N-Glycans were isolated from human induced pluripotent stem cells (iPSCs), iPSC-derived cardiomyocytes (iPSC-CMs), and human cardiomyocytes (hCMCs), and analyzed. The exposed N-acetylglucosamine (GlcNAc) and the nonreduced terminal fucose types of N-glycans decreased, whereas the exposed galactose or the α2-3 NeuAc types increased in the iPSCs during cardiomyogenic differentiation. More bisecting GlcNAc and the triantennary structures were found in the iPSC-CMs than hCMCs or iPSCs, and MGAT3 expression was higher in iPSCs and iPSC-CMs than in hCMCs.
Keywords: Induced pluripotent stem cell, Induced pluripotent stem cell-derived cardiomyocyte, N-Glycan
Abstract
Cell-surface glycans vary widely, depending on cell properties. Previously, we reported that the pattern of N-glycan expression on murine induced pluripotent stem cells (iPSCs) changed toward that of the cardiac tissue during cardiomyogenic differentiation. In this study, N-glycans were isolated from human iPSCs, iPSC-derived cardiomyocytes (iPSC-CMs), and human cardiomyocytes (hCMCs). Their structures were analyzed by a mapping technique based on high-performance liquid chromatography elution positions and matrix-assisted laser desorption/ionization time-of-flight mass-spectrometric data. Of 52 isolated N-glycans, the structures of 38 were clearly identified. In addition, 11 structures were partially identified because the binding style and fucose binding site at the nonreduced terminal could not be identified. Quantitation of each type of N-glycan, based on the terminal glycosylation process, revealed that the exposed N-acetylglucosamine (GlcNAc) and the nonreduced terminal fucose types decreased, whereas the exposed galactose or the α2-3 NeuAc types increased in the iPSCs during cardiomyogenic differentiation. However, the bisecting GlcNAc and the triantennary structures were found in relative abundance in the iPSC-CMs in comparison with hCMCs or iPSCs. Expression of MGAT3, a glycosyltransferase-encoding gene that produces the bisecting GlcNAc structures, was higher in iPSCs and iPSC-CMs than in hCMCs. These findings will prove useful in understanding the directional precision of cardiomyogenic differentiation in vitro.
Significance
This study focused on N-glycans produced in human induced pluripotent stem cells (iPSCs) and iPSC-derived cardiomyocytes to investigate their change on cardiomyogenic differentiation in vitro. This shows that the expression pattern of N-glycans in human iPSCs changed toward the pattern observed in human cardiomyocytes upon cardiomyogenic differentiation. Structural differences were also observed in the bisecting N-acetylglucosamine and the triantennary structures upon cardiomyogenic differentiation. The findings of this study will help in understanding the directional precision of cardiomyogenic differentiation in vitro.
Introduction
Cell-surface glycans have several important biological functions [1–3]. Their expression patterns on embryonic stem cells [4] or on induced pluripotent stem cells (iPSCs) [5] undergo changes during cell differentiation. Here, we focused on N-glycans produced by human iPSCs and iPSC-derived cardiomyocytes (iPSC-CMs) to investigate their change on cardiomyogenic differentiation in vitro.
Materials and Methods
Cardiomyogenic Differentiation of iPSCs
Human iPSC lines 201B7, 606A1, and 253G1 were used (gifts from Dr. Shinya Yamanaka, Kyoto University) [6, 7]. Cardiomyogenic differentiation of iPSCs was performed according to Kawamura et al. [8, 9], and beating cell aggregates were collected for further analysis (supplemental online Fig. 1). Human cardiomyocytes (hCMCs) purchased from PromoCell (Heidelberg, Germany, http://www.promocell.com) were used as a control.
Structural and Quantitative Identification of N-Glycans
The structures and quantities of N-glycans in iPSCs, iPSC-CMs, and hCMCs were identified according to Kawamura et al. [5] (supplemental online Fig. 2).
Semiquantitative Polymerase Chain Reaction
Total RNA, extracted using the RNeasy RNA isolation Kit (Qiagen, Venlo, The Netherlands, https://www.qiagen.com), was reverse transcribed using Omniscript reverse transcriptase (Qiagen) and analyzed by real-time polymerase chain reaction (RT-PCR) on the ABI PRISM 7700 thermocycler using SYBR Green dye (Applied Biosystems; Thermo Fisher Scientific, Waltham, MA, http://www.appliedbiosystems.com). The primers for each gene were selected based on previous reports [10–13] (supplemental online Table 4).
Results
Cardiomyocytes Derived From Human iPSCs
Cardiomyogenic differentiation was performed for human iPSCs by a previously reported protocol [8, 9] (supplemental online Fig. 1A); the beating cell aggregates were collected via pipette, to be used as iPSC-CMs. The iPSC-CMs showed significantly higher expression for the cardiac marker genes of CTNT and αMHC, and significantly lower expression for undifferentiated marker genes of OCT3/4, NANOG, and LIN28 than iPSCs, by semiquantitative real-time PCR (supplemental online Fig. 1B). Furthermore, more than 85% of the 253G1-CMs were positive for troponin T (supplemental online Fig. 1C).
Isolation of N-Glycans
N-glycan protein extracted from undifferentiated iPSCs (201B7, 12.78 mg; 606A1, 8.94 mg; 253G1, 11.51 mg); iPSC-CMs (201B7-CM, 7.43 mg; 606A1-CM, 0.76 mg; 253G1-CM, 2.79 mg); and hCMCs (2.08 mg) were separated based on increasing acidity on a diethylaminoethyl column. Peak 1 represented a neutral fraction; peak 2, a monosialyl fraction; peak 3, a di-sialyl fraction; and peak 4, a trisialyl fraction (supplemental online Fig. 2).
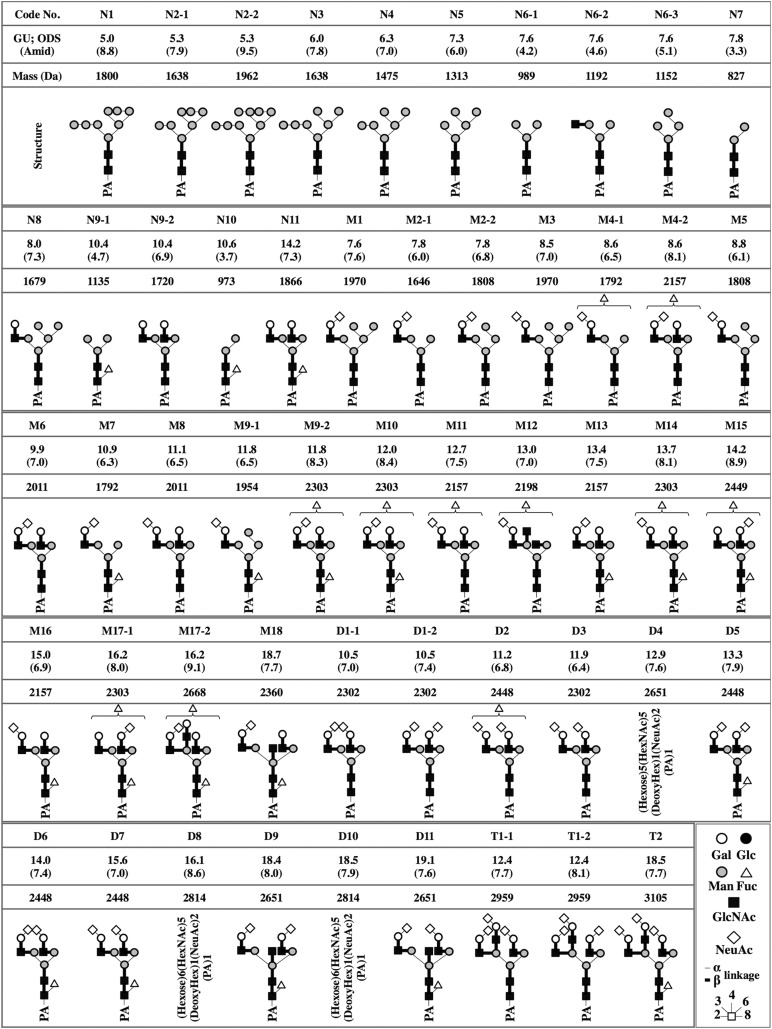
An octadecyl (ODS) column (Shimadzu Corp., Kyoto, Japan, http://www.shimadzu.com) separated peak 1 into fractions N1–N11, peak 2 into fractions M1–M18, peak 3 into fractions D1–D11, and peak 4 into fractions T1 and T2 (supplemental online Figs. 3–7). Upon further fractionation using an amide column (Tosoh Corp., Tokyo, Japan, http://www.tosoh.com), samples were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Fractions N2, N9, M2, M4, M9, M17, D1, and T1 contained 2 types of N-glycans, whereas the N6 fraction contained 3 types of N-glycans (data not shown). In total, 52 different N-glycans were isolated. Their relative quantities are summarized in supplemental online Tables 1–3.
N-Glycan Structures
Structures of 38 of the 52 isolated N-glycans were clearly identified based on coinciding coordinates of known references in the GALAXY database [14]. However, the structures of M4-1, M4-2, M9-2, M10, M11, M12, M14, M15, M17-1, M17-2, and D2, found to contain one or two fucose molecules based on MALDI-TOF-MS, could not be clearly identified. However, structures in which a single fucose could be removed from the nonreduced terminal end were predicted by comparing the glucose units obtained after subtraction of a value corresponding to the nonreduced terminal fucose to reference values in the GALAXY database. Structure of D11 was identified in the GALAXY reference 1A1-211.4 after trimming by a α2-3 sialidase. D4, D8, and D10 could not be identified (Fig. 1).
Figure 1.
Chemical structures of the neutral, monosialyl, disialyl, and trisialyl PA-oligosaccharides in induced pluripotent stem cells (iPSCs), iPSC-derived cardiomyocytes, and human cardiomyocytes. GUs were calculated from the peak elution times on the ODS and the amide column (data not shown). Average mass was calculated from the mlz values of [M+Na]+ or [M+H]+ ions for neutral, [M-H]− ion for monosialyl, and [M-H]− and [M+Na-2H]− ions for the disialyl and trisialyl PA-oligosaccharides. Symbolic representations of the various glycans and their linkages are shown at the lower right. Abbreviations: Fuc, fucose; Gal, galactose; Glc, glucose; GlcNAc, N-acetylglucosamine; GU, glucose unit; Man, mannose; Mass, average mass; NeuAc, N-acetylneuraminic acid; ODS, octadecyl; PA, pyridylamino.
Relative Quantities of N-Glycans in the Biosynthetic Pathway
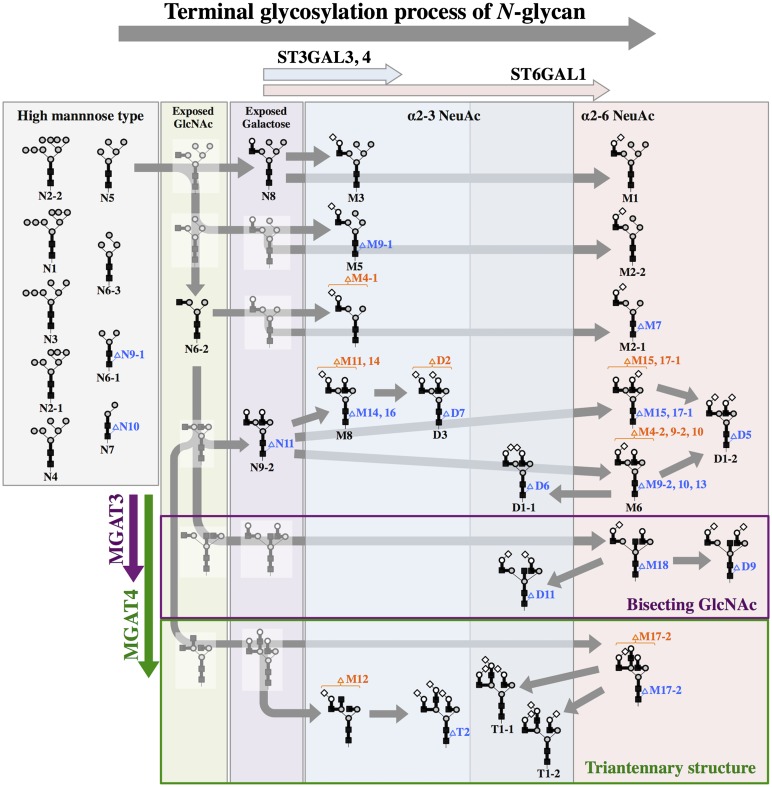
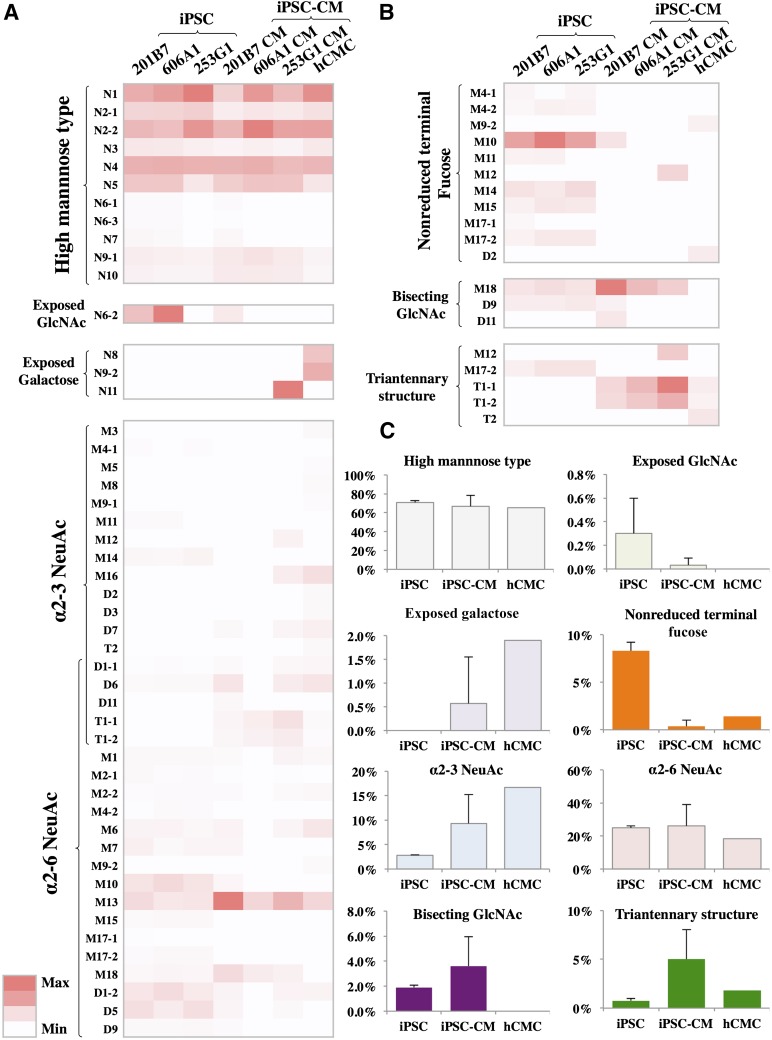
N-Glycan biosynthetic pathways have been studied in detail [15]. Based on terminal glycosylations, the N-glycans were categorized into the high mannose, the exposed N-acetylglucosamine (GlcNAc), the exposed galactose, the α2-3 or the α2-6 NeuAc, and the nonreduced terminal fucose types (Fig. 2). Relative quantities of the high mannose type and the α2-6 NeuAc type did not differ significantly among the three different cells (Fig. 3). In contrast, relative quantities of the exposed GlcNAc type, exposed galactose type, the α2-3 NeuAc type, and the nonreduced terminal fucose type differed in each cell (Fig. 3).
Figure 2.
N-Glycan biosynthetic pathways in induced pluripotent stem cells (iPSCs), iPSC-derived cardiomyocytes, and human cardiomyocytes. The code numbers, symbolic representations, and linkages of N-glycans are defined in Figure 1. The hypothetical pathway map is constructed based on the generally accepted N-glycan biosynthetic pathways [14]. ST3GAL3, ST3GAL4, ST6GAL1, MGAT3, and MGAT4 indicate glycosyltransferases presumed to be functional at those points.
Figure 3.
Relative quantities of N-glycans expressed at each stage of the biosynthetic pathway. The relative quantity of each N-glycan within the different cells was calculated from the area under the peak (supplemental online Figs. 4–7). (A): The high mannose-type glycans (N1-N5, N6-1, N6-3, and N7); the exposed GlcNAc type (N6-2); the exposed galactose type (N8, N9-2, and N11); the α2-3NeuAc type (M3, M5, M8, M9-1, M11, M12, M14, M16, D1-1, D2, D3, D6, D7, D11, T1-1, T1-2, and T2); and the α2-6NeuAc type (M1, M2-1, M2-2, M4-2, M6, M7, M9-2, M10, M13, M15, M17-1, M17-2, M18, D1-1, D1-2, D5, D6, D9, D11, T1-1, and T1-2) are shown on a heat map. (B): The nonreduced terminal fucose type (M4-1, M4-2, M9-2, M10, M11, M12, M14, M15, M17-1, M17-2, and D2); the bisecting GlcNAc type (M18, D9, and D11); and the triantennary structure type (M17-2, T1-1, T1-2, and T2) in each cell are shown on a heat map. The maximum and minimum values were settled for each group of N-glycans. (C): Relative quantities of N-glycans expressed at each stage of the biosynthetic pathway are expressed as mean ± SD of 201B7, 606A1, and 253G1 for iPSC, or 201B7-CM, 606A1-CM, and 253G1-CM for iPSC-CM. Abbreviations: GlcNAC, N-acetylglucosamine; hCMC, human cardiomyocyte; iPSC, induced pluripotent stem cell; iPSC-CM, induced pluripotent stem cell-derived cardiomyocyte; max, maximum; min, minimum.
Structurally Different N-Glycans in the iPSC-CMs
Two relatively abundant and special structures, the bisecting GlcNAc type (M18, D9, and D11) and the triantennary structure type (M12, M17-2, T1-1, T1-2, and T2), were observed in the iPSC-CMs. The former, which was not recognized in the hCMCs, was abundant in the iPSC-CMs (3.6%), and occurred relatively less frequently in the iPSCs (1.9%). The latter type was abundantly expressed in the iPSC-CMs (5.0%) in comparison with iPSCs (0.8%) or hCMCs (1.8%) (Fig. 3C).
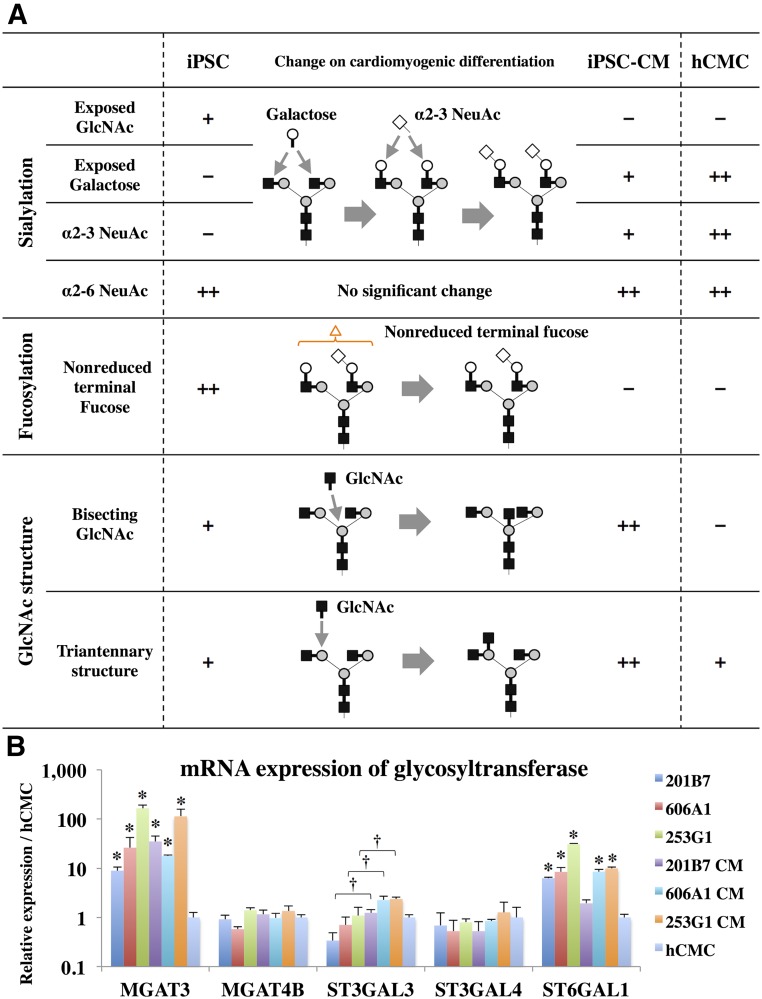
Expression of Glycosyltransferases
The expression of MGAT3, which produces the bisecting GlcNAc structures, was higher in iPSCs and iPSC-CMs compared with that in hCMC. However, the expression of MGAT4B, which produces the triantennary structures, showed similar levels in each cell (Fig. 4B). MGAT4A was not expressed in any of the iPSCs, the iPSC-CMs, or hCMCs (data not shown).
Figure 4.
Structural change of N-glycan in human iPSCs during cardiomyogenic differentiation. (A): Structural change of N-glycan in human iPSCs during cardiomyogenic differentiation about sialylation, fucosylation, and GlcNAc structure are indicated. Symbolic representations of the glycans and their linkages are shown in Figure 1. (B): The mRNA expression of MGAT3, MGAT4B, ST3GAL3, ST3GAL4, and ST6GAL1 in each cell type was measured by real-time polymerase chain reaction. Results are expressed as the mean ± SD. ∗, p < .05 versus hCMCs, †, statistical significance between iPSC and iPSC-CM, p < .05. All experiments were performed in three independent triplicates by the relative standard curve method. Statistical comparison of the data were performed by the Student’s t test. Abbreviations: GlcNAC, N-acetylglucosamine; hCMC, human cardiomyocyte; iPSC, induced pluripotent stem cell; iPSC-CM, induced pluripotent stem cell-derived cardiomyocyte.
The expression of ST3GAL3, which produces the α2-3NeuAc structures, was significantly increased in the iPSC-CMs compared with the iPSCs in each cell line (Fig. 4B). With the exception of 201B7-CM, the expression of ST6GAL1, responsible for the production of α2-6NeuAc in the iPSCs and the iPSC-CMs, was significantly higher compared with hCMCs (Fig. 4B).
Discussion
We reported previously about the changes in N-glycan phenotypes toward cardiac tissue by cardiomyogenic differentiation within iPSCs, using a murine model [5]. The high mannose and the exposed GlcNAc types decreased, whereas the exposed galactose and the sialylated types increased during cardiomyogenic differentiation. Furthermore, this study revealed that the expression pattern of terminally glycosylated N-glycans in human iPSCs changed toward the pattern of hCMCs upon cardiomyogenic differentiation, especially about the terminal sialylation and fucosylation.
The exposed GlcNAc type in the iPSCs was decreased through the process of terminal sialylation, and, following increase of the exposed galactose type and the α2-3 NeuAc type, was observed in the iPSC-CMs (Figs. 3C, 4A). The increase of the α2-3 NeuAc type on cardiomyogenic differentiation may be related to the higher expression of ST3GAL3 in the iPSC-CMs as compared with the iPSCs of each cell line (Fig. 4B).
The nonreduced terminal fucose, whose accurate position could not be identified in this study, was relatively abundant in the iPSCs and markedly rare in the iPSC-CMs and hCMCs. This finding is supported by a previous report in which 1,3-fucosyltransferase was required for the maintenance of a stem cell population [16].
Although the Galα1-6Gal structures observed in the murine iPSC-CMs [5] were not present in each human cell, several structural differences in N-glycans expressed in iPSC-CMs and hCMCs, were observed (Figs. 3, 4A). Specifically, the bisecting GlcNAc structures, mediated by MGAT3, were observed in iPSCs or iPSC-CMs, but not in hCMCs. This difference was confirmed from the expression level of MGAT3: the iPSCs or iPSC-CMs were expressed at 10- to 50-fold higher levels of MGAT3 than in hCMCs (Fig. 4B). The expression of MGAT3 is restricted to a few organs, such as the brain, testis, and adrenal gland cells [17]. Therefore, the presence of bisecting GlcNAcs and MGAT3 enzyme activity may be related to erroneous direction of cardiomyogenic differentiation. Although the level of MGAT4B expression was similar in each cell, the triantennary structures, mediated by MGAT4, were abundant in iPSC-CMs as compared with iPSCs or hCMCs.
Conclusion
The in vitro expression pattern of N-glycans in human iPSCs changed toward that of hCMCs upon cardiomyogenic differentiation, especially about the terminal sialylation and fucosylation. Important changes in structural differences of the bisecting GlcNAc and the triantennary structures were observed. Our findings will help in understanding the directional precision of cardiomyogenic differentiation in vitro.
Supplementary Material
Acknowledgments
We thank Drs. Shinya Yamanaka, Keisuke Okita, and Yoshinori Yoshida of the Center for iPS Cell Research and Application, Kyoto University, for kindly providing the human iPSCs. This study was supported by the Japan Science and Technology Agency as a part of the project “Center for the Development of Myocardial Regenerative Treatments Using iPS Cells.”
Author Contributions
T.K.: provision of study materials, collection and/or assembly of data, and manuscript writing; Shigeru Miyagawa: financial support, data analysis and interpretation; S.F., N.K., A.K., A.S., A.M., H.E., K.T., and H.O.: data analysis and interpretation; E.I.: provision of study materials; Shuji Miyagawa: conception and design, collection and/or assembly of data, and manuscript writing; Y.S.: conception and design of study, and financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Amano M, Yamaguchi M, Takegawa Y, et al. Threshold in stage-specific embryonic glycotypes uncovered by a full portrait of dynamic N-glycan expression during cell differentiation. Mol Cell Proteomics. 2010;9:523–537. doi: 10.1074/mcp.M900559-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura T, Miyagawa S, Fukushima S, et al. N-Glycans: Phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9:e111064. doi: 10.1371/journal.pone.0111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki T, Futaki S, Suemori H, et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(suppl 1):S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura M, Miyagawa S, Fukushima S, et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013;128(suppl 1):S87–S94. doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- 10.Vagin O, Tokhtaeva E, Yakubov I, et al. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na,K-ATPase beta1 subunit. J Biol Chem. 2008;283:2192–2202. doi: 10.1074/jbc.M704713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anugraham M, Jacob F, Nixdorf S, et al. Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: Glycan structures reflect gene expression and DNA methylation status. Mol Cell Proteomics. 2014;13:2213–2232. doi: 10.1074/mcp.M113.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tano K, Yasuda S, Kuroda T, et al. A novel in vitro method for detecting undifferentiated human pluripotent stem cells as impurities in cell therapy products using a highly efficient culture system. PLoS One. 2014;9:e110496. doi: 10.1371/journal.pone.0110496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu TY, Lin B, Kim J, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, Kato K. GALAXY (glycoanalysis by the three axes of MS and chromatography): A web application that assists structural analyses of N-glycans. Trends Glycosci Glycotechnol. 2003;15:235–251. [Google Scholar]
- 15.Stanley P, Schachter H, Taniguchi N. N-Glycans. In: Varki A, Cummings RD, Esko JD et al., eds. Essentials of Glycobiology, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009 [Google Scholar]
- 16.Kumar A, Torii T, Ishino Y, et al. The Lewis X-related α1,3-fucosyltransferase, Fut10, is required for the maintenance of stem cell populations. J Biol Chem. 2013;288:28859–28868. doi: 10.1074/jbc.M113.469403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagawa S, Murakami H, Takahagi Y, et al. Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. J Biol Chem. 2001;276:39310–39319. doi: 10.1074/jbc.M104359200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.