The therapeutic effects of ultrasound-guided human umbilical cord blood (UBC)-derived mesenchymal stem cell (MSC) injection to regenerate a full-thickness subscapularis tendon tear in a rabbit model were studied. Gross morphology and histology of the injected tendon were evaluated, and improvement in functional ability was assessed by motion analysis. Histology revealed that UCB-derived MSCs induced regeneration of the rotator cuff tendon; motion analysis showed improved walking capacity.
Keywords: Tendon, Rotator cuff, Mesenchymal stem cells, Ultrasound, Injection
Abstract
Rotator cuff tendon tear is one of the most common causes of chronic shoulder pain and disability. In this study, we investigated the therapeutic effects of ultrasound-guided human umbilical cord blood (UCB)-derived mesenchymal stem cell (MSC) injection to regenerate a full-thickness subscapularis tendon tear in a rabbit model by evaluating the gross morphology and histology of the injected tendon and motion analysis of the rabbit’s activity. At 4 weeks after ultrasound-guided UCB-derived MSC injection, 7 of the 10 full-thickness subscapularis tendon tears were only partial-thickness tears, and 3 remained full-thickness tendon tears. The tendon tear size and walking capacity at 4 weeks after UCB-derived MSC injection under ultrasound guidance were significantly improved compared with the same parameters immediately after tendon tear. UCB-derived MSC injection under ultrasound guidance without surgical repair or bioscaffold resulted in the partial healing of full-thickness rotator cuff tendon tears in a rabbit model. Histology revealed that UCB-derived MSCs induced regeneration of rotator cuff tendon tear and that the regenerated tissue was predominantly composed of type I collagens. In this study, ultrasound-guided injection of human UCB-derived MSCs contributed to regeneration of the full-thickness rotator cuff tendon tear without surgical repair. The results demonstrate the effectiveness of local injection of MSCs into the rotator cuff tendon.
Significance
The results of this study suggest that ultrasound-guided umbilical cord blood-derived mesenchymal stem cell injection may be a useful conservative treatment for full-thickness rotator cuff tendon tear repair.
Introduction
Rotator cuff tendon tear is one of the most common causes of chronic shoulder pain and disability. The incidence of the full-thickness rotator cuff tendon tear ranges from 5% to 40% [1]. Chronic tendon degeneration is currently considered the primary contributing factor in the pathogenesis of rotator cuff tendon tear [2]. Treatment for rotator cuff tendon tear is divided into conservative therapy (exercise, electrotherapy, acupuncture, manual therapy, taping, and steroid and platelet-rich plasma [PRP] injections) and surgery [3]. The surgical repair of a tendon tear is one of the most common orthopedic procedures [4]. However, the failure rates for rotator cuff tendon repair ranged from 20% to 90% [5] and success of the procedure depends on the patient’s age, tear size, chronicity, muscle atrophy and fatty degeneration, tendon quality, operative technique, and postoperative rehabilitation treatment [6]. Revision rotator cuff surgery has been shown to have a less successful outcome than primary repair [7].
Since current therapeutic approaches do not lead to physiological restoration of the rotator cuff tendon tear and the quality at the distal end of the rotator cuff tendon tear is suboptimal [8], a new biological treatment strategy is needed to regenerate the torn tendon and reduce scar formation. The new treatment should create an optimized environment for the restoration of the torn tendon. Mesenchymal stem cells (MSCs) have been shown to differentiate into various cell types, such as chondrocytes, neural cells, and endothelial cells, both in vivo and in vitro [9–13], and they are extracted from various sources, including bone marrow, adipose tissue, and umbilical cord blood (UCB). UCB is a promising source of human MSCs because of its easy availability, high proliferation capacity, and low immunogenicity [10, 13, 14].
A previous study showed that surgically implanting tissue-engineered MSC-collagen composites significantly improves the biomechanical properties of tendon repair tissues in full thickness, full length, central defects created in the patellar tendons of the animals providing the cells [15]. In cases of the rotator cuff tear, it has been shown that repairing a full-thickness infraspinatus tendon tear using a bioabsorbable material with bone marrow-derived MSCs can regenerate tendon-bone insertion and the tendon belly, and increase the mechanical strength of the regenerated tendon in a rabbit model [4]. In patients with rotator cuff tendon tear, ultrasound and magnetic resonance imaging have similar accuracy in the diagnosis of rotator cuff tear [16].
In a previous study of ultrasound-guided platelet-rich plasma (PRP) injection in patients with rotator cuff disease, the authors precisely localized the rotator cuff lesion using ultrasound and successfully performed the PRP injection [3]. In this study, we investigated the therapeutic effects of ultrasound-guided human UCB-derived MSC injection to regenerate a full-thickness subscapularis tendon tear in a rabbit model. We evaluated the gross morphology and histology of the injected tendon and motion analysis was used to assess improvement in functional ability of the rabbit’s activity.
Materials and Methods
Animal Model
Twelve-week-old male New Zealand white rabbits (n = 30) were housed in separate metal cages at 23°C ± 2°C and a relative humidity of 45°C ± 10%. They had free access to tap water and were fed a commercial rabbit diet. None of the rabbits received additional exercise; they were allowed to do normal activities in a 65 × 45 × 30 cm cage. Animal experiments were performed in accordance with internationally accredited guidelines and approved by the Catholic University of Daegu School of Medicine Animal Care and Use Committee.
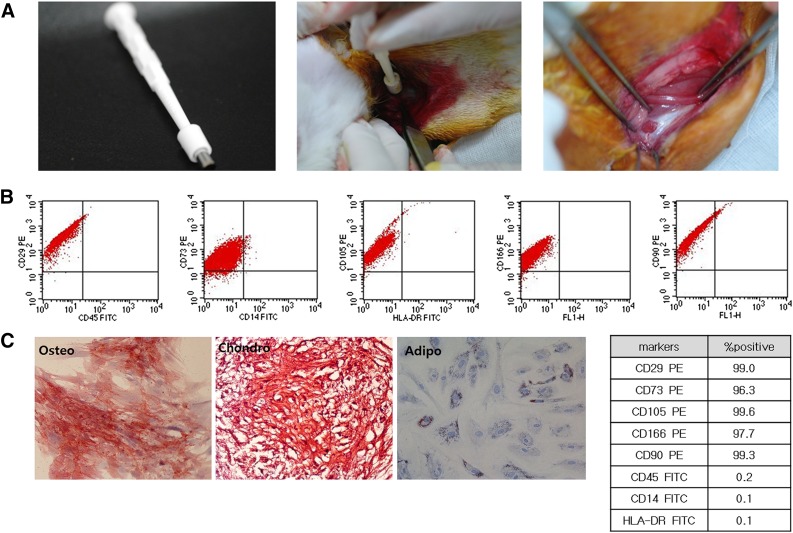
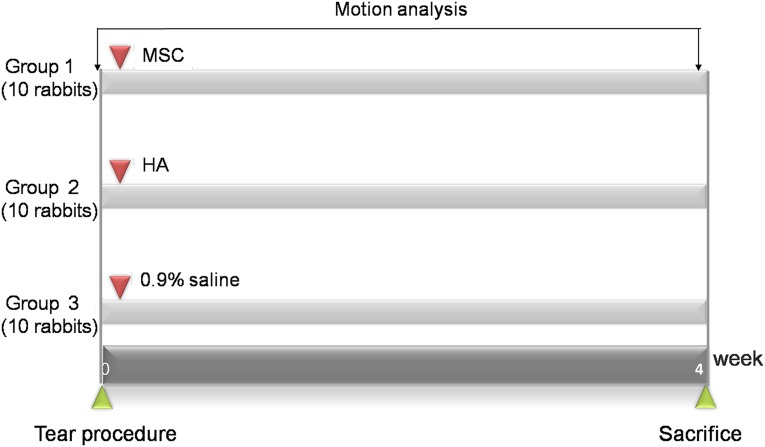
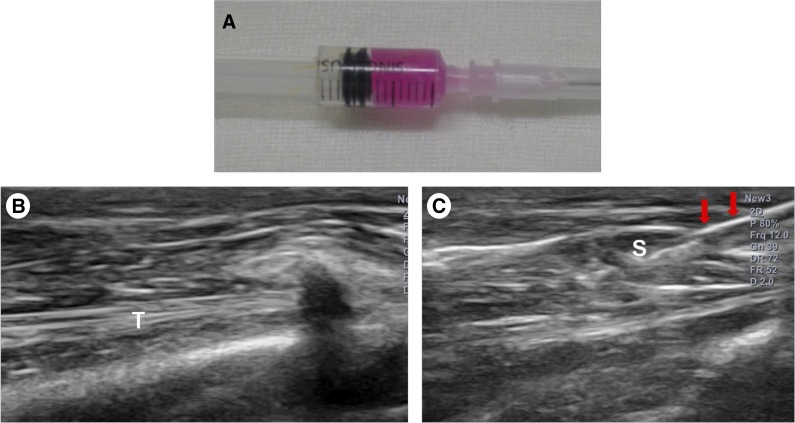
Anesthesia was induced using isoflurane (JW Pharmaceutical, Goyang, Korea, http://www.cwp.co.kr/pharma/en/intro/pharma.jsp) vaporized in oxygen and delivered via a large animal cycling system. Under general anesthesia, a 5-mm diameter, full-thickness rotator cuff tear on the insertion site of left subscapularis tendon was created by punch biopsy excision using a 5-mm biopsy punch (SFM Medical Devices, Wächtersbach, Germany, http://sfm.de) (Fig. 1A). After the excision was made, the wound was immediately closed by subcutaneous and skin sutures. The 30 rabbits were randomly allocated into 3 different treatment groups with 10 rabbits in each group. After the site of the full-thickness subscapularis tendon tear was confirmed via ultrasound, group 1 was injected with 0.1 ml of human UCB-derived MSCs (Cartistem; Medipost Co. Ltd., Seoul, Korea, http://www.medi-post.com), group 2 was injected with 0.1 ml of hyaluronic acid (Hyruan-plus; LG Life Science, Daejeon, Korea, www.lgls.com), and group 3 was injected with 0.1 ml of normal saline (Fig. 2). All injections were performed under ultrasound guidance by a physiatrist with a commercially available ultrasound system with a 3–12 MHz multifrequency linear transducer (E-CUBE 15; Alpinion Medical Systems, Seoul, Korea, http://www.alpinion.com) (Fig. 3B, 3C). No medication was administered and all rabbits underwent immobilization in equinus position, using an elastic bandage, for 2 days after the injection.
Figure 1.
Creation of the rotator cuff tear and mesenchymal stem cell (MSC) differentiation. (A): Three panels show the techniques and equipment required to create a 5-mm diameter full-thickness rotator cuff tear by punch biopsy excision. (B): Characterization of flow cytometry. (C): Differentiation data of the MSCs. Osteo: 2 weeks, alkaline phosphatase; magnification, ×200. Chondro: 4 weeks, Safranin O; magnification, ×200. Adipo: 4 weeks, Oil Red O; magnification, ×200. Abbreviations: adipo, adipocyte; chondro, chondrycyte; FITC, fluorescein isothiocyanate; HLA, human leukocyte antigen; osteo, osteocyte.
Figure 2.
Time line of saline, HA, and MSC injection. MSCs (0.1 ml; group 1), HA (0.1 ml; group 2), and saline (0.1 ml; group 3) were injected under ultrasound guidance into the left shoulder subscapularis full-thickness tears immediately after the tears were created. The motion analysis was performed before the creation of tendon tear and 4 weeks after injection of 3 different solutions. All rabbits were euthanized by carbon monoxide inhalation at 4 weeks after injection of 3 different solutions. Abbreviations: HA, hyaluronic acid, MSC, human umbilical cord blood-derived mesenchymal stem cell.
Figure 3.
Human umbilical cord blood-derived mesenchymal stem cell (MSC) and ultrasound images. (A): Human umbilical cord blood-derived MSCs. (B): Injection was made in the left shoulder subscapularis (SCC) full-thickness tears under ultrasound guidance. (C): Longitudinal ultrasound image showed the needle (arrows) in the left shoulder SCC of the rabbit. Abbreviations: S, mesenchymal stem cell; T, tendon.
Mesenchymal Stem Cell Preparation
UCB was collected from the umbilical vein after neonatal delivery with informed consent from pregnant mothers, and MSCs were isolated and cultivated from human UCB [2, 17]. The cells expressed CD105 PE (99.6%), CD90 PE (99.3%), CD29 PE (99.0%), CD166 PE (97.7%), CD73 PE (96.3%), CD45 fluorescein isothiocyanate (FITC) (0.2%), CD14 FITC (0.1%), and human leukocyte antigen (HLA)-DR FITC (0.1%) (Fig. 1B, 1C). The cells also expressed pluripotent markers such as octamer-binding transcription factor 4 (30.5%) and stage-specific embryonic antigen 4 (SSEA-4; 67.7%). Human UCB-derived MSCs differentiated into various cell types, such as respiratory epithelium, osteoblasts, chondrocytes, and adipocytes, with specific in vitro induction stimuli (Fig. 1C) [13, 14, 18]. We confirmed the differentiation potential and karyotypic stability of the human UCB-derived MSCs up to the 11th passage. The human UCB-derived MSCs were mixed with viscous hyaluronic acid (HA) (Fig. 3A)
Gross Morphology and Histological Examination
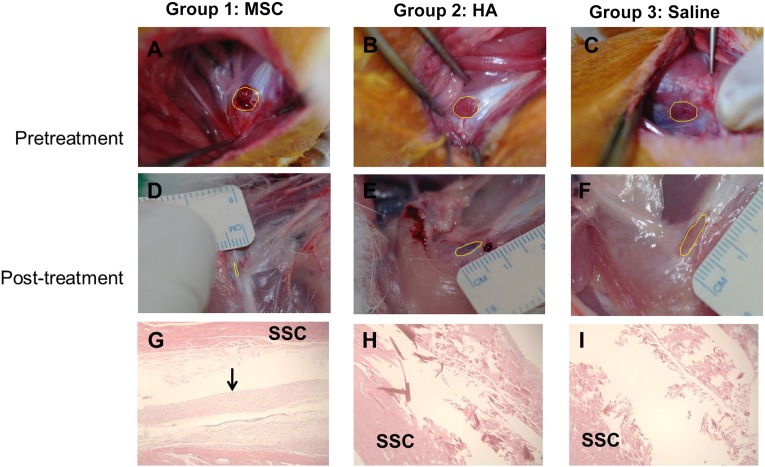
Gross morphologic examinations were conducted at prior injection and when all 30 rabbits were euthanized 4 weeks postinjection. The severity of the tendon tear was classified as a partial- or full-thickness tear. The surface area of subscapularis tendon tear was photographed with a clear plastic ruler near the center of the tear site and its size was calculated using image J software (National Institutes of Health, Bethesda, Maryland, http://imagej.nih.gov/ij) by tracing the outlined tear edge at 4 weeks postinjection (Fig. 4A–4C). The tendon’s injection site was removed and fixed with 10% neutral buffered formalin. Standard frontal sections of 4 μm were prepared and stained with hematoxylin and eosin, Masson’s trichrome, and immunohistochemical staining of type I collagen. Histological changes were analyzed by light microscopy.
Figure 4.
Gross morphological (A–F) and histological (G–I) findings of the subscapularis tendons in groups 1, 2, and 3. The polygon in each of the first six images depicts the area of the full-thickness subscapularis tendon tear. (A–C): Pretreatment images. (D–F): Posttreatment images. (G): Parallel arrangement of hypercellular fibroblastic bundles (arrow) was noted in group 1. (H, I): Histological findings in groups 2 and 3 showed absence of fiber bundles. Group 1 received a 0.1-ml injection of MSCs; group 2, 0.1 ml of HA; group 3, 0.1 ml of saline. Hematoxylin-and-eosin stain, ×40. Abbreviations: MSC, human umbilical cord blood-derived mesenchymal stem cell; HA, hyaluronic acid; SSC, subscapularis muscle.
Immunohistochemical studies were conducted using mouse anti-collagen 1 monoclonal antibody (Abcam, Cambridge, U.K., http://www.abcam.com). The paraffin-embedded sections among three groups were cleared, dehydrated, and washed with phosphate-buffered saline (PBS). Endogenous peroxidases were inhibited by preincubation in 0.3% hydrogen peroxide in PBS for 30 minutes, and nonspecific protein binding was blocked in PBS containing 10% normal goat serum (Vector Laboratories, Burlingame, CA, http://vectorlabs.com) for 30 minutes. The sections were incubated in primary antibodies (1:50) for 3 hours at room temperature and washed three times with PBS. The secondary antibody (1:100), biotinylated anti-mouse immunoglobulin G (Vector Laboratories) was placed on the sections for 1 hour at room temperature and washed 3 times with PBS. Then, avidin-biotin-peroxidase complex (Vector Laboratories) was placed on the sections for 1 hour and washed 3 times with PBS, followed by a peroxidase reaction using 0.05 mol/l Tris hydrochloride (pH 7.6) containing 0.01% hydrogen peroxide and 0.05% 3,3′-diaminobenzidine (Sigma Aldrich, St. Louis, MO, https://www.sigmaaldrich.com). The sections were counterstained with hematoxylin and then mounted.
The slides were investigated with the Axiophot photomicroscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com) and AxioCam MRc5 (Carl Zeiss). Each slide was evaluated according to the intensity of positive immunostaining, which was graded as −, +, ++, or +++, denoting negative, slightly positive, moderately positive, and strongly positive staining, respectively [19]. We tested the fate of the human UCB-derived MSCs. We used a HLA ABC stain to track human UCB-derived MSCs.
Motion Analysis
Motion analysis of the rabbits was conducted at preinjection and 4 weeks postinjection. Rabbits were habituated for 30 minutes to the open field before motion analysis was performed. They were placed on a 3 × 3 m arena and allowed to freely explore the field for 5 minutes. Their movements were individually assessed using a video-tracking system equipped with a camera (Smart; Panlab, Barcelona, Spain, http://www.panlab.com) that records the rabbit’s horizontal activity. We measured walking distance, fast-walking time, and mean walking speed for 5 minutes.
Statistical Analyses
Statistical analyses were performed using the SPSS V. 19.0 software (IBM Corp., Armonk, NY, http://www-01.ibm.com). The Kruskal-Wallis test was used to compare the sizes of the subscapularis tendon tear and motion analysis data among the three groups. The Wilcoxon signed-rank test was used to assess the differences in motion analysis data pre- and postinjection within each group. A p value less than .05 was considered statistically significant.
Results
At 4 weeks postinjection, a partial-thickness subscapularis tendon tear was observed in 7 rabbits (70%) and full-thickness tendon tear in 3 rabbits (30%) in group 1. In groups 2 and 3, a full-thickness tendon tear was observed in all 10 rabbits.
The mean tendon tear size of groups 1, 2, and 3 at 4 weeks postinjection was 2.94 ± 0.18 mm2, 12.95 ± 0.52 mm2, and 14.33 ± 0.33 mm2, respectively. In all three groups, the tendon tear sizes at postinjection were decreased compared with those at preinjection. The mean tendon tear size in group 1 was significantly smaller than those in groups 2 and 3 (p < .05) (Fig. 4D–4F). There was no significant difference of tendon tear size between groups 2 and 3.
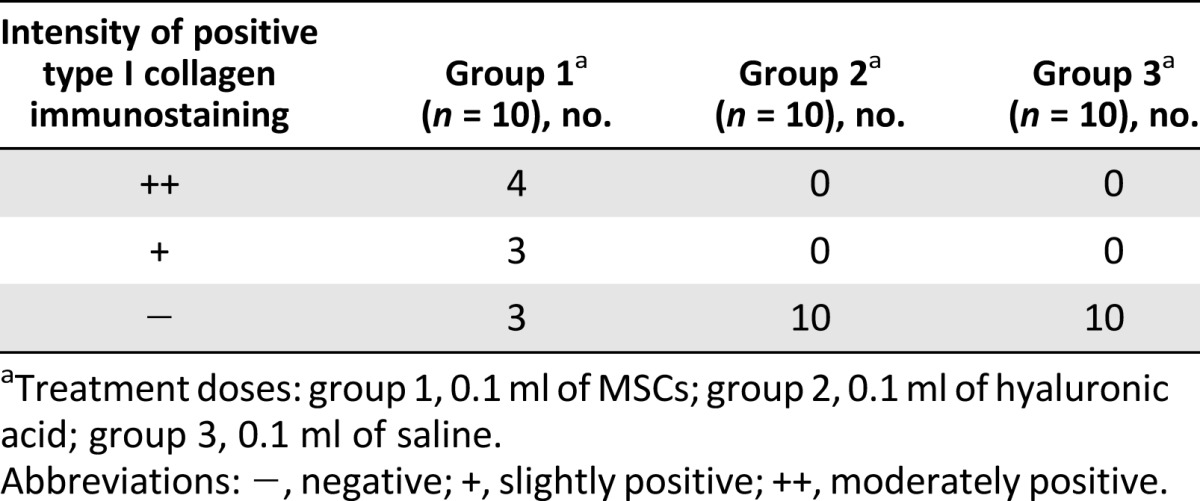
By hematoxylin and eosin staining, parallel arrangement of hypercellular fibroblastic bundles was observed in group 1 (Fig. 4G) but not in groups 2 and 3 (Fig. 4H, 4I). As seen with Masson’s trichrome staining, regenerated collagen fibers were connected to adjacent muscle fibers and stained with anti-type 1 collagen antibody in group 1 (Fig. 5A–5C). The intensity of positive type I collagen immunostaining in group 1 was classified as follows: moderate expression in 4, slight in 3, and negative in 3 rabbits at 4 weeks postinjection. Negative expression was found in all 10 rabbits of groups 2 and 3 (Table 1). The analysis of cell rejection was performed in the group of rabbits in which cells were implanted. The microscopic assessment of the tissue around the implanted cells revealed no evidence of immune rejection, such as vasculitis and lymphocyte infiltration. Therefore, we found no evidence of cell rejection reaction in the specimens from the implanted-cells group. The cell tracking experiment with the HLA ABC stain showed that human UCB-derived MSCs injected at the tendon tear site were detected in the implanted-cells group at 4 weeks after injection.
Figure 5.
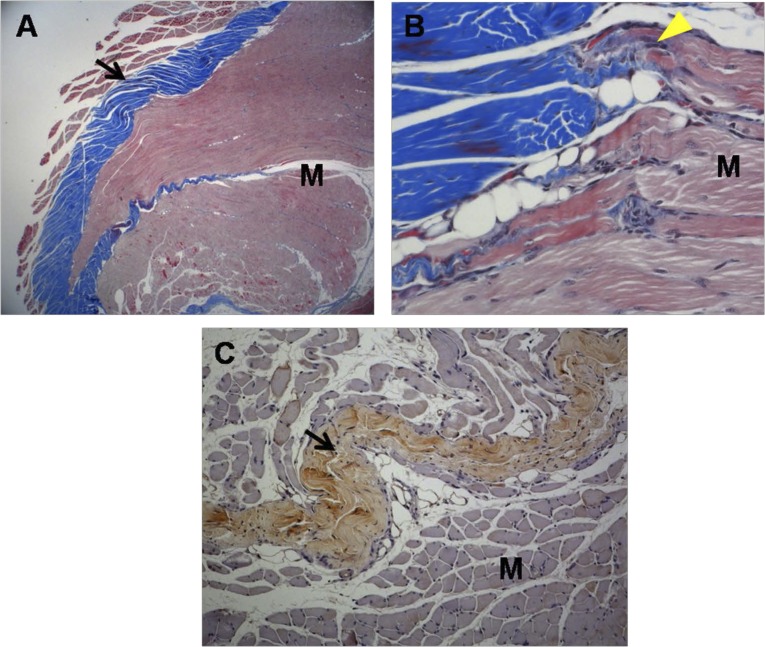
Histological micrographs of tissue from group 1 rabbits. (A): Newly regenerated tendons are shown in the blue-stained fibers (black arrow; Masson’s trichrome stain; magnification, ×12.5). (B): Regenerated tendon fibers (yellow arrowhead; Masson’s trichrome stain; magnification, ×250) are connected to adjacent M fibers. (C): The regenerated tendon fibers (black arrow) stained with anti-type 1 collagen antibody. The defect was reconstructed with human umbilical cord blood-derived mesenchymal stem cells (magnification, ×100). Abbreviation: M, muscle.
Table 1.
Immunoreactivity of type I collagen according to treatment groups at 4 weeks after injection
There was no significant difference in the walking distance, the fast-walking time, and the mean walking speed among the three groups at preinjection. The mean walking distances in groups 1, 2, and 3 at 4 weeks postinjection were 7,088.65 ± 2,202.08 cm, 4,149.79 ± 985.75 cm, and 4,255.01 ± 955.09 cm, respectively. The mean fast-walking times for groups 1, 2, and 3 were 12.57% ± 7.04%, 5.70% ± 1.55% and 5.51% ± 1.55%, respectively. The mean walking speeds for groups 1, 2, and 3 were 10.82 ± 4.38 cm/second, 5.89 ± 1.26 cm/second, and 5.88 ± 1.32 cm/second, respectively (Fig. 6A–6C). The mean walking distances at 4 weeks postinjection in all 3 groups were significantly longer than those at preinjection. In group 1, the fast-walking time and mean walking speed at postinjection were faster and higher than at preinjection. However, there was no significant difference in the fast-walking time and the mean walking speed between preinjection and postinjection in groups 2 and 3. The walking distance, fast-walking time, and mean walking speed at 4 weeks postinjection were significantly longer, faster, and higher in group 1 compared with those in groups 2 and 3 (Fig. 6A–6C). No adverse effects were observed after the injections.
Figure 6.
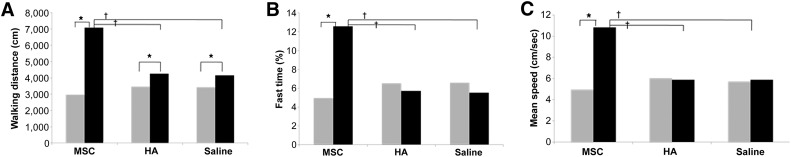
Graphs comparing walking distance (A), fast-walking time (B), and mean walking speed (C) among three groups. ∗, p < .05 by Wilcoxon signed-rank test; †, p < .05 by Kruskal-Wallis test among 3 groups. Gray bars, preinjection; black bars, 4 weeks postinjection. Abbreviations: HA, hyaluronic acid; MSC, human umbilical cord blood-derived mesenchymal stem cell.
Discussion
At 4 weeks after ultrasound-guided, UCB-derived MSC injection, 7 of the 10 full-thickness subscapularis tendon tears were only partial-thickness tears and 3 remained full-thickness tendon tears. However, the full-thickness tendon tear was unchanged in all 10 rabbits after HA and normal saline injection. The 5-mm-diameter tendon tear size before injection decreased after 3 kinds of injections, but the tendon tear size after MSC injection was significantly smaller than those after HA and normal saline injection.
Histological analysis revealed that the regenerated tissues were predominantly composed of type I collagen, which is the main component of extracellular matrices in tendons that connect to adjacent muscle fibers. Motion analysis revealed the locomotor function of rabbits after UCB-derived MSC injection was better compared with rabbits injected with HA and normal saline. This is the first report to demonstrate that ultrasound-guided, human UCB-derived MSC injection can induce regeneration of the full-thickness rotator cuff tendon tear and functional improvement without surgical repair in rabbit models.
Rotator cuff tendon tear is a common cause of shoulder pain and morbidity. Previous studies have shown that a surgically repaired rotator cuff tendon has a persistent defect at the repair site in 11%–94% of cases [20, 21]. Poor blood supply, intrinsic tendon degeneration, muscle atrophy, and fatty infiltration can contribute to tendon failure after surgical repair. Therefore, new biological treatments such as growth factors and cytokines, gene therapy, and tissue engineering with stem cells are required to enhance tendon healing [22]. Although the efficacy of these biological agents has yet to be proven, they are promising and might increase the chances for a successful outcome in treatment of tendon pathology. A recent animal study has demonstrated that growth factors and cytokines might have regenerative effects in rotator cuff tendon healing [23]. However, further studies are necessary to delineate the best method for their use in terms of time, administration method, and dosage.
The main cause for tendon failure after surgical repair is immature fibrovascular scar formation that contains a high amount of type III collagens. Type III collagen is mechanically weaker than type I collagen, the main component of extracellular matrices in tendons [24, 25]. In the maturation and remodeling stage of the tendon healing process, which is characterized by scar tissue maturation, the immature type III collagen is replaced by type I collagen with the formation of dense connective tissue. In our study, the hypercellular fibroblastic bundles composed of type I collagens were observed in the regenerated tendon at 4 weeks after human UCB-derived MSC injection. This means that the MSC injection promoted the normal tendon healing process in the repair of full-thickness tendon tears.
Our study revealed that human UCB-derived MSCs injected at the tendon tear site were detected in the implanted-cells group at 4 weeks after injection. The precise mechanism by which UCB-derived MSCs enhance the tendon regeneration is unclear. It is suggested that tendon regeneration might be attributable to the one of two roles of MSCs. MSCs may work through either differentiation into cells capable of synthesizing tendon matrix or secretion of factors that induce adjacent cells to synthesize tendon matrix [26, 27]. The study by Kryger et al. [28] reported that MSCs survived well on decellularized tendon matrices, and that equine MSCs, cultured on fresh acellular equine tendon sections, not only survived, proliferated, and invaded the matrix but also upregulated the glycoprotein cartilage oligomeric matrix protein gene expression, which has a restricted distribution in tendon primarily designed to withstand load. In addition, the other in vitro study showed that long-term contact with MSCs for 3 weeks changed the morphology of implanted cells and resulted in many similarities between tenocytes and MSCs lined up with the collagen fascicles [29]. Therefore, we suggest that even a small number of surviving MSCs can have the therapeutic effects of tendon regeneration primarily through direct tissue repair rather than secretion of key bioactive mediators.
After MSC injection, 7 of the 10 full-thickness subscapularis tendon tears turned into partial-thickness tears, but 3 full-thickness tendon tears remained unchanged. This partial regeneration might be caused by an insufficient therapeutic effect due to low injection volume of UCB-derived MSCs, short-term follow-up period, large tear size, or short-term immobilization after injection.
In our study, human UCB-derived MSCs were used for tendon repair in rabbits. UCB-derived MSCs are known to be less immunogenic because they have low expression levels of HLA-major histocompatibility complex (MHC) class I and lack of MHC class II molecules that induce the immune evasion in the allogenic transplantation [30]. In addition, the recent study using human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model showed that no evidence of immune rejection [31]. This finding is in accordance with our finding.
We performed the motion analysis of rabbits instead of the mechanical properties of a regenerated tendon after the injection. Motion analysis assesses the activity of rabbits to determine whether UCB-derived MSC injection had a better functional effect on the healing of full-thickness rotator cuff tendon tears compared with HA or normal saline injection. The walking distance, fast-walking time, and the mean walking speed in the UCB-derived MSC injection group were significantly farther, faster, and greater than those in HA and normal saline injection groups at 4 weeks postinjection. These results suggest that the regenerated rotator cuff tendon function in walking ability was improved after UCB-derived MSC injection than HA and normal saline injections.
In our study, the full-thickness subscapularis tendon tear in rabbits was used. The subscapularis tendon tear in rabbits is commonly used as a model to study rotator cuff tendon regeneration, since the subscapularis tendon in rabbits is similar to the supraspinatus tendon in humans [4, 32].
Since the normal tendon healing process after injury is slow and leads to fibrotic scarring and adhesion, it is not functionally sufficient to repair the injured tendon tissue. This limitation has triggered an intense investigation into cell-mediated tendon therapies, given the low activity/low cell numbers of tendons. In cell-mediated treatment, a cell suspension can be injected at the site of lesion without surgical repairs. There is a report regarding successful results using platelet-rich plasma or stem cell injection without surgical repair in lateral epicondylitis [33]. The effect of cell-mediated treatment combined with surgical repair has been reported mostly in rotator cuff tears. In studies of stem cell therapy with surgical repair, stem cell injection is only an adjuvant therapy, and the effect of surgical repair overshadows the exact role of stem cell injection for the regeneration of the injured tendon. Our previous study [34] reported that ultrasound-guided platelet-rich plasma injection in rotator cuff disease had a positive therapeutic effect on healing of the rotator cuff tendon. Therefore, we thought stem cell injection has more regenerative potential for rotator cuff tendon tears than platelet-rich plasma injection. This is the first study to demonstrate the therapeutic effects of ultrasound-guided, human UCB-derived MSC injection to regenerate a full-thickness subscapularis tendon tear without surgical repair in a rabbit model.
There are some limitations in our study. First, we made an acute, full-thickness rotator cuff tendon tear using punch biopsy excision to resemble an acute traumatic tear. Therefore, it did not reflect chronic degenerative rotator cuff tendon tear in humans. Second, the partial-thickness and various sized full-thickness tendon tears were not used for the tendon regeneration. Third, the rabbits underwent immobilization for 2 days after the injection. Postoperative immobilization using a sling after the surgical repair of the rotator cuff is recommended for the first 4–6 weeks postoperatively to reduce stress on the operated tendon and improve microcirculation [35]. It is uncertain that 2 days of immobilization after injection was sufficient to heal the tendon tear in the rabbit model. Fourth, rehabilitative treatment after the injection was not performed. The rehabilitative treatment protects the surgical repair in the early stages of the tendon healing process and restores the function of the shoulder joint [36]. Fifth, we did not perform the biomechanical test of the regenerative tendon.
Therefore, future studies need to address the biomechanical property of the injected tendon, the long-term effects of UCB-derived MSCs, differences in various full-thickness tendon tear size, the appropriate immobilization period after injection, the optimal dose of UCB-derived MSCs, comparison with surgical repair, the most effective rehabilitation protocol after injection, and human trials.
Conclusion
UCB-derived MSC injection under ultrasound guidance without surgical repair or bioscaffold resulted in the partial healing of full-thickness rotator cuff tendon tears in a rabbit model. Histology revealed that UCB-derived MSCs induced regeneration of rotator cuff tendon tears and that the regenerated tissue was predominantly composed of type I collagens. In addition, motion analysis showed better walking capacity after MSC injection than HA or normal saline injection. These results suggest that ultrasound-guided UCB-derived MSC injection may be a useful conservative treatment for full-thickness rotator cuff tendon tear repair.
Acknowledgments
This study was supported by a Korea of Health & Welfare grant, Republic of Korea (Projects 090-091-3000-3033-320 and 090-091-3000-3033-340-320-01). We thank professor and anatomist Yong-Suk Moon for collecting and analyzing histological data presented in this article.
Author Contributions
G.-Y.P. and S.C.L.: conception and design, financial support, manuscript writing, final approval of manuscript; D.R.K.: conception and design, provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baydar M, Akalin E, El O, et al. The efficacy of conservative treatment in patients with full-thickness rotator cuff tears. Rheumatol Int. 2009;29:623–628. doi: 10.1007/s00296-008-0733-2. [DOI] [PubMed] [Google Scholar]
- 3.Berger MJ, Adams SD, Tigges BM, et al. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480–487. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- 4.Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 5.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 6.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 7.Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 8.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: A review. Clin Orthop Relat Res. 2010;468:1476–1484. doi: 10.1007/s11999-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cofield RH, Parvizi J, Hoffmeyer PJ, et al. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83-A:71–77. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Edwards SL, Lynch TS, Saltzman MD, et al. Biologic and pharmacologic augmentation of rotator cuff repairs. J Am Acad Orthop Surg. 2011;19:583–589. doi: 10.5435/00124635-201110000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Grumet RC, Hadley S, Diltz MV, et al. Development of a new model for rotator cuff pathology: The rabbit subscapularis muscle. Acta Orthop. 2009;80:97–103. doi: 10.1080/17453670902807425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 16.Hayatsu K, De Deyne PG. Muscle adaptation during distraction osteogenesis in skeletally immature and mature rabbits. J Orthop Res. 2001;19:897–905. doi: 10.1016/S0736-0266(01)00002-X. [DOI] [PubMed] [Google Scholar]
- 17.Isaac C, Gharaibeh B, Witt M, et al. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21:181–190. doi: 10.1016/j.jse.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Lafosse L, Brozska R, Toussaint B, et al. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89:1533–1541. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 19.Sohn DS, Moon JW, Lee WH, et al. Comparison of new bone formation in the maxillary sinus with and without bone grafts: immunochemical rabbit study. Int J Oral Maxillofac Implants. 2011;26:1033–1042. [PubMed] [Google Scholar]
- 20.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 21.Lee JK, Lee MK, Jin HJ, et al. Efficient intracytoplasmic labeling of human umbilical cord blood mesenchymal stromal cells with ferumoxides. Cell Transplant. 2007;16:849–857. doi: 10.3727/000000007783465271. [DOI] [PubMed] [Google Scholar]
- 22.Longo UG, Lamberti A, Maffulli N, et al. Tissue engineered biological augmentation for tendon healing: A systematic review. Br Med Bull. 2011;98:31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 23.Longo UG, Rizzello G, Berton A, et al. Biological strategies to enhance rotator cuff healing. Curr Stem Cell Res Ther. 2013;8:464–470. doi: 10.2174/1574888x113086660065. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 27.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 28.Kryger GS, Chong AK, Costa M, et al. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32:597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang HW, Goh JC, Lee EH. Viability of allogeneic bone marrow stromal cells following local delivery into patella tendon in rabbit model. Cell Transplant. 2004;13:649–657. [PubMed] [Google Scholar]
- 30.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 31.Jang KM, Lim HC, Jung WY, et al. Efficacy and safety of human umbilical cord blood-derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31:1530–1539. doi: 10.1016/j.arthro.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Yang SE, Ha CW, Jung M, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 33.Connell D, Datir A, Alyas F, et al. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med. 2009;43:293–298. doi: 10.1136/bjsm.2008.056457. [DOI] [PubMed] [Google Scholar]
- 34.Rha DW, Park GY, Kim YK, et al. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: A randomized controlled trial. Clin Rehabil. 2013;27:113–122. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 35.Yokoya S, Mochizuki Y, Nagata Y, et al. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36:1298–1309. doi: 10.1177/0363546508314416. [DOI] [PubMed] [Google Scholar]
- 36.Yokoya S, Mochizuki Y, Natsu K, et al. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40:1259–1268. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]