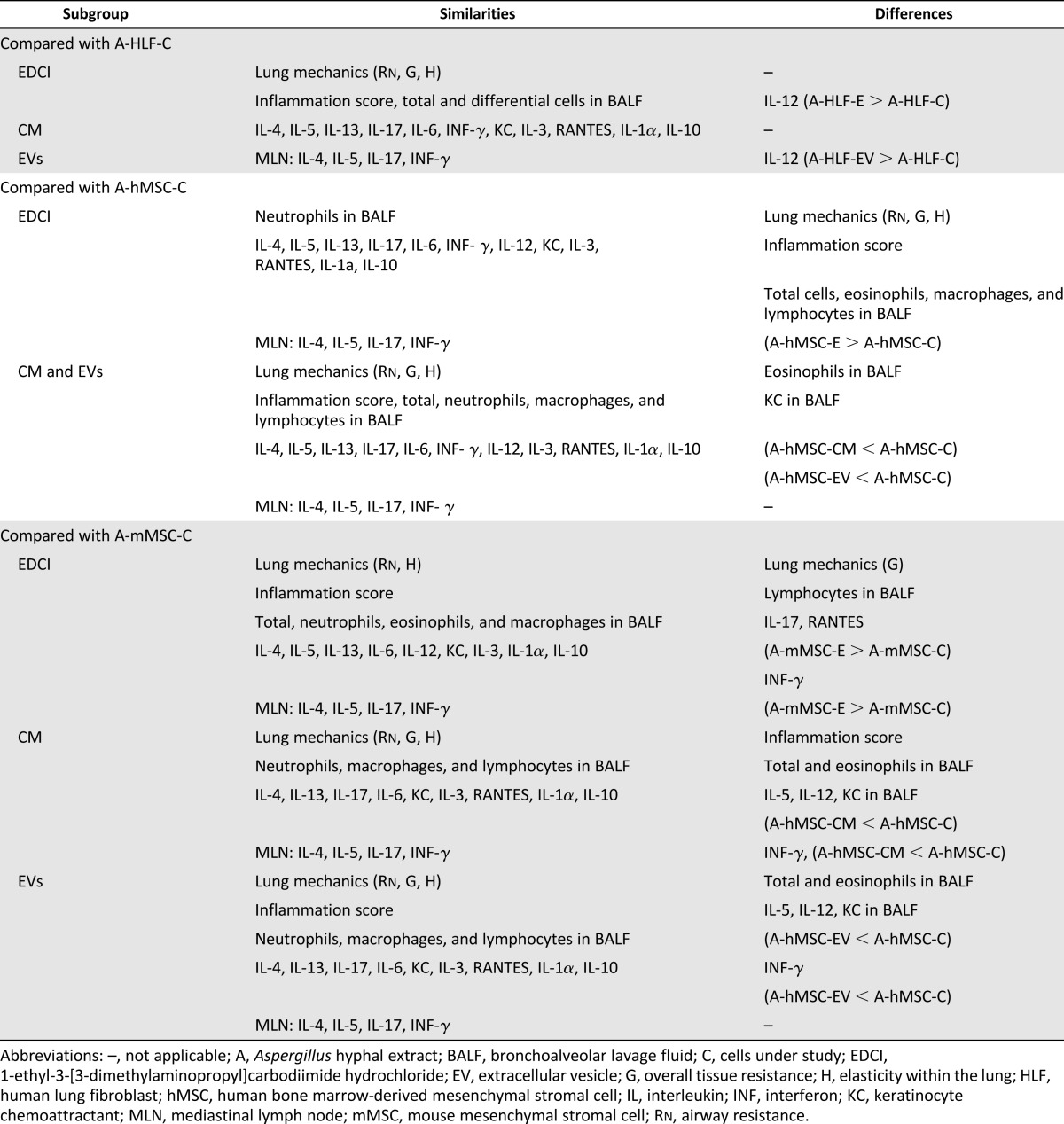
AHE sensitization and challenge resulted in a significant increase in IL-4, IL-5, and IL-17 release by mixed MLN cultures following ex vivo antigen stimulation (Fig. 6A). This was most notable at 48 hours, particularly for the increase in IL-17 levels. No significant changes in levels of IFN-γ were observed. Systemic administration of either mMSCs, hMSCs, or their respective CM or EVs, but not HLFs or their CM or EVs, decreased levels of IL-4, IL-5, and IL-17 with no notable difference observed between effects of cells, CM, or EVs. EDCI treatment of mMSCs and hMSCS had no effect on levels of IL-4 or IL-5 but resulted in less decrease in the AHE-provoked increase in IL-17. In contrast, hMSCs, mMSCs, and their respective CM or EVs promoted an increase in IFN-γ release. Neither EDCI-treated hMSCs nor mMSCs were effective in increasing IFN-γ levels. Neither HLFs nor their CM or EVs had an effect on IFN-γ levels. The results of the different effects of cells, CM, or EVs for all outcome measures are summarized in Table 1.
Figure 6.
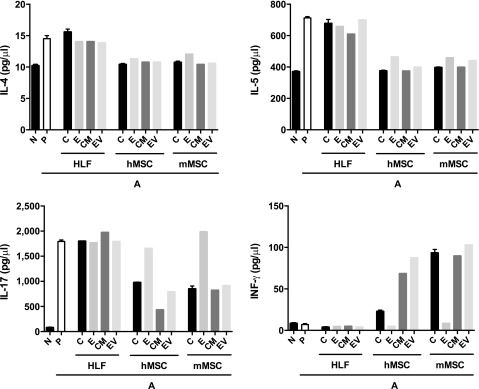
Systemic administration of human or mouse MSCs or their respective conditioned media or extracellular vesicles significantly alters IL-4, IL-5, IL-17, and INF-γ production in ex vivo restimulation of mediastinal lymphocytes. Shown is the assessment of IL-4, IL-5, IL-17, and INF-γ levels in supernatants from pooled mixed mediastinal lymph node cell populations restimulated ex vivo for 48 hours with Aspergillus hyphal-extract antigen (n = 6 for all treatment combinations except the following: 17 N, 15 A-P, 10 A-hMSC-C). Data are presented as mean ± SD. p ≤ .05. Abbreviations: A, Aspergillus hyphal extract-exposed mice; CM, conditioned media; E, EDCI-treated cells; EV, extracellular vesicle; HLF, human lung fibroblast; hMSC, human mesenchymal stromal cell; IL, interleukin; INF, interferon; mMSC, mouse mesenchymal stromal cell; N, naïve mice; P, phosphate-buffered saline.
Table 1.
Summary of similarities and disparities among subgroups of the same cell type
Discussion
The current study demonstrates that systemic administration at the onset of antigen challenge of CM and, in particular, the EV fraction isolated from either cultured human and mouse bone marrow-derived MSCs were effective (in some cases, more effective) than administration of the hMSCs or mMSCs themselves in mitigating allergic airway hyperresponsiveness and lung inflammation, and also altered the phenotype of antigen-specific CD4 T cells in a model of severe, acute, mixed Th2/Th17-mediated eosinophilic and neutrophilic airway allergic inflammation in immunocompetent mice. Blocking release of EVs and soluble proteins removed many of the protective effects of both hMSCs and mMSCs. Neither HLFs nor CM or EVs obtained from HLFs had any effect on lung mechanics and inflammation except on a few isolated BALF cytokine levels.
There is an increasing number of reports in preclinical models of lung and other diseases in which conditioned media obtained from MSCs were as effective as the cells themselves in mitigating specific, model-dependent inflammatory endpoints [6–12]. This suggests that among different postulated mechanisms of MSC actions, soluble mediators and other components released by the MSCs play significant roles. This is further supported by previous findings that blocking release of soluble mediators can significantly abrogate the protective effects of the MSCs in mouse models of Th2 eosinophilic- and Th2/Th17 neutrophilic-mediated allergic airway inflammation [26, 30]. However, this is not a consistent finding for all inflammatory endpoints and suggests that cell-cell contact or other mechanisms may, in fact, play a role in mitigating certain inflammatory pathways. Moreover, mechanisms of MSC actions are likely to differ for specific inflammatory conditions.
While an abundance of available data suggests specific soluble anti-inflammatory or antibacterial peptides as key mediators, alone or in combination, recent focus on the extracellular vesicle fraction released by the MSCs further suggests that mRNAs, miRNAs, mitochondria, and other components of the EVs also play important roles in ameliorating inflammation and injury [14, 18]. In preclinical models of lung diseases, EVs released by MSCs were effective in ameliorating injury in murine models of pulmonary hypertension, as well as acute lung injury in adult mice [15–17]. However, a challenge in this field is to improve and standardize methods for EV isolation, characterization, and subsequent study in experimental systems [37, 38]. Currently, EVs are mostly isolated by differential ultracentrifugation from the supernatants of cultured cells grown in either serum-free media or in media with fetal calf serum depleted of EVs [17, 40]. Characterization of the isolated EVs has been accomplished using a variety of techniques, including biochemical (immunoblotting), mass spectrometry, dynamic light scattering, or by imaging techniques, including transmission electron microscopy [40]. There remains controversy regarding the nomenclature of EVs [38]. Attempts at consensus have suggested that EVs that originate from multivesicular endosomes with a diameter of 30–150 nm be designated exosomes, whereas EVs that bud from the cell surface and are generally larger, with diameters of 100–1,000 nm, be designated microparticles [38, 40–42].
The EVs used in the current study appeared as heterogeneously spheroid bodies by TEM, with diameters ranging from approximately 40 nm to 1,000 nm, consistent with a mix of exosomes and microvesicle particles [38]. Parallel assessments by particle-size tracking analyses demonstrated that the majority of the particles were exosomes, with the majority having a size range of approximately 50–150 nm. At present, we cannot distinguish whether the observed effects in this allergic airway inflammation model of the EVs from MSCs of either human or mouse origin reflected actions of the exosomes, microvesicles, or a combination of both. Future careful study is needed to differentially assess the relative contributions of each. The novel finding that EDCI inhibits EV production from cells will be a valuable tool in attempting to further determine the role of EVs in different lung injury models.
Future careful study is also required to determine which EV contents are responsible for mitigating the observed effects in this model. There are likely multiple EV components (adherence or internal factors) that may contribute to the benefits of this therapy, and these components may differ if obtained from MSCs exposed to other inflammatory microenvironments (e.g., microarray analyses of human MSC EVs, more than 700 unique transcripts for genes involved in cell differentiation, transcription, proliferation, adhesion, migration, and immune regulation) [43, 44]. miRNA encoding Let7 has been implicated as mediating the protective effects of mMSC-derived EVs in a mouse model of hyperoxia exposure [15]. We are assessing the EV fractions from human and mouse MSCs to determine which components are responsible for the protective effects in the AHE model of allergic airway inflammation.
Another notable finding of these studies is that CM or EVs obtained from hMSCs were as, if not more, effective than CM or EVs from syngeneic mMSCs in ameliorating experimentally induced, mixed Th2/Th17 AHR and lung inflammation in an immunocompetent mouse model. A growing number of preclinical studies demonstrate that xenogeneic administration of human MSCs is both feasible and can be effective in mitigating disease-specific endpoints in different preclinical lung disease models in immunocompetent mice [8, 45–49]. There is less information about CM or EVs from human MSCs in immunocompetent preclinical mouse models, but these are also likely to be effective, as demonstrated in the current study. These results further bolster study of xenogeneic hMSC administration in preclinical models of lung diseases in immunocompetent mice and provide a powerful tool with which to investigate the pathways by which the MSCs are exerting protective effects.
There are several specific mechanisms suggested by which the hMSCs or mMSCs or their CM or EVs might be acting in this model. A reduction in AHE-induced increases in soluble Th2 (IL-4 and IL-5) and Th17 (IL-17) cytokines in BALF and in mixed lymphocyte cultures is accompanied by an increase in IFN-γ. Systemic administration of the MSCs or their CM or EVs also resulted in a decrease in IL-12(p40), a key subunit of IL-23 that functions as an autocrine regulator of the Th17 phenotype. This suggests that one mechanism by which systemic administration of either hMSCs or mMSCs or of their respective CM or EVs ameliorates Th2/Th17-mediated allergic airway inflammation is to shift the Th2/Th17 inflammatory response in the lung toward a counter-regulatory Th1 response, as observed in previous studies using murine MSCs [26, 30, 33]. AHE-stimulated increases in BALF levels of the neutrophil chemoattractants KC and RANTES were significantly reduced [30, 50, 51]. Furthermore, higher levels of IL-10, an important anti-inflammatory cytokine, were found after treatment. Additional study is necessary to further elucidate these mechanisms.
Although there were some quantitative differences between the results produced by hMSCs versus mMSCs or by their respective CM or EVs, the effects on inflammation patterns were qualitatively similar. However, blocking release of soluble mediators and of EVs with EDCI more completely abrogated the effects of hMSCs compared with mMSCs, suggesting different potential mechanisms of mMSC versus hMSC actions. These data suggest that hMSCs, mMSCs, and their respective CM or EVs similarly function by suppression of differentiation and/or activation of Th2/Th17 antigen-specific CD4 T cells by either upregulation of counter-regulatory Th1 antigen-specific T cells and/or secretion of soluble anti-inflammatory mediators, including IL-10 [52, 53]. Besides differences in soluble mediators production, we hypothesize that human MSCs may act more through paracrine effects (CM and EV), and that mouse MSCs, besides their paracrine effects, can act through cell-to-cell interactions. Further study is required to identify the specific differences between hMSCs and mMSCs in this regard.
Conclusion
Systemic administration of conditioned media and EVs from both human and mouse bone marrow-derived MSCs is as effective, if not more so, than the cells themselves in mitigating Th2/Th17-mediated airway hyperresponsiveness and lung inflammation in a preclinical model of allergic airway inflammation provoked by mucosal sensitization and challenge with Aspergillus hyphal extract. These results add to the growing number of observations that conditioned media and, in particular, EVs released by the MSCs can convey many of the protective actions of the MSCs. MSC-secreted EVs have been shown to have anti-inflammatory effects in more than one model of lung disease, despite the fact that the underlying inflammation is different between the models. It appears, therefore, that their major immunomodulatory action may be one of restoration of a balance perturbed by disease rather than the suppression of a specific type of inflammation. Importantly, our results also demonstrate effective xenogeneic actions of human MSC-derived conditioned media and EVs in an immunocompetent model of lung disease, and provide further impetus for using immunocompetent mouse models to investigate mechanisms of MSC actions.
Supplementary Material
Acknowledgments
We thank Nirav Daphtary and Minara Aliyeva of the Vermont Lung Center Core facility for assistance with Flexivent technical support; Joseph Platz, Vikas Anathy, and Matthew Poynter, for constructive ideas; and Michele von Turkovich of the UVM Imaging Facility for assistance with the TEM images. This research was supported by NIH ARRA award RC4HL106625; National Heart, Lung, and Blood Institute (NHLBI) Grants R21HL108689 (D.J.W.), NHLBI RO1 HL096702, NHBLI R21HL110023-01, and NHBLI R21HL117090 (C.S.); Environmental Pathology Training Grant T32ES007122 from the National Institute of Environmental Health Sciences; Vermont Lung Center CoBRE Grant P20RR15557; and the Brazilian National Counsel of Technological and Scientific Development (CNPq)-Science Without Borders. Some cells used in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from the National Center for Research Resources of the NIH (Grant P40RR017447). K.T. and A.M.H. received funding from Shipley Foundation.
Author Contributions
F.F.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Z.D.B., M.G., D.S., K.L.R., D.E.W., A.C., and M.A.: collection and/or assembly of data, final approval of manuscript; S.A.M., S.K., P.R.M.R.: conception and design, final approval of manuscript; K.T.: data analysis, final approval of manuscript; A.M.H.: data analysis and interpretation, final approval of manuscript; D.H.M.: provision of study material or patients, manuscript writing, final approval of manuscript; D.J.W.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.A.M. has a compensated patent licensed by United Therapeutics Corp. and uncompensated research funding from the Sponsored Research Administration shared between Boston Children's Hospital and United Therapeutics Corp. D.H.M. receives compensated research funding from a Novartis cord blood expansion trial. D.J.W. receives compensated research funding from Athersys Inc. and from United Therapeutics Corp. to conduct research studies. The other authors indicated no potential conflicts of interest.
References
- 1.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46:207–216. doi: 10.1165/rcmb.2010-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 9.Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Lee JH, Kim HJ, et al. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012;302:L891–L908. doi: 10.1152/ajplung.00288.2011. [DOI] [PubMed] [Google Scholar]
- 11.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goolaerts A, Pellan-Randrianarison N, Larghero J, et al. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L975–L985. doi: 10.1152/ajplung.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliotta JM, Lee D, Puente N, et al. Progenitor/stem cell fate determination: Interactive dynamics of cell cycle and microvesicles. Stem Cells Dev. 2012;21:1627–1638. doi: 10.1089/scd.2011.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HC, Liu XB, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thébaud B, Stewart DJ. Exosomes: Cell garbage can, therapeutic carrier, or Trojan horse? Circulation. 2012;126:2553–2555. doi: 10.1161/CIRCULATIONAHA.112.146738. [DOI] [PubMed] [Google Scholar]
- 19.Sdrimas K, Kourembanas S. MSC microvesicles for the treatment of lung disease: A new paradigm for cell-free therapy. Antioxid Redox Signal. 2014;21:1905–1915. doi: 10.1089/ars.2013.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho KS, Park HK, Park HY, et al. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 21.Cho KS, Roh HJ. Immunomodulatory effects of adipose-derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther. 2010;5:111–115. doi: 10.2174/157488810791268681. [DOI] [PubMed] [Google Scholar]
- 22.Bonfield TL, Koloze M, Lennon DP, et al. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HK, Cho KS, Park HY, et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010;19:1811–1818. doi: 10.1089/scd.2009.0513. [DOI] [PubMed] [Google Scholar]
- 24.Nemeth K, Keane-Myers A, Brown JM, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firinci F, Karaman M, Baran Y, et al. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11:1120–1126. doi: 10.1016/j.intimp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin M, Sueblinvong V, Eisenhauer P, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Jang AS, Kwon JH, et al. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res. 2011;3:205–211. doi: 10.4168/aair.2011.3.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou-Yang HF, Huang Y, Hu XB, et al. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011;236:1461–1467. doi: 10.1258/ebm.2011.011221. [DOI] [PubMed] [Google Scholar]
- 30.Lathrop MJ, Brooks EM, Bonenfant NR, et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Translational Medicine. 2014;3:194–205. doi: 10.5966/sctm.2013-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allard JB, Poynter ME, Marr KA, et al. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 32.Allard JB, Rinaldi L, Wargo MJ, et al. Th2 allergic immune response to inhaled fungal antigens is modulated by TLR-4-independent bacterial products. Eur J Immunol. 2009;39:776–788. doi: 10.1002/eji.200838932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz FF, Borg ZD, Goodwin M, et al. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Translational Medicine. 2015;4:615–624. doi: 10.5966/sctm.2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 35.National Lung, Heart, and Blood Program. About PACT. Available at https://secure.emmes.com/pactweb/Facilities. Accessed November 15, 2013.
- 36.Reed W, Noga SJ, Gee AP, et al. Production Assistance for Cellular Therapies (PACT): Four-year experience from the United States National Heart, Lung, and Blood Institute (NHLBI) contract research program in cell and tissue therapies. Transfusion. 2009;49:786–796. doi: 10.1111/j.1537-2995.2008.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine (Lond) 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: Design and evaluation. IEEE Trans Biomed Eng. 1995;42:860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 40.Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 41.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Booth AM, Fang Y, Fallon JK, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 45.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 46.Pati S, Gerber M, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim ES, Chang YS, Choi SJ, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Han ZB, Liao W, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 49.Lim R, Milton P, Murphy SV, et al. Human mesenchymal stem cells reduce lung injury in immunocompromised mice but not in immunocompetent mice. Respiration. 2013;85:332–341. doi: 10.1159/000343078. [DOI] [PubMed] [Google Scholar]
- 50.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 51.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghannam S, Pène J, Moquet-Torcy G, et al. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 53.Qu X, Liu X, Cheng K, et al. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol. 2012;40:761–770. doi: 10.1016/j.exphem.2012.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.